
Filed Pursuant to Rule 497
Registration No. 333-214767
This preliminary prospectus supplement relates to an effective registration statement under the Securities Act of 1933, as amended, but is not complete and may be changed. This preliminary prospectus supplement is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED OCTOBER 10, 2017
PRELIMINARY PROSPECTUS SUPPLEMENT
(To prospectus dated September 7, 2017)
$

% Notes due
We are an internally-managed, non-diversified, closed-end investment company that has elected to be regulated as a business development company under the Investment Company Act of 1940, as amended, or the 1940 Act. Our investment objective is to maximize our portfolio’s total return by generating current income from our debt investments and capital appreciation from our warrant and equity-related investments.
We are offering $ in aggregate principal amount of % notes due , or the “Notes.” The Notes will mature on . We will pay interest on the Notes on and of each year, beginning on , 2018. We may redeem the Notes in whole or in part at any time or from time to time, at the redemption price set forth under “Description of Notes and the Offering—Optional Redemption” in this prospectus supplement. In addition, holders of the Notes can require us to repurchase the Notes at 100% of their principal amount upon the occurrence of a Change of Control Repurchase Event (as defined herein). The Notes will be issued in minimum denominations of $2,000 and integral multiples of $1,000 in excess thereof.
The Notes will be our unsecured obligations and rank pari passu, or equally in right of payment, with all outstanding and future unsecured unsubordinated indebtedness issued by Hercules Capital, Inc.
An investment in the Notes involves risks that are described in the “Supplementary Risk Factors ” section beginning on page S-14 in this prospectus supplement and the “Risk Factors” section beginning on page 14 of the accompanying prospectus.
This prospectus supplement and the accompanying prospectus contain important information you should know before investing in the Notes. Please read this prospectus supplement and the accompanying prospectus before investing and keep it for future reference. We file annual, quarterly and current reports, proxy statements and other information about us with the Securities and Exchange Commission, or the SEC. This information is available free of charge by contacting us at 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301, or by telephone by calling collect at (650) 289-3060 or on our website at www.htgc.com. The information on the websites referred to herein is not incorporated by reference into this prospectus supplement or the accompanying prospectus. The SEC also maintains a website at www.sec.gov that contains information about us.
| Per Note | Total | |||||||
| Public offering price(1) |
% | $ | ||||||
| Sales load (underwriting discounts and commissions) |
% | $ | ||||||
| Proceeds to us (before expenses)(2) |
% | $ | ||||||
| (1) | The public offering price set forth above does not include accrued interest, if any. Interest on the Notes will accrue from October , 2017 and must be paid by the purchaser if the Notes are delivered after October , 2017. |
| (2) | Before deducting expenses payable by us related to this offering, estimated at $ . See “Underwriting” in this prospectus supplement for complete details of underwriters’ compensation. |
THE NOTES ARE NOT DEPOSITS OR OTHER OBLIGATIONS OF A BANK AND ARE NOT INSURED BY THE FEDERAL DEPOSIT INSURANCE CORPORATION OR ANY OTHER GOVERNMENT AGENCY.
Neither the SEC nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Delivery of the Notes in book-entry form only through The Depository Trust Company will be made on or about October , 2017.
Joint Book-Running Managers
| Citigroup | Jefferies |
The date of this prospectus supplement is October , 2017.
You should rely only on the information contained in this prospectus supplement and the accompanying prospectus. We have not, and the underwriters have not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this prospectus supplement and the accompanying prospectus is accurate only as of the date on the front cover of this prospectus supplement or such prospectus, as applicable. Our business, financial condition, results of operations and prospects may have changed since that date.
This document is in two parts. The first part is this prospectus supplement, which describes the terms of this offering and also adds to and updates information contained in the accompanying prospectus. The second part is the accompanying prospectus, which gives more general information and disclosure. To the extent the information contained in this prospectus supplement differs from the information contained in the accompanying prospectus, the information in this prospectus supplement shall control. You should read this prospectus supplement and the accompanying prospectus together with the additional information described under the heading, “Available Information” before investing in our Notes.
TABLE OF CONTENTS
Prospectus Supplement
| Page | ||||
| S-1 | ||||
| S-12 | ||||
| S-14 | ||||
| S-19 | ||||
| S-20 | ||||
| S-21 | ||||
| S-22 | ||||
| S-34 | ||||
| S-38 | ||||
| S-43 | ||||
| S-43 | ||||
| S-43 | ||||
Prospectus
| Page | ||||
| 1 | ||||
| 10 | ||||
| 12 | ||||
| 14 | ||||
| 61 | ||||
| 62 | ||||
| 63 | ||||
| 66 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
67 | |||
| 125 | ||||
| 138 | ||||
S-i
| Page | ||||
| 162 | ||||
| 165 | ||||
| 176 | ||||
| 181 | ||||
| 201 | ||||
| 203 | ||||
| 204 | ||||
| 214 | ||||
| 220 | ||||
| 224 | ||||
| 229 | ||||
| 230 | ||||
| 237 | ||||
| 239 | ||||
| 241 | ||||
| 243 | ||||
| 256 | ||||
| 258 | ||||
| 258 | ||||
| 258 | ||||
| 258 | ||||
| 259 | ||||
| F-1 | ||||
S-ii
This summary highlights some of the information in this prospectus supplement and may not contain all of the information that is important to you. For a more complete understanding of this offering, we encourage you to read this entire prospectus supplement and the accompanying prospectus and the documents that are referenced in this prospectus supplement and the accompanying prospectus, together with any accompanying supplements. In this prospectus supplement and the accompanying prospectus, unless the context otherwise requires, the “Company,” “Hercules Capital,” “Hercules,” “we,” “us” and “our” refer to Hercules Capital, Inc. and our wholly-owned subsidiaries.
Our Company
We are a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences and sustainable and renewable technology industries. Our investment objective is to maximize our portfolio’s total return by generating current income from our debt investments and capital appreciation from our warrant and equity-related investments. We are an internally-managed, non-diversified closed-end investment company that has elected to be regulated as a business development company, or BDC, under the 1940 Act. Effective January 1, 2006, we elected to be treated for tax purposes as a regulated investment company, or RIC, under the Internal Revenue Code of 1986, as amended, or the Code.
As of June 30, 2017, our total assets were approximately $1.6 billion, of which our investments comprised $1.4 billion at fair value and $1.5 billion at cost. Since inception through June 30, 2017, we have made debt and equity commitments of approximately $6.9 billion to our portfolio companies.
We also make investments in qualifying small businesses through our two wholly owned small business investment companies, or SBICs. Our SBIC subsidiaries, Hercules Technology II, L.P., or HT II, and Hercules Technology III, L.P., or HT III, hold approximately $104.8 million and $271.5 million in assets, respectively, and accounted for approximately 5.8% and 14.9% of our total assets, respectively, prior to consolidation at June 30, 2017. At June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries. See “Regulation—Small Business Administration Regulations” in the accompanying prospectus for additional information regarding our SBIC subsidiaries.
As of June 30, 2017, our investment professionals, including Manuel A. Henriquez, our co-founder, Chairman, President and Chief Executive Officer, are currently comprised of 34 professionals who have, on average, more than 15 years of experience in venture capital, structured finance, commercial lending or acquisition finance with the types of technology-related companies that we are targeting. We believe that we can leverage the experience and relationships of our management team to successfully identify attractive investment opportunities, underwrite prospective portfolio companies and structure customized financing solutions.
S-1
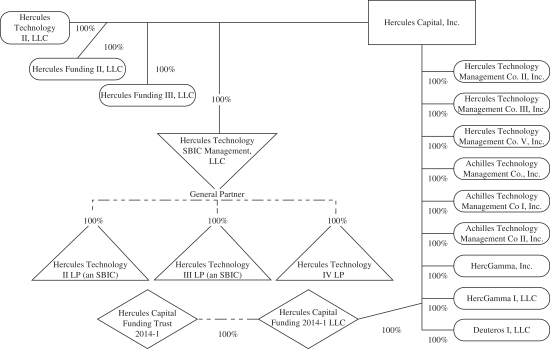
Organizational Chart
The following chart summarizes our organizational structure as of June 30, 2017. This chart is provided for illustrative purposes only.
Our Market Opportunity
We believe that technology-related companies compete in one of the largest and most rapidly growing sectors of the U.S. economy and that continued growth is supported by ongoing innovation and performance improvements in technology products as well as the adoption of technology across virtually all industries in response to competitive pressures. We believe that an attractive market opportunity exists for a specialty finance company focused primarily on investments in structured debt with warrants in technology-related companies for the following reasons:
| • | Technology-related companies have generally been underserved by traditional lending sources; |
| • | Unfulfilled demand exists for structured debt financing to technology-related companies due to the complexity of evaluating risk in these investments; and |
| • | Structured debt with warrants products are less dilutive and complement equity financing from venture capital and private equity funds. |
Technology-Related Companies are Underserved by Traditional Lenders. We believe many viable technology-related companies backed by financial sponsors have been unable to obtain sufficient growth financing from traditional lenders, including financial services companies such as commercial banks and finance companies because traditional lenders have continued to consolidate and have adopted a more risk-averse approach to lending. More importantly, we believe traditional lenders are typically unable to underwrite the risk associated with these companies effectively.
S-2
The unique cash flow characteristics of many technology-related companies typically include significant research and development expenditures and high projected revenue growth thus often making such companies difficult to evaluate from a credit perspective. In addition, the balance sheets of these companies often include a disproportionately large amount of intellectual property assets, which can be difficult to value. Finally, the speed of innovation in technology and rapid shifts in consumer demand and market share add to the difficulty in evaluating technology-related companies.
Due to the difficulties described above, we believe traditional lenders generally refrain from entering the structured debt financing marketplace, instead preferring the risk-reward profile of asset based lending. Traditional lenders generally do not have flexible product offerings that meet the needs of technology-related companies. The financing products offered by traditional lenders typically impose on borrowers many restrictive covenants and conditions, including limiting cash outflows and requiring a significant depository relationship to facilitate rapid liquidation.
Unfulfilled Demand for Structured Debt Financing to Technology-Related Companies. Private debt capital in the form of structured debt financing from specialty finance companies continues to be an important source of funding for technology-related companies. We believe that the level of demand for structured debt financing is a function of the level of annual venture equity investment activity.
We believe that demand for structured debt financing is currently underserved. The venture capital market for the technology-related companies in which we invest has been active. Therefore, to the extent we have capital available, we believe this is an opportune time to be active in the structured lending market for technology-related companies.
Structured Debt with Warrants Products Complement Equity Financing From Venture Capital and Private Equity Funds. We believe that technology-related companies and their financial sponsors will continue to view structured debt securities as an attractive source of capital because it augments the capital provided by venture capital and private equity funds. We believe that our structured debt with warrants products provides access to growth capital that otherwise may only be available through incremental investments by existing equity investors. As such, we provide portfolio companies and their financial sponsors with an opportunity to diversify their capital sources. Generally, we believe many technology-related companies at all stages of development target a portion of their capital to be debt in an attempt to achieve a higher valuation through internal growth. In addition, because financial sponsor-backed companies have reached a more mature stage prior to reaching a liquidity event, we believe our investments could provide the debt capital needed to grow or recapitalize during the extended period sometimes required prior to liquidity events.
Our Business Strategy
Our strategy to achieve our investment objective includes the following key elements:
Leverage the Experience and Industry Relationships of Our Management Team and Investment Professionals. We have assembled a team of experienced investment professionals with extensive experience as venture capitalists, commercial lenders, and originators of structured debt and equity investments in technology-related companies.
Mitigate Risk of Principal Loss and Build a Portfolio of Equity-Related Securities. We expect that our investments have the potential to produce attractive risk-adjusted returns through current income, in the form of interest and fee income, as well as capital appreciation from warrant and equity-related securities. We believe that we can mitigate the risk of loss on our debt investments through the combination of loan principal
S-3
amortization, cash interest payments, relatively short maturities (typically between 24 – 48 months), security interests in the assets of our portfolio companies, and on select investment covenants requiring prospective portfolio companies to have certain amounts of available cash at the time of our investment and the continued support from a venture capital or private equity firm at the time we make our investment.
Provide Customized Financing Complementary to Financial Sponsors’ Capital. We offer a broad range of investment structures and possess expertise and experience to effectively structure and price investments in technology-related companies.
Invest at Various Stages of Development. We provide growth capital to technology-related companies at all stages of development, including select publicly listed companies and select special opportunity lower middle market companies that require additional capital to fund acquisitions, recapitalizations and refinancings and established-stage companies.
Benefit from Our Efficient Organizational Structure. We believe that the perpetual nature of our corporate structure enables us to be a long-term partner for our portfolio companies in contrast to traditional investment funds, which typically have a limited life. In addition, because of our access to the equity markets, we believe that we may benefit from a lower cost of capital than that available to private investment funds.
Deal Sourcing Through Our Proprietary Database. We have developed a proprietary and comprehensive structured query language based database system to track various aspects of our investment process including sourcing, originations, transaction monitoring and post-investment performance.
Recent Developments
Evaluation of Alternative Management Structures
On May 3, 2017, we filed preliminary proxy materials with the SEC for a special meeting of stockholders to seek approval for a proposed advisory agreement with Hamilton Advisers LLC. However, after receiving feedback from our stockholders, on May 15, 2017, we decided to postpone the proposed special meeting of stockholders indefinitely and formally withdrew the proxy materials containing our proposal seeking stockholder approval of our plans to externalize our management structure to expand our ongoing review process. We, along with our professional advisors, are currently evaluating alternatives with respect to our management structure. The evaluation process is still ongoing, and we are continuing to move forward in evaluating various options, but we currently have no definitive timeline for completion. While internal management has served us well since our formation, our board of directors, or the Board of Directors, has concluded that there are limitations to that management structure that may operate to our disadvantage. To that end, we are exploring the possibility of externalizing our management structure as a means of addressing those concerns; and, we are also examining various alternatives that could be pursued with respect to externalization if we determine that externalization is the proper course to follow. We and our independent directors are working with advisors to assist in these efforts. This program will result in us incurring additional and unusual expense until this project is concluded. Should we determine to pursue externalization, which would be subject to approval by our stockholders, it could involve some disruption (at least on a temporary basis) and expense during the period of transition.
Distribution Declaration
On July 26, 2017, our Board of Directors declared a cash distribution of $0.31 per share to be paid on August 21, 2017 to stockholders of record as of August 14, 2017. This distribution represented our forty-eighth consecutive distribution since our initial public offering, bringing the total cumulative distribution to date to $13.40 per share.
S-4
Closed and Pending Commitments
As of October 6, 2017, we have:
| • | Closed debt and equity commitments of approximately $166.4 million to new and existing portfolio companies and funded approximately $158.7 million subsequent to June 30, 2017. |
| • | Pending commitments (signed non-binding term sheets) of approximately $60.0 million. The table below summarizes our year-to-date closed and pending commitments as follows: |
| Closed Commitments and Pending Commitments (in millions) |
||||
| January 1— June 30, 2017 Closed Commitments |
$ | 397.0 | ||
| July 1—October 6, 2017 Closed Commitments (a) |
$ | 166.4 | ||
| Pending Commitments (as of October 6, 2017)(b) |
$ | 60.0 | ||
|
|
|
|||
| Closed and Pending Commitments as of October 6, 2017 |
$ | 623.4 | ||
|
|
|
| a. | Closed Commitments may include renewals of existing credit facilities. Not all Closed Commitments result in future cash requirements. Commitments generally fund over the two succeeding quarters from close. |
| b. | Not all pending commitments (signed non-binding term sheets) are expected to close and they do not necessarily represent any future cash requirements. |
Year-to-Date Momentum Continues with New Originations and Closed Commitments on Pace to Exceed 2016
| • | Closed total new debt and equity commitments of approximately $154.0 million to seven (7) companies including five (5) new and two (2) existing portfolio companies in Q3 2017. Closed total new debt and equity commitments of approximately $552.0 million for the first nine months of 2017. |
| • | “Early loan pay-offs,” or unscheduled principal repayments of approximately $115.0 million, consisting of a large amount of older loans which typically have lower call premiums, for Q3 2017. Early loan pay-offs for the first nine months of 2017 of approximately $382.0 million. |
Portfolio Company Developments
As of October 6, 2017, we held warrants or equity positions in 6 companies that have filed registration statements on Form S-1 with the SEC in contemplation of potential initial public offerings, including ForeScout Technologies, Inc., and 5 companies which filed confidentially under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). There can be no assurance that these companies will complete their initial public offerings in a timely manner or at all. In addition, subsequent to June 30, 2017, our portfolio companies announced or completed the following liquidity events:
| 1. | In August 2017, our portfolio companies Cempra, Inc. (NASDAQ: CEMP), a clinical-stage pharmaceutical company focused on developing differentiated anti-infectives for acute care and community settings to meet critical medical needs in the treatment of infectious diseases, and Melinta Therapeutics, Inc., a privately held company focused on discovering, developing, and commercializing novel antibiotics to treat serious bacterial infections, announced that the companies had entered into a definitive agreement under which Melinta will merge with a subsidiary of Cempra. The merger is expected to create a NASDAQ-listed company committed to discovering, developing and commercializing important anti-infective therapies for patients and physicians in areas of significant unmet need. The merger is expected to close in the fourth quarter of 2017, subject to the approval of the stockholders of each company as well as other customary conditions. We committed $40.0 million in venture debt financing to Cempra from 2011 to 2014. We initially committed $30.0 million in venture debt financing to Melinta in December 2014 and currently hold 1,194,448 shares of Preferred Series 4 stock as of June 30, 2017. |
S-5
| 2. | In August 2017, our portfolio company CashStar, Inc., a leading provider of gift card commerce solutions at the forefront of mobile payments and digital gifting innovation, was acquired by Blackhawk Network, Inc., a global financial technology company and a leader in connecting brands and people through branded value solutions, for $175.0 million in cash. We initially committed $8.0 million in venture debt financing in June 2013 and currently hold warrants for 727,272 shares of Preferred Series C-2 stock as of June 30, 2017. |
| 3. | In September 2017, our portfolio company Cloud Technology Partners, Inc., a born-in-the-cloud services company with strong enterprise experience, announced that Hewlett Packard Enterprise intends to acquire the company to accelerate IT services growth as they transition from a traditional hardware business to a hybrid IT strategy. Terms of the deal were not disclosed. We initially committed $14.4 million in venture debt financing in December 2016 and currently hold warrants for 113,960 shares of Preferred Series C stock as of June 30, 2017. |
| 4. | In September 2017, our portfolio company Exicure, Inc., a clinical stage biotechnology company developing a new class of immunomodulatory and gene slicing drugs against validated targets, announced the closing of a $20 million private placement financing and the completion of a reverse merger transaction, with Max-1 Acquisition Corporation, a blank check company. Following the reverse merger transaction, Max-1 changed its name to Exicure, Inc., and will continue the historical business of Exicure. |
General Information
Our principal executive offices are located at 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301, and our telephone number is (650) 289-3060. We also have offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT, and San Diego, CA. We maintain a website on the Internet at www.htgc.com. We make available, free of charge, on our website our proxy statement, annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information contained on our website is not incorporated by reference into this prospectus supplement or the accompanying prospectus, and you should not consider that information to be part of this prospectus supplement or the accompanying prospectus.
We file annual, quarterly and current periodic reports, proxy statements and other information with the SEC under the Securities Exchange Act of 1934, as amended, or the Exchange Act. This information is available at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information about the operation of the SEC’s public reference room by calling the SEC at (202) 551-8090. In addition, the SEC maintains an Internet website, at www.sec.gov, that contains reports, proxy and information statements, and other information regarding issuers, including us, who file documents electronically with the SEC.
S-6
The Offering
This prospectus supplement sets forth certain terms of the Notes that we are offering pursuant to this prospectus supplement and supplements the accompanying prospectus that is attached to the back of this prospectus supplement. This section outlines the specific legal and financial terms of the Notes. You should read this section together with the more general description of the Notes under the heading “Description of Notes” in this prospectus supplement and in the accompanying prospectus under the heading “Description of Our Debt Securities” before investing in the Notes. Capitalized terms used in this prospectus supplement and not otherwise defined shall have the meanings ascribed to them in the accompanying prospectus or in the indenture governing the Notes (as amended from time to time, the “indenture”).
| Issuer |
Hercules Capital, Inc. |
| Title of the securities |
% Notes due |
| Aggregate principal amount being offered |
$ |
| Initial public offering price |
% of the aggregate principal amount. |
| Interest Rate |
% |
| Yield to Maturity |
% |
| Trade Date |
October , 2017 |
| Issue Date |
October , 2017 |
| Stated Maturity Date |
, |
| Day Count Basis |
360-day year of twelve 30-day months |
| Interest payment dates for the Notes |
Each and commencing , 2018. If an interest payment date falls on a non-business day, the applicable interest payment will be made on the next business day and no additional interest will accrue as a result of such delayed payment. |
| Regular Record Dates for Interest |
Each and . |
| Specified Currency |
U.S. Dollars |
| Place of Payment |
New York City or such other office designated by the Trustee |
| Ranking of Notes |
The Notes will be our unsecured obligations that rank senior in right of payment to all of our existing and future indebtedness that is expressly subordinated, or junior, in right of payment to the Notes. The Notes will not be guaranteed by any of our current or future subsidiaries. The Notes will rank pari passu, or equally, in right of payment with all of our existing and future liabilities that are not so subordinated, or junior. The Notes will effectively rank subordinated, |
S-7
| or junior, to any of our secured indebtedness (including unsecured indebtedness that we later secure) to the extent of the value of the assets securing such indebtedness. The Notes will rank structurally subordinated, or junior, to all existing and future indebtedness (including trade payables) incurred by our subsidiaries, financing vehicles or similar facilities. |
| As of June 30, 2017, our total consolidated indebtedness was approximately $766.4 million, which included: |
| • | approximately $ 258.5 million of 6.25% notes due 2024 (the “2024 Notes”); approximately $230.0 million in aggregate principal amount of 4.375% convertible notes due 2022 (the “2022 Convertible Notes”). |
| • | indebtedness and other obligations of any of our subsidiaries, including, without limitation, the indebtedness of HT II and HT III, borrowings under the $120.0 million revolving senior secured credit facility with Wells Fargo Capital Finance, LLC, (the “Wells Facility”), borrowings under the $75.0 million revolving senior secured credit facility with MUFG Union Bank, N. A. (the “Union Bank Facility,” and together with the Wells Facility, the “Credit Facilities”) and the approximately $87.7 million of fixed-rate asset-backed notes (the “Asset-Backed Notes”), each as of June 30, 2017. Note that there were no borrowings outstanding under the Wells Facility or Union Bank Facility as of June 30, 2017. |
| We expect to use a portion of the proceeds of this offering to repurchase or redeem all or a portion of our 2024 Notes, see “Use of Proceeds.” After giving effect to the issuance of the Notes and assuming the proceeds therefrom are used to repurchase or redeem all or a portion of our 2024 Notes, our total consolidated indebtedness would have been approximately $ million aggregate principal amount outstanding as of June 30, 2017. See “Capitalization.” |
| Denominations |
We will issue the Notes in denominations of $2,000 and integral multiples of $1,000 in excess thereof. |
| Business Day |
Each Monday, Tuesday, Wednesday, Thursday and Friday that is not a day on which banking institutions in New York City, or in such other place of payment designated by the Trustee, are authorized or required by law or executive order to close. |
| Optional Redemption |
We may redeem some or all of the Notes at any time, or from time to time, at a redemption price equal to the greater of (1) 100% of the principal amount of the Notes to be redeemed or (2) the sum of the present values of the remaining scheduled payments of principal and interest (exclusive of accrued and unpaid interest to the date of redemption) on the Notes to be redeemed, discounted to the redemption date on a semi-annual basis (assuming a 360-day year |
S-8
| consisting of twelve 30-day months) using the applicable Treasury Rate plus basis points, plus, in each case, accrued and unpaid interest to the redemption date; provided, however, that if we redeem any Notes on or after , (the date falling prior to the maturity date of the Notes), the redemption price for the Notes will be equal to 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest, if any, to, but excluding, the date of redemption. |
| You may be prevented from exchanging or transferring the Notes when they are subject to redemption. |
| If we are redeeming less than all of the Notes, the particular Notes to be redeemed will be selected in accordance with the applicable procedures of the Trustee and, so long as the Notes are registered to The Depository Trust Company or its nominee, DTC; provided, however, that no such partial redemption shall reduce the portion of the principal amount of a Note not redeemed to less than $2,000. Unless we default in payment of the redemption price, on and after the date of redemption, interest will cease to accrue on the Notes or portions of the Notes called for redemption. |
| Sinking Fund |
The Notes will not be subject to any sinking fund. A sinking fund is a reserve fund accumulated over a period of time for the retirement of debt. |
| Offer to Purchase upon a Change of Control Repurchase Event |
If a Change of Control Repurchase Event occurs prior to maturity, holders will have the right, at their option, to require us to repurchase for cash some or all of the Notes at a repurchase price equal to 100% of the principal amount of the Notes being repurchased, plus accrued and unpaid interest to, but not including, the repurchase date. |
| Defeasance and Covenant Defeasance |
The Notes are subject to defeasance by us, which means that, subject to the satisfaction of certain conditions, including, but not limited to, (i) depositing in trust for the benefit of the holders of the Notes a combination of money and/or U.S. government or U.S. government agency notes or bonds that will generate enough cash to make interest, principal and any other payments on the Notes on their various due dates and (ii) delivering to the Trustee an opinion of counsel as described herein under “Description of Notes—Satisfaction and Discharge; Defeasance,” we can legally release ourselves from all payment and other obligations on the Notes. |
| The Notes are subject to covenant defeasance by us, which means that, subject to the satisfaction of certain conditions, including, but not limited to, (i) depositing in trust for the benefit of the holders of the Notes a combination of money and/or U.S. government or U.S. government agency notes or bonds that will generate enough cash to make interest, principal and any other payments on the Notes on their various due dates and (ii) delivering to the Trustee an opinion of counsel as described herein under “Description of Notes— |
S-9
| Satisfaction and Discharge; Defeasance,” we will be released from some of the restrictive covenants in the indenture. |
| Form of Notes |
The Notes will be represented by global securities that will be deposited and registered in the name of DTC or its nominee. Except in limited circumstances, you will not receive certificates for the Notes. Beneficial interests in the Notes will be represented through book-entry accounts of financial institutions acting on behalf of beneficial owners as direct and indirect participants in DTC. Investors may elect to hold interests in the Notes through either DTC, if they are a participant, or indirectly through organizations which are participants in DTC. |
| Trustee, Paying Agent and Security Registrar |
U.S. Bank National Association |
| Other Covenants |
In addition to the covenants described in the prospectus attached to this prospectus supplement, the following covenants shall apply to the Notes: |
| • | We agree that for the period of time during which the Notes are outstanding, we will not violate, whether or not we are subject to, Section 18(a)(1)(A) as modified by Section 61(a)(1) of the 1940 Act or any successor provisions, giving effect to any exemptive relief granted to us by the SEC (even if we are no longer subject to the 1940 Act). Currently, these provisions generally prohibit us from making additional borrowings, including through the issuance of additional debt or the sale of additional debt securities, unless our asset coverage, as defined in the 1940 Act, equals at least 200% after such borrowings. See “Risk Factor—Risks Related to our Business Structure—Legislation may allow us to incur additional leverage,” in the accompanying prospectus. |
| • | If, at any time, we are not subject to the reporting requirements of Sections 13 or 15(d) of the Exchange Act to file any periodic reports with the SEC, we agree to furnish to holders of the Notes and the Trustee, for the period of time during which the Notes are outstanding, our audited annual consolidated financial statements, within 90 days of our fiscal year end, and unaudited interim consolidated financial statements, within 45 days of our fiscal quarter end (other than our fourth fiscal quarter). All such financial statements will be prepared, in all material respects, in accordance with applicable United States generally accepted accounting principles (“GAAP”), as applicable. |
| Events of Default |
If an event of default (as described herein under “Description of Notes”) on the Notes occurs, the principal amount of the Notes, plus accrued and unpaid interest, may be declared immediately due and payable, subject to conditions set forth in the indenture. These amounts automatically become due and payable in the case of certain types of bankruptcy or insolvency events involving us. |
S-10
| No Established Trading Market |
The Notes are a new issue of securities with no established trading market. The Notes will not be listed on any securities exchange or quoted on any automated dealer quotation system. Although the underwriters have informed us that they intend to make a market in the Notes, as permitted by applicable laws and regulations, they are not obligated to do so and may discontinue any such market making activities at any time without notice. See “Underwriting.” Accordingly, we cannot assure you that a liquid market for the Notes will develop or be maintained. |
| Global Clearance and Settlement Procedures |
Interests in the Notes will trade in DTC’s Same Day Funds Settlement System, and any permitted secondary market trading activity in such Notes will, therefore, be required by DTC to be settled in immediately available funds. None of the issuer, the Trustee or the paying agent will have any responsibility for the performance by DTC or its participants or indirect participants of their respective obligations under the rules and procedures governing their operations. |
| Further Issuances |
We have the ability to issue additional debt securities under the indenture with terms different from the Notes and, without the consent of the holders thereof, to reopen the Notes and issue additional Notes. |
| Use of Proceeds |
We estimate that the net proceeds we receive from the sale of the $ million aggregate principal amount of Notes in this offering will be approximately $ million after deducting the underwriting discount of approximately $ million payable by us and estimated offering expenses of approximately $ payable by us. We expect to use the net proceeds from this offering (i) to repurchase or redeem all or a portion of our 2024 Notes, (ii) to fund investments in debt and equity securities in accordance with our investment objective, and (iii) for other general corporate purposes. |
| Governing Law |
The Notes and the indenture will be governed by and construed in accordance with the laws of the State of New York. |
S-11
The matters discussed in this prospectus supplement and the accompanying prospectus, as well as in future oral and written statements by management of Hercules Capital, Inc., that are forward-looking statements are based on current management expectations that involve substantial risks and uncertainties which could cause actual results to differ materially from the results expressed in, or implied by, these forward-looking statements. Forward-looking statements relate to future events or our future financial performance. We generally identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Important assumptions include our ability to originate new investments, achieve certain margins and levels of profitability, the availability of additional capital, and the ability to maintain certain debt to asset ratios. In light of these and other uncertainties, the inclusion of a projection or forward-looking statement in this prospectus should not be regarded as a representation by us that our plans or objectives will be achieved. The forward-looking statements contained in this prospectus supplement and the accompanying prospectus include statements as to:
| • | our current and future management structure; |
| • | our future operating results; |
| • | our business prospects and the prospects of our prospective portfolio companies; |
| • | the impact of investments that we expect to make; |
| • | our informal relationships with third parties including in the venture capital industry; |
| • | the expected market for venture capital investments and our addressable market; |
| • | the dependence of our future success on the general economy and its impact on the industries in which we invest; |
| • | our ability to access debt markets and equity markets; |
| • | the ability of our portfolio companies to achieve their objectives; |
| • | our expected financings and investments; |
| • | our regulatory structure and tax status; |
| • | our ability to operate as a BDC, a SBIC and a RIC; |
| • | the adequacy of our cash resources and working capital; |
| • | the timing of cash flows, if any, from the operations of our portfolio companies; |
| • | the timing, form and amount of any distributions; |
| • | the impact of fluctuations in interest rates on our business; |
| • | the valuation of any investments in portfolio companies, particularly those having no liquid trading market; and |
| • | our ability to recover unrealized losses. |
For a discussion of factors that could cause our actual results to differ from forward-looking statements contained in this prospectus supplement and the accompanying prospectus, please see the discussion under “Supplementary Risk Factors” in this prospectus supplement and “Risk Factors” in the accompanying prospectus. We undertake no obligation to update any forward-looking statement to reflect events or circumstances occurring after the date of this prospectus supplement.
You should not place undue reliance on these forward-looking statements. The forward-looking statements made in this prospectus supplement relate only to events as of the date on which the statements are made and are
S-12
excluded from the safe harbor protection provided by Section 27A of the Securities Act of 1933, as amended, or the Securities Act.
Industry and Market Data
We have compiled certain industry estimates presented in this prospectus supplement and the accompanying prospectus from internally generated information and data. While we believe our estimates are reliable, they have not been verified by any independent sources. The estimates are based on a number of assumptions, including increasing investment in venture capital and private equity-backed companies. Actual results may differ from projections and estimates, and this market may not grow at the rates projected, or at all. If this market fails to grow at projected rates, our business and the market price of our securities, including the Notes, could be materially adversely affected.
S-13
Investing in our securities involves a number of significant risks. Before you invest in our securities, you should be aware of various risks, including those described below and those set forth in the accompanying prospectus. You should carefully consider these risk factors, together with all of the other information included in this prospectus supplement and the accompanying prospectus, before you decide whether to make an investment in our securities. The risks set out below and in the accompanying prospectus are not the only risks we face. Additional risks and uncertainties not presently known to us or not presently deemed material by us may also impair our operations and performance. If any of the following events occur, our business, financial condition, results of operations and cash flows could be materially and adversely affected which could materially adversely affect our ability to repay principal and interest on the Notes. In addition, the market price of the Notes and our net asset value could decline, and you may lose all or part of your investment. The risk factors described below, together with those set forth in the accompanying prospectus, are the principal risk factors associated with an investment in our securities, including the Notes, as well as those factors generally associated with an investment company with investment objectives, investment policies, capital structure or trading markets similar to ours.
Risks Related to the Notes
The Notes will be unsecured and therefore will be effectively subordinated to any secured indebtedness we have currently incurred or may incur in the future.
The Notes will not be secured by any of our assets or any of the assets of our subsidiaries. As a result, the Notes are effectively subordinated to any secured indebtedness we or our subsidiaries have currently incurred and may incur in the future (or any indebtedness that is initially unsecured to which we subsequently grant security) to the extent of the value of the assets securing such indebtedness. In any liquidation, dissolution, bankruptcy or other similar proceeding, the holders of any of our existing or future secured indebtedness and the secured indebtedness of our subsidiaries may assert rights against the assets pledged to secure that indebtedness in order to receive full payment of their indebtedness before the assets may be used to pay other creditors, including the holders of the Notes. As of June 30, 2017, we had no outstanding borrowings under our Union Bank Facility, which is secured by debt investments in our portfolio companies and related assets, and no outstanding borrowings under our Wells Facility, which is secured by loans in the borrowing base for the Wells Facility.
The Notes will be structurally subordinated to the indebtedness and other liabilities of our subsidiaries.
The Notes are obligations exclusively of Hercules Capital, Inc. and not of any of our subsidiaries. None of our subsidiaries is a guarantor of the Notes and the Notes are not required to be guaranteed by any subsidiaries we may acquire or create in the future. A significant portion of the indebtedness required to be consolidated on our balance sheet is held through our SBIC subsidiaries. For example, at June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries. The assets of such subsidiaries are not directly available to satisfy the claims of our creditors, including holders of the Notes. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity and Capital Resources” in the accompanying prospectus for more detail on the SBA-guaranteed debentures.
Except to the extent we are a creditor with recognized claims against our subsidiaries, all claims of creditors (including trade creditors), if any, of our subsidiaries will have priority over our equity interests in such subsidiaries (and therefore the claims of our creditors, including holders of the Notes) with respect to the assets of such subsidiaries. Even if we are recognized as a creditor of one or more of our subsidiaries, our claims would still be effectively subordinated to any security interests in the assets of any such subsidiary and to any indebtedness or other liabilities of any such subsidiary senior to our claims. Consequently, the Notes will be structurally subordinated to all indebtedness and other liabilities (including trade payables) of any of our subsidiaries and any subsidiaries that we may in the future acquire or establish as financing vehicles or otherwise.
S-14
As of June 30, 2017, we had no outstanding borrowings under our Wells Facility, no outstanding borrowings under our Union Bank Facility and approximately $190.2 million of indebtedness outstanding incurred by our SBIC subsidiaries, HT II and HT III. All of such indebtedness would be structurally senior to the Notes. In addition, our subsidiaries may incur substantial additional indebtedness in the future, all of which would be structurally senior to the Notes.
The indenture under which the Notes will be issued will contain limited protection for holders of the Notes.
The indenture under which the Notes will be issued offers limited protection to holders of the Notes. The terms of the indenture and the Notes do not restrict our or any of our subsidiaries’ ability to engage in, or otherwise be a party to, a variety of corporate transactions, circumstances or events that could have an adverse impact on your investment in the Notes. In particular, the terms of the indenture and the Notes will not place any restrictions on our or our subsidiaries’ ability to:
| • | issue securities or otherwise incur additional indebtedness or other obligations, including (1) any indebtedness or other obligations that would be equal in right of payment to the Notes, (2) any indebtedness or other obligations that would be secured and therefore rank effectively senior in right of payment to the Notes to the extent of the values of the assets securing such debt, (3) indebtedness of ours that is guaranteed by one or more of our subsidiaries and which therefore is structurally senior to the Notes and (4) securities, indebtedness or obligations issued or incurred by our subsidiaries that would be senior to our equity interests in our subsidiaries and therefore rank structurally senior to the Notes with respect to the assets of our subsidiaries, in each case other than an incurrence of indebtedness or other obligation that would cause a violation of Section 18(a)(1)(A) as modified by Section 61(a)(1) of the 1940 Act or any successor provisions, whether or not we continue to be subject to such provisions of the 1940 Act, but giving effect to any exemptive relief granted to us by the SEC (currently, these provisions generally prohibit us from making additional borrowings, including through the issuance of additional debt or the sale of additional debt securities, unless our asset coverage, as defined in the 1940 Act, equals at least 200% after such borrowings); |
| • | pay distributions on, or purchase or redeem or make any payments in respect of, capital stock or other securities ranking junior in right of payment to the Notes; |
| • | sell assets (other than certain limited restrictions on our ability to consolidate, merge or sell all or substantially all of our assets); |
| • | enter into transactions with affiliates; |
| • | create liens (including liens on the shares of our subsidiaries) or enter into sale and leaseback transactions; |
| • | make investments; or |
| • | create restrictions on the payment of distributions or other amounts to us from our subsidiaries. |
Furthermore, the terms of the indenture and the Notes do not protect holders of the Notes in the event that we experience changes (including significant adverse changes) in our financial condition, results of operations or credit ratings, as they do not require that we or our subsidiaries adhere to any financial tests or ratios or specified levels of net worth, revenues, income, cash flow, or liquidity.
Our ability to recapitalize, incur additional debt and take a number of other actions that are not limited by the terms of the Notes may have important consequences for you as a holder of the Notes, including making it more difficult for us to satisfy our obligations with respect to the Notes or negatively affecting the trading value of the Notes.
Certain of our current debt instruments include more protections for their holders than the indenture and the Notes. See “Risk Factors—In addition to regulatory requirements that restrict our ability to raise capital, our
S-15
Credit Facilities and the 2024 Notes contain various covenants which, if not complied with, could accelerate repayment under the facility or require us to repurchase the 2024 Notes thereby materially and adversely affecting our liquidity, financial condition, results of operations and ability to pay distributions” in the accompanying prospectus. In addition, other debt we issue or incur in the future could contain more protections for its holders than the indenture and the Notes, including additional covenants and events of default. The issuance or incurrence of any such debt with incremental protections could affect the market for, and trading levels and prices of, the Notes.
Our amount of debt outstanding may increase as a result of this offering. Our current indebtedness could adversely affect our business, financial condition and results of operations and our ability to meet our payment obligations under the Notes and our other debt.
The use of debt could have significant consequences on our future operations, including:
| • | making it more difficult for us to meet our payment and other obligations under the Notes and our other outstanding debt; |
| • | resulting in an event of default if we fail to comply with the financial and other restrictive covenants contained in our financing arrangements, which event of default could result in substantially all of our debt becoming immediately due and payable; |
| • | reducing the availability of our cash flow to fund investments, acquisitions and other general corporate purposes, and limiting our ability to obtain additional financing for these purposes; |
| • | subjecting us to the risk of increased sensitivity to interest rate increases on our indebtedness with variable interest rates, including borrowings under our financing arrangements; and |
| • | limiting our flexibility in planning for, or reacting to, and increasing our vulnerability to, changes in our business, the industry in which we operate and the general economy. |
Any of the above-listed factors could have an adverse effect on our business, financial condition and results of operations and our ability to meet our payment obligations under the Notes and our other debt.
Our ability to meet our payment and other obligations under our financing arrangements depends on our ability to generate significant cash flow in the future. This, to some extent, is subject to general economic, financial, competitive, legislative and regulatory factors as well as other factors that are beyond our control. We cannot assure you that our business will generate cash flow from operations, or that future borrowings will be available to us under our financing arrangements or otherwise, in an amount sufficient to enable us to meet our payment obligations under the Notes and our other debt and to fund other liquidity needs. If we are not able to generate sufficient cash flow to service our debt obligations, we may need to refinance or restructure our debt, including the Notes, sell assets, reduce or delay capital investments, or seek to raise additional capital. If we are unable to implement one or more of these alternatives, we may not be able to meet our payment obligations under the Notes and our other debt.
We may not be able to repurchase the Notes upon a Change of Control Repurchase Event.
Upon the occurrence of a Change of Control Repurchase Event, as defined in the indenture, as supplemented, subject to certain conditions, we will be required to offer to repurchase all outstanding Notes at 100% of their principal amount, plus accrued and unpaid interest. The source of funds for that purchase of Notes will be our available cash or cash generated from our operations or other potential sources, including borrowings, investment repayments, sales of assets or sales of equity. We cannot assure you that sufficient funds from such sources will be available at the time of any Change of Control Repurchase Event to make required repurchases of Notes tendered. The terms of certain of our and our subsidiaries’ financing arrangements provide that certain change of control events will constitute an event of default thereunder entitling the lenders to accelerate any
S-16
indebtedness outstanding under the our and our subsidiaries’ financing arrangements at that time and to terminate the financing arrangements. In addition, the indenture governing our 2022 Convertible Notes contains a provision that would require us to offer to purchase the 2022 Convertible Notes upon the occurrence of a fundamental change. A failure to purchase any tendered 2022 Convertible Notes would constitute an event of default under the indenture for the 2022 Convertible Notes, which would, in turn, constitute a default under the Credit Facilities and the indenture. Our and our subsidiaries’ future debt instruments may also contain similar restrictions and provisions. If the holders of the Notes exercise their right to require us to repurchase Notes upon a Change of Control Repurchase Event, the financial effect of this repurchase could cause a default under our and our subsidiaries’ future debt instruments, even if the Change of Control Repurchase Event itself would not cause a default. It is possible that we will not have sufficient funds at the time of the Change of Control Repurchase Event to make the required repurchase of the Notes and/or our other debt. See “Description of Notes—Offer to Repurchase Upon a Change of Control Repurchase Event.”
An active trading market for the Notes may not develop or be maintained, which could limit the market price of the Notes or your ability to sell them.
The Notes are a new issue of debt securities for which there currently is no trading market. We do not intend to apply for listing of the Notes on any securities exchange or for quotation of the Notes on any automated dealer quotation system. If no active trading market develops, you may not be able to resell your Notes at their fair market value or at all. If the Notes are traded after their initial issuance, they may trade at a discount from their initial offering price depending on prevailing interest rates, the market for similar securities, our credit ratings, general economic conditions, our financial condition, performance and prospects and other factors. The underwriters have advised us that they intend to make a market in the Notes, but they are not obligated to do so. The underwriters may discontinue any market-making in the Notes at any time at their sole discretion. Accordingly, we cannot assure you that a liquid trading market will develop or be maintained for the Notes, that you will be able to sell your Notes at a particular time or that the price you receive when you sell will be favorable. To the extent an active trading market does not develop or is not maintained, the liquidity and trading price for the Notes may be harmed. Accordingly, you may be required to bear the financial risk of an investment in the Notes for an indefinite period of time.
A downgrade, suspension or withdrawal of a credit rating assigned by a rating agency to us or our unsecured debt, if any, or change in the debt markets could cause the liquidity or market value of the Notes to decline significantly.
Our credit ratings are an assessment by rating agencies of our ability to pay our debts when due. Consequently, real or anticipated changes in our credit ratings will generally affect the market value of the Notes. These credit ratings may not reflect the potential impact of risks relating to the structure or marketing of the Notes. Credit ratings are not a recommendation to buy, sell or hold any security, and may be revised or withdrawn at any time by the issuing organization in its sole discretion. Neither we nor any underwriter undertakes any obligation to maintain our credit ratings or to advise holders of Notes of any changes in our credit ratings. There can be no assurance that our credit ratings will remain for any given period of time or that such credit ratings will not be lowered or withdrawn entirely by the rating agencies if in their judgment future circumstances relating to the basis of the credit ratings, such as adverse changes in our company, so warrant. The conditions of the financial markets and prevailing interest rates have fluctuated in the past and are likely to fluctuate in the future, which could have an adverse effect on the market prices of the Notes.
If we Default on our obligations to pay our other indebtedness, we may not be able to make payments on the Notes.
Any default under the agreements governing our indebtedness, including a default under the Wells Facility, the Union Bank Facility, the 2024 Notes, the 2022 Convertible Notes and the Asset-Backed Notes or other indebtedness to which we may be a party, that is not waived by the required lenders or holders, and the remedies
S-17
sought by the holders of such indebtedness, could make us unable to pay principal, premium, if any, and interest on the Notes and substantially decrease the market value of the Notes. If we are unable to generate sufficient cash flow and are otherwise unable to obtain funds necessary to meet required payments of principal, premium, if any, and interest on our indebtedness, or if we otherwise fail to comply with the various covenants, including financial and operating covenants, in the instruments governing our indebtedness, we could be in default under the terms of the agreements governing such indebtedness. In the event of such default, the holders of such indebtedness could elect to declare all the funds borrowed thereunder to be due and payable, together with accrued and unpaid interest, the lenders under the Wells Facility and the Union Bank Facility or other debt we may incur in the future could elect to terminate their commitments, cease making further loans and institute foreclosure proceedings against our assets, and we could be forced into bankruptcy or liquidation. If our operating performance declines, we may in the future need to seek to obtain waivers from the required lenders under the Wells Facility or Union Bank Facility or the required holders of our 2024 Notes, 2022 Convertible Notes, Asset-Backed Notes or other debt that we may incur in the future to avoid being in default. If we breach our covenants under the Wells Facility, Union Bank Facility, the 2024 Notes, the 2022 Convertible Notes, the Asset-Backed Notes or other debt and seek a waiver, we may not be able to obtain a waiver from the required lenders or holders. If this occurs, we would be in default under the Wells Facility or Union Bank Facility, the 2024 Notes, the 2022 Convertible Notes, the Asset-Backed Notes or other debt, as applicable, the lenders or holders could exercise their rights as described above, and we could be forced into bankruptcy or liquidation. If we are unable to repay debt, lenders having secured obligations, including the lenders under the Wells Facility and the Union Bank Facility, could proceed against the collateral securing the debt. Because the Wells Facility and the Union Bank Facility have, and any future credit facilities will likely have, customary cross-default provisions, if the indebtedness under the Notes, the Wells Facility, Union Bank Facility, the 2024 Notes, the 2022 Convertible Notes or the Asset-Backed Notes or under any future credit facility is accelerated, we may be unable to repay or finance the amounts due. See “Specific Terms of the Notes and the Offering” in this prospectus supplement.
FATCA withholding may apply to payments to certain foreign entities.
Payments made under the Notes to a foreign financial institution or non-financial foreign entity (including such an institution or entity acting as an intermediary) may be subject to a U.S. withholding tax of 30% under U.S. Foreign Account Tax Compliance Act provisions of the Code (commonly referred to as “FATCA”). This U.S. withholding tax generally applies to payments of interest on the Notes as well as, after December 31, 2018, to any payments of gross proceeds (including principal payments) from the sale, redemption, retirement or other disposition of the Notes, unless the foreign financial institution or non-financial foreign entity complies with certain information reporting, withholding, identification, certification and related requirements imposed by FATCA. Depending upon the status of a holder and the status of an intermediary through which any Notes are held, the holder could be subject to this 30% U.S. withholding tax in respect of any interest paid on the Notes as well as any proceeds from the sale, redemption, retirement or other disposition of the Notes. Persons located in jurisdictions that have entered into an intergovernmental agreement with the United States to implement FATCA may be subject to different rules. You should consult your own tax advisors regarding FATCA and how it may affect your investment in the Notes. See “United States Federal Income Tax Matters—Taxation of Note Holders—FATCA” in this prospectus supplement for further information.
S-18
We estimate that the net proceeds we will receive from the sale of the $ million aggregate principal amount of Notes in this offering will be approximately $ , based on a public offering of % of par, after deducting the underwriting discount of approximately $ million payable by us and estimated offering expenses of approximately $ payable by us.
We expect to use the net proceeds from this offering (i) to repurchase or redeem all or a portion of our 2024 Notes, (ii) to fund investments in debt and equity securities in accordance with our investment objective, and (iii) for other general corporate purposes.
As of June 30, 2017, the aggregate principal balance of the 2024 Notes was approximately $258.5 million. The 2024 Notes bear interest at a rate of 6.25% per year, payable quarterly and mature, unless earlier repurchased or redeemed, on July 30, 2024.
We intend to seek to invest the net proceeds received in this offering as promptly as practicable after receipt thereof consistent with our investment objective. We anticipate that substantially all of the net proceeds from any offering of our securities will be used as described above within three to six months, depending on market conditions. We anticipate that the remainder will be used for working capital and general corporate purposes, including potential payments or distributions to shareholders. Pending such uses and investments, we will invest a portion of the net proceeds of this offering primarily in cash, cash equivalents, U.S. government securities or high-quality debt securities maturing in one year or less from the time of investment. Our ability to achieve our investment objectives may be limited to the extent that the net proceeds of this offering, pending full investment, are held in lower yielding short-term instruments.
The amount of net proceeds may be more or less than the amount described in this preliminary prospectus supplement depending on the amount of Notes we sell in the offering, which will be determined at pricing. To the extent that we receive more than the amount described in this preliminary prospectus supplement, we intend to use the net proceeds for investment in portfolio companies in accordance with our investment objective and strategies and for working capital and general corporate purposes. To the extent we receive less, the amount we have available for such purposes will be reduced.
S-19
The following table sets forth (i) our actual capitalization as of June 30, 2017, and (ii) our capitalization as adjusted to give effect to the sale of $ aggregate principal amount of Notes in this offering, after deducting the underwriting discounts and commissions of approximately $ million payable by us and estimated offering expenses of approximately $ payable by us and the application of the net proceeds therefrom as described under “Use of Proceeds.” You should read this table together with the “Use of Proceeds” section and our statement of assets and liabilities included elsewhere in this prospectus supplement.
| As of June 30, 2017 | ||||||||
| Actual | As Adjusted |
|||||||
| (in thousands) | ||||||||
| Investments at fair value |
$ | 1,395,469 | $ | |||||
| Cash and cash equivalents |
$ | 160,412 | $ | |||||
|
Debt(1): |
||||||||
| Accounts payable and accrued liabilities |
$ | 22,193 | $ | |||||
| Long-term SBA debentures |
187,824 | |||||||
| 2022 Convertible Notes |
222,898 | |||||||
| 2021 Asset-Backed Notes |
86,865 | |||||||
| 2024 Notes |
251,478 | |||||||
| Notes offered herein |
0 | |||||||
|
|
|
|
|
|||||
| Total debt |
$ | 771,258 | $ | |||||
|
|
|
|
|
|||||
| Stockholders’ equity: |
||||||||
| Common stock, par value $0.001 per share; 200,000,000 shares authorized; 83,844,624 shares issued and outstanding |
$ | 83 | $ | |||||
| Capital in excess of par value |
892,930 | |||||||
| Unrealized depreciation on investments |
(106,941 | ) | ||||||
| Accumulated realized gains on investments |
35,128 | |||||||
| Distributions in excess of investment income |
(3,749 | ) | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
$ | 817,451 | $ | |||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 1,588,709 | $ | |||||
|
|
|
|
|
|||||
| (1) | The above table reflects the principal amount of indebtedness outstanding as of June 30, 2017. As of October 6, 2017, indebtedness under the Wells Facility, the Union Bank Facility, the 2022 Convertible Notes, the 2024 Notes, and the Asset-Backed Notes were $0 million, $0 million, $230.0 million, $258.5 million and $65.5 million, respectively. The net proceeds from the sale of the Notes are expected to be used to fund investments in debt and equity securities in accordance with our investment objective, to retire certain debt obligations, and for other general corporate purposes. See “Use of Proceeds.” |
S-20
RATIO OF EARNINGS TO FIXED CHARGES
The following contains our ratio of earnings to fixed charges for the periods indicated, computed as set forth below. You should read these ratios of earnings to fixed charges in connection with our consolidated financial statements, including the notes to those statements, included in this prospectus supplement:
| For the six- months ended June 30, 2017 |
For the year ended December 31, 2016 |
For the year ended December 31, 2015 |
For the year ended December 31, 2014 |
For the year ended December 31, 2013 |
For the year ended December 31, 2012 |
|||||||||||||||||||
| Earnings to Fixed Charges(1) |
2.20 | 2.85 | 2.16 | 3.10 | 3.83 | 2.97 | ||||||||||||||||||
For purposes of computing the ratios of earnings to fixed charges, earnings represent net increase in stockholders’ equity resulting from operations plus fixed charges. Fixed charges include interest and credit facility fees expense and amortization of debt issuance costs.
| (1) | Earnings include net realized and unrealized gains or losses. Net realized and unrealized gains or losses can vary substantially from period to period. |
S-21
The following description of the particular terms of the % Notes due 20 supplements and, to the extent inconsistent with, replaces the description of the general terms and provisions of the debt securities set forth in the accompanying prospectus.
We will issue the Notes under a base indenture dated as of March 6, 2012, between us and U.S. Bank National Association, as trustee (the “trustee”), as supplemented by a separate supplemental indenture to be dated as of the settlement date for the Notes. As used in this section, all references to the “indenture” mean the base indenture as supplemented by the supplemental indenture. The terms of the Notes include those expressly set forth in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as amended, or the TIA.
The following description is a summary of the material provisions of the Notes and the indenture and does not purport to be complete. This summary is subject to and is qualified by reference to all the provisions of the Notes and the indenture, including the definitions of certain terms used in the indenture. We urge you to read these documents because they, and not this description, define your rights as a holder of the Notes.
For purposes of this description, references to “we,” “our” and “us” refer only to Hercules Capital, Inc. and not to any of its current or future subsidiaries and references to “subsidiaries” refer only to our consolidated subsidiaries and exclude any investments held by Hercules Capital in the ordinary course of business which are not, under GAAP, consolidated on the financial statements of Hercules Capital and its subsidiaries.
General
The Notes:
| • | will be our general unsecured, senior obligations; |
| • | will initially be issued in an aggregate principal amount of $ million; |
| • | will mature on , unless earlier redeemed or repurchased, as discussed below; |
| • | will bear cash interest from , 2017 at an annual rate of % payable semi-annually on and of each year, beginning on , 2018; |
| • | will not be subject to any sinking fund; |
| • | will be subject to redemption at our option as described under “—Optional Redemption;” |
| • | will be subject to repurchase by us at the option of the holders following a Change of Control Repurchase Event (as defined below under “—Offer to Repurchase Upon a Change of Control Repurchase Event”), at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest to, but excluding, the date of repurchase; |
| • | will be issued in denominations of $2,000 and integral multiples of $1,000 thereof; and |
| • | will be represented by one or more registered Notes in global form, but in certain limited circumstances may be represented by Notes in definitive form. See “—Book-Entry, Settlement and Clearance.” |
The indenture does not limit the amount of debt that may be issued by us or our subsidiaries under the indenture or otherwise. The indenture does not contain any financial covenants and does not restrict us from paying distributions or issuing or repurchasing our other securities. Other than restrictions described under “—Offer to Repurchase Upon a Change of Control Repurchase Event” and “—Merger, Consolidation or Sale of Assets” below, the indenture does not contain any covenants or other provisions designed to afford holders of the Notes protection in the event of a highly leveraged transaction involving us or in the event of a decline in our credit rating as the result of a takeover, recapitalization, highly leveraged transaction or similar restructuring involving us that could adversely affect such holders.
S-22
We may, without the consent of the holders, issue additional Notes under the indenture with the same terms (except for the issue date, public offering price and, if applicable, the initial interest payment date) and with the same CUSIP numbers as the Notes offered hereby in an unlimited aggregate principal amount; provided that if such additional Notes are not fungible with the Notes offered hereby (or any other tranche of additional Notes) for U.S. federal income tax purposes, then such additional Notes will have different CUSIP numbers from the Notes offered hereby (and any such other tranche of additional Notes).
We do not intend to apply to list the Notes on any securities exchange or any automated dealer quotation system.
Payments on the Notes; Paying Agent and Registrar; Transfer and Exchange
We will pay the principal of, and interest on, Notes in global form registered in the name of or held by DTC or its nominee in immediately available funds to DTC or its nominee, as the case may be, as the registered holder of such Global Note (as defined below).
Payment of principal of (and premium, if any) and any such interest on the Notes will be made at the corporate trust office of the trustee in such coin or currency of the United States of America as at the time of payment is legal tender for payment of public and private debts; provided, however, that at our option payment of interest may be made by check mailed to the address of the person entitled thereto as such address shall appear in the security register.
A holder of Notes may transfer or exchange Notes at the office of the registrar in accordance with the indenture. The registrar and the trustee may require a holder, among other things, to furnish appropriate endorsements and transfer documents. No service charge will be imposed by us, the trustee or the registrar for any registration of transfer or exchange of Notes, but we may require a holder to pay a sum sufficient to cover any transfer tax or other similar governmental charge required by law or permitted by the indenture.
The registered holder of a Note will be treated as its owner for all purposes.
Interest
The Notes will bear cash interest at a rate of % per year until maturity. Interest on the Notes will accrue from , 2017 or from the most recent date on which interest has been paid or duly provided for. Interest will be payable semiannually in arrears on and of each year, beginning on , 2018.
Interest will be paid to the person in whose name a Note is registered at 5:00 p.m. New York City time (the “close of business”) on or , as the case may be, immediately preceding the relevant interest payment date. Interest on the Notes will be computed on the basis of a 360-day year composed of twelve 30-day months.
If any interest payment date, redemption date, the maturity date or any earlier required repurchase date upon a Change of Control Repurchase Event (defined below) of a Note falls on a day that is not a business day, the required payment will be made on the next succeeding business day and no interest on such payment will accrue in respect of the delay. The term “business day” means, with respect to any Note, any day other than a Saturday, a Sunday or a day on which banking institutions in New York are authorized or obligated by law or executive order to close.
Ranking
The Notes will be our unsecured obligations that rank senior in right of payment to all of our existing and future indebtedness that is expressly subordinated, or junior, in right of payment to the Notes. The Notes will not be guaranteed by any of our current or future subsidiaries. The Notes will rank pari passu, or equally, in right of
S-23
payment with all of our existing and future liabilities that are not so subordinated, or junior. The Notes will effectively rank subordinated, or junior, to any of our secured indebtedness (including unsecured indebtedness that we later secure) to the extent of the value of the assets securing such indebtedness. The Notes will rank structurally subordinated, or junior, to all existing and future indebtedness (including trade payables) incurred by our subsidiaries, financing vehicles or similar facilities. In the event of our bankruptcy, liquidation, reorganization or other winding up, our assets that secure secured debt will be available to pay obligations on the Notes only after all indebtedness under such secured debt has been repaid in full from such assets. We advise you that there may not be sufficient assets remaining to pay amounts due on any or all the Notes then outstanding.
As of June 30, 2017, our total consolidated indebtedness was approximately $766.4 million aggregate principal amount outstanding, of which approximately $87.7 million was secured indebtedness. After giving effect to the issuance of the Notes, our total consolidated indebtedness would have been approximately $ million aggregate principal amount outstanding as of June 30, 2017. See “Capitalization.”
Optional Redemption
We may redeem some or all of the Notes at any time, or from time to time. If we choose to redeem any Notes prior to maturity, we will pay a redemption price equal to the greater of the following amounts, plus, in each case, accrued and unpaid interest to the redemption date:
| • | 100% of the principal amount of the Notes to be redeemed, or |
| • | the sum of the present values of the remaining scheduled payments of principal and interest (exclusive of accrued and unpaid interest to the date of redemption) on the Notes to be redeemed, discounted to the redemption date on a semi-annual basis (assuming a 360-day year consisting of twelve 30- day months) using the applicable Treasury Rate plus basis points; |
provided, however, that if we redeem any Notes on or after (the date falling one month prior to the maturity date of the Notes), the redemption price for the Notes will be equal to 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest, if any, to, but excluding, the date of redemption.
If we choose to redeem any Notes, we will deliver a notice of redemption to holders of Notes not less than 30 nor more than 60 days before the redemption date. If we are redeeming less than all of the Notes, the particular Notes to be redeemed will be selected in accordance with the applicable procedures of the trustee and, so long as the Notes are registered to DTC or its nominee, DTC; provided, however, that no such partial redemption shall reduce the portion of the principal amount of a Note not redeemed to less than $2,000. Unless we default in payment of the redemption price, on and after the redemption date, interest will cease to accrue on the Notes or portions of the Notes called for redemption.
For purposes of calculating the redemption price in connection with the redemption of the Notes, on any redemption date, the following terms have the meanings set forth below:
“Treasury Rate” means, with respect to any redemption date, the rate per annum equal to the semi-annual equivalent yield-to-maturity of the Comparable Treasury Issue (computed as of the third business day immediately preceding the redemption), assuming a price for the Comparable Treasury Issue (expressed as a percentage of its principal amount) equal to the Comparable Treasury Price for such redemption date. The redemption price and the Treasury Rate will be determined by us.
“Comparable Treasury Issue” means the United States Treasury security selected by the Reference Treasury Dealer as having a maturity comparable to the remaining term of the Notes to be redeemed that would be utilized, at the time of selection and in accordance with customary financing practice, in pricing new issues of corporate debt securities of comparable maturity to the remaining term of the Notes being redeemed.
S-24
“Comparable Treasury Price” means (1) the average of the remaining Reference Treasury Dealer Quotations for the redemption date, after excluding the highest and lowest Reference Treasury Dealer Quotations, or (2) if the Quotation Agent obtains fewer than four such Reference Treasury Dealer Quotations, the average of all such quotations.
“Quotation Agent” means a Reference Treasury Dealer selected by us.
“Reference Treasury Dealer” means each of (1) Citigroup Global Markets Inc. and (2) Jefferies LLC, or their respective affiliates which are primary U.S. government securities dealers and their respective successors; provided, however, that if any of the foregoing or their affiliates shall cease to be a primary U.S. government securities dealer in the United States (a “Primary Treasury Dealer”), we shall select another Primary Treasury Dealer.
“Reference Treasury Dealer Quotations” means, with respect to each Reference Treasury Dealer and any redemption date, the average, as determined by the Quotation Agent, of the bid and asked prices for the Comparable Treasury Issue (expressed in each case as a percentage of its principal amount) quoted in writing to the Quotation Agent by such Reference Treasury Dealer at 3:30 p.m. New York time on the third business day preceding such redemption date.
All determinations made by any Reference Treasury Dealer, including the Quotation Agent, with respect to determining the redemption price will be final and binding absent manifest error.
Offer to Repurchase Upon a Change of Control Repurchase Event
If a Change of Control Repurchase Event occurs, unless we have exercised our right to redeem the Notes in full, we will make an offer to each holder of Notes to repurchase all or any part (in minimum denominations of $2,000 and integral multiples of $1,000 principal amount) of that holder’s Notes at a repurchase price in cash equal to 100% of the aggregate principal amount of Notes repurchased plus any accrued and unpaid interest on the Notes repurchased to, but not including, the date of purchase. Within 30 days following any Change of Control Repurchase Event or, at our option, prior to any Change of Control, but after the public announcement of the Change of Control, we will mail a notice to each holder describing the transaction or transactions that constitute or may constitute the Change of Control Repurchase Event and offering to repurchase Notes on the payment date specified in the notice, which date will be no earlier than 30 days and no later than 60 days from the date such notice is mailed. The notice shall, if mailed prior to the date of consummation of the Change of Control, state that the offer to purchase is conditioned on the Change of Control Repurchase Event occurring on or prior to the payment date specified in the notice. We will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with the repurchase of the Notes as a result of a Change of Control Repurchase Event. To the extent that the provisions of any securities laws or regulations conflict with the Change of Control Repurchase Event provisions of the Notes, we will comply with the applicable securities laws and regulations and will not be deemed to have breached our obligations under the Change of Control Repurchase Event provisions of the Notes by virtue of such conflict.
On the Change of Control Repurchase Event payment date, subject to extension if necessary to comply with the provisions of the 1940 Act, we will, to the extent lawful:
| (1) | accept for payment all Notes or portions of Notes properly tendered pursuant to our offer; |
| (2) | deposit with the paying agent an amount equal to the aggregate purchase price in respect of all Notes or portions of Notes properly tendered; and |
| (3) | deliver or cause to be delivered to the trustee the Notes properly accepted, together with an officers’ certificate stating the aggregate principal amount of Notes being purchased by us. |
S-25
The paying agent will promptly remit to each holder of Notes properly tendered the purchase price for the Notes, and the trustee will promptly authenticate and mail (or cause to be transferred by book-entry) to each holder a new Note equal in principal amount to any unpurchased portion of any Notes surrendered; provided that each new Note will be in a minimum principal amount of $2,000 or an integral multiple of $1,000 in excess thereof.
We will not be required to make an offer to repurchase the Notes upon a Change of Control Repurchase Event if a third party makes an offer in the manner, at the times and otherwise in compliance with the requirements for an offer made by us and such third party purchases all Notes properly tendered and not withdrawn under its offer.
The source of funds that will be required to repurchase Notes in the event of a Change of Control Repurchase Event will be our available cash or cash generated from our operations or other potential sources, including funds provided by a purchaser in the Change of Control transaction, borrowings, sales of assets or sales of equity. We cannot assure you that sufficient funds from such sources will be available at the time of any Change of Control Repurchase Event to make required repurchases of Notes tendered. The terms of certain of our and our subsidiaries’ financing arrangements provide that certain change of control events will constitute an event of default thereunder entitling the lenders to accelerate any indebtedness outstanding under the our and our subsidiaries’ financing arrangements at that time and to terminate the financing arrangements. In addition, the indenture governing our 2022 Convertible Notes contains a provision that would require us to offer to purchase the 2022 Convertible Notes upon the occurrence of a fundamental change. A failure to purchase any tendered 2022 Convertible Notes would constitute an event of default under the indenture for the 2022 Convertible Notes, which would, in turn, constitute a default under the Credit Facilities and the indenture. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity and Capital Resources” in the accompanying prospectus for a general discussion of our indebtedness. Our and our subsidiaries’ future debt instruments may contain similar restrictions and provisions. If the holders of the Notes exercise their right to require us to repurchase Notes upon a Change of Control Repurchase Event, the financial effect of this repurchase could cause a default under our and our subsidiaries’ future debt instruments, even if the Change of Control Repurchase Event itself would not cause a default. It is possible that we will not have sufficient funds at the time of the Change of Control Repurchase Event to make the required repurchase of the Notes and/or our other debt. See “Risk Factors—Risks Relating to the Notes—We may not be able to repurchase the Notes upon a Change of Control Repurchase Event.”
The definition of “Change of Control” includes a phrase relating to the direct or indirect sale, transfer, conveyance or other disposition of “all or substantially all” of our properties or assets and those of our subsidiaries taken as a whole. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise, established definition of the phrase under applicable law. Accordingly, the ability of a holder of Notes to require us to repurchase the Notes as a result of a sale, transfer, conveyance or other disposition of less than all of our assets and the assets of our subsidiaries taken as a whole to another person or group may be uncertain.
For purposes of the Notes:
“Below Investment Grade Rating Event” means the Notes are downgraded below Investment Grade by the Rating Agency on any date from the date of the public notice of an arrangement that results in a Change of Control until the end of the 60-day period following public notice of the occurrence of a Change of Control (which period shall be extended so long as the rating of the Notes is under publicly announced consideration for possible downgrade by the Rating Agency); provided that a Below Investment Grade Rating Event otherwise arising by virtue of a particular reduction in rating shall not be deemed to have occurred in respect of a particular Change of Control (and thus shall not be deemed a Below Investment Grade Rating Event for purposes of the definition of Change of Control Repurchase Event hereunder) if the Rating Agency making the reduction in rating to which this definition would otherwise apply does not announce or publicly confirm or inform the trustee
S-26
in writing at its request that the reduction was the result, in whole or in part, of any event or circumstance comprised of or arising as a result of, or in respect of, the applicable Change of Control (whether or not the applicable Change of Control shall have occurred at the time of the Below Investment Grade Rating Event).
“Change of Control” means the occurrence of any of the following:
| (1) | the direct or indirect sale, lease, transfer, conveyance or other disposition (other than by way of merger or consolidation) in one or a series of related transactions, of all or substantially all of the assets of Hercules Capital and its Controlled Subsidiaries taken as a whole to any “person” or “group” (as those terms are used in Section 13(d)(3) of the Exchange Act), other than to any Permitted Holders; provided that, for the avoidance of doubt, a pledge of assets pursuant to any secured debt instrument of Hercules Capital or its Controlled Subsidiaries shall not be deemed to be any such sale, lease, transfer, conveyance or disposition; |
| (2) | the consummation of any transaction (including, without limitation, any merger or consolidation) the result of which is that any “person” or “group” (as those terms are used in Section 13(d)(3) of the Exchange Act) (other than any Permitted Holders) becomes the “beneficial owner” (as defined in Rules 13d-3 and 13d-5 under the Exchange Act), directly or indirectly, of more than 50% of the outstanding Voting Stock of Hercules Capital, measured by voting power rather than number of shares; or |
| (3) | the approval by Hercules Capital’s stockholders of any plan or proposal relating to the liquidation or dissolution of Hercules Capital. |
“Change of Control Repurchase Event” means the occurrence of a Change of Control and a Below Investment Grade Rating Event.
“Controlled Subsidiary” means any subsidiary of Hercules Capital, 50% or more of the outstanding equity interests of which are owned by Hercules Capital and its direct or indirect subsidiaries and of which Hercules Capital possesses, directly or indirectly, the power to direct or cause the direction of the management or policies, whether through the ownership of voting equity interests, by agreement or otherwise.
“Investment Grade” means a rating of BBB– or better by S&P (or its equivalent under any successor rating categories of S&P) or the equivalent of any other Rating Agency, as applicable, or in each case, the equivalent under any successor category of such Rating Agency.
“Kroll” means Kroll Bond Rating Agency, Inc., or any successor thereto.
“Permitted Holders” means (i) us and (ii) one or more of our Controlled Subsidiaries.
“Rating Agency” means:
| (1) | S&P; and |
| (2) | if S&P ceases to rate the Notes or fails to make a rating of the Notes publicly available for reasons outside of our control, a “nationally recognized statistical rating organization” as defined in Section (3)(a)(62) of the Exchange Act selected by us as a replacement agency for S&P which may include Kroll. |
“S&P” means Standard & Poor’s Ratings Services, a Standard & Poor’s Financial Services LLC business, or any successor thereto.
“Voting Stock” as applied to stock of any person, means shares, interests, participations or other equivalents in the equity interest (however designated) in such person having ordinary voting power for the election of a majority of the directors (or the equivalent) of such person, other than shares, interests, participations or other equivalents having such power only by reason of the occurrence of a contingency.
S-27
Covenants
In addition to the covenants described in the base indenture, the following covenants shall apply to the Notes. To the extent of any conflict or inconsistency between the base indenture and the following covenants, the following covenants shall govern:
Merger, Consolidation or Sale of Assets
The indenture will provide that we will not merge or consolidate with or into any other person (other than a merger of a wholly owned subsidiary into us), or sell, transfer, lease, convey or otherwise dispose of all or substantially all our property (provided that, for the avoidance of doubt, a pledge of assets pursuant to any secured debt instrument of Hercules Capital or its Controlled Subsidiaries shall not be deemed to be any such sale, transfer, lease, conveyance or disposition) in any one transaction or series of related transactions unless:
| • | we are the surviving person (the “Surviving Person”) or the Surviving Person (if other than us) formed by such merger or consolidation or to which such sale, transfer, lease, conveyance or disposition is made shall be a corporation or limited liability company organized and existing under the laws of the United States of America or any state or territory thereof; |
| • | the Surviving Person (if other than us) expressly assumes, by supplemental indenture in form reasonably satisfactory to the trustee, executed and delivered to the trustee by such Surviving Person, the due and punctual payment of the principal of, and premium, if any, and interest on, all the Notes outstanding, and the due and punctual performance and observance of all the covenants and conditions of the indenture to be performed by us; |
| • | immediately before and immediately after giving effect to such transaction or series of related transactions, no default or event of default shall have occurred and be continuing; and |
| • | we shall deliver, or cause to be delivered, to the trustee, an officers’ certificate and an opinion of counsel, each stating that such transaction and the supplemental indenture, if any, in respect thereto, comply with this covenant and that all conditions precedent in the indenture relating to such transaction have been complied with. |
For the purposes of this covenant, the sale, transfer, lease, conveyance or other disposition of all the property of one or more of our subsidiaries, which property, if held by us instead of such subsidiaries, would constitute all or substantially all of our property on a consolidated basis, shall be deemed to be the transfer of all or substantially all of our property.
Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve “all or substantially all” of the properties or assets of a person. As a result, it may be unclear as to whether the merger, consolidation or sale of assets covenant would apply to a particular transaction as described above absent a decision by a court of competent jurisdiction. Although these types of transactions are permitted under the indenture, certain of the foregoing transactions could constitute a Change of Control that results in a Change of Control Repurchase Event permitting each holder to require us to repurchase the Notes of such holder as described above.
An assumption by any person of obligations under the Notes and the indenture might be deemed for U.S. federal income tax purposes to be an exchange of the Notes for new Notes by the holders thereof, resulting in recognition of gain or loss for such purposes and possibly other adverse tax consequences to the holders. Holders should consult their own tax advisors regarding the tax consequences of such an assumption.
S-28
Other Covenants
| • | We agree that for the period of time during which the Notes are outstanding, we will not violate, whether or not we are subject to, Section 18(a)(1)(A) as modified by Section 61(a)(1) of the 1940 Act or any successor provisions, giving effect to any exemptive relief granted to us by the SEC (even if we are no longer subject to the 1940 Act). |
| • | If, at any time, we are not subject to the reporting requirements of Sections 13 or 15(d) of the Exchange Act to file any periodic reports with the SEC, we agree to furnish to holders of the Notes and the trustee, for the period of time during which the Notes are outstanding, our audited annual consolidated financial statements, within 90 days of our fiscal year end, and unaudited interim consolidated financial statements, within 45 days of our fiscal quarter end (other than our fourth fiscal quarter). All such financial statements will be prepared, in all material respects, in accordance with GAAP, as applicable. |
Events of Default
Each of the following is an event of default:
| (1) | default in the payment of any interest upon any Note when due and payable and the default continues for a period of 30 days; |
| (2) | default in the payment of the principal of (or premium, if any, on) any Note when it becomes due and payable at its maturity including upon any redemption date or required repurchase date; |
| (3) | our failure for 60 consecutive days after written notice from the trustee or the holders of at least 25% in principal amount of the Notes then outstanding has been received to comply with any of our other agreements contained in the Notes or indenture; |
| (4) | default by us or any of our significant subsidiaries, as defined in Article 1, Rule 1-02 of Regulation S-X under the Exchange Act (but excluding any subsidiary which is (a) a non-recourse or limited recourse subsidiary, (b) a bankruptcy remote special purpose vehicle or (c) is not consolidated with Hercules Capital for purposes of GAAP), with respect to any mortgage, agreement or other instrument under which there may be outstanding, or by which there may be secured or evidenced, any indebtedness for money borrowed in excess of $50 million in the aggregate of us and/or any such subsidiary, whether such indebtedness now exists or shall hereafter be created (i) resulting in such indebtedness becoming or being declared due and payable or (ii) constituting a failure to pay the principal or interest of any such debt when due and payable at its stated maturity, upon required repurchase, upon declaration of acceleration or otherwise, unless, in either case, such indebtedness is discharged, or such acceleration is rescinded, stayed or annulled, within a period of 30 calendar days after written notice of such failure is given to us by the trustee or to us and the trustee by the holders of at least 25% in aggregate principal amount of the Notes then outstanding; |
| (5) | Pursuant to Section 18(a)(1)(C)(ii) and Section 61 of the 1940 Act, on the last business day of each of 24 consecutive calendar months, any class of securities shall have an asset coverage (as such term is used in the 1940 Act) of less than 100% giving effect to any exemptive relief granted to us by the SEC; or |
| (6) | certain events of bankruptcy, insolvency, or reorganization involving us occur and remain undischarged or unstayed for a period of 60 days. |
If an event of default occurs and is continuing, then and in every such case (other than an event of default specified in item (6) above) the trustee or the holders of at least 25% in principal amount of the outstanding Notes may declare the entire principal amount of Notes to be due and immediately payable, by a notice in writing to us (and to the trustee if given by the holders), and upon any such declaration such principal or specified portion thereof shall become immediately due and payable. Notwithstanding the foregoing, in the case of the events of bankruptcy, insolvency or reorganization described in item (6) above, 100% of the principal of and accrued and unpaid interest on the Notes will automatically become due and payable.
S-29
At any time after a declaration of acceleration with respect to the Notes has been made and before a judgment or decree for payment of the money due has been obtained by the trustee, the holders of a majority in principal amount of the outstanding Notes, by written notice to us and the trustee, may rescind and annul such declaration and its consequences if (i) we have paid or deposited with the trustee a sum sufficient to pay all overdue installments of interest, if any, on all outstanding Notes, the principal of (and premium, if any, on) all outstanding Notes that have become due otherwise than by such declaration of acceleration and interest thereon at the rate or rates borne by or provided for in such Notes, to the extent that payment of such interest is lawful interest upon overdue installments of interest at the rate or rates borne by or provided for in such Notes, and all sums paid or advanced by the trustee and the reasonable compensation, expenses, disbursements and advances of the trustee, its agents and counsel, and (ii) all events of default with respect to the Notes, other than the nonpayment of the principal of (or premium, if any, on) or interest on such Notes that have become due solely by such declaration of acceleration, have been cured or waived. No such rescission will affect any subsequent default or impair any right consequent thereon.
No holder of Notes will have any right to institute any proceeding, judicial or otherwise, with respect to the indenture, or for the appointment of a receiver or trustee, or for any other remedy under the indenture, unless
| (i) | such holder has previously given written notice to the trustee of a continuing event of default with respect to the Notes, |
| (ii) | the holders of not less than 25% in principal amount of the outstanding Notes shall have made written request to the trustee to institute proceedings in respect of such event of default; |
| (iii) | such holder or holders have offered to the trustee reasonable indemnity against the costs, expenses and liabilities to be incurred in compliance with such request; |
| (iv) | the trustee for 60 days after its receipt of such notice, request and offer of indemnity has failed to institute any such proceeding; and |
| (v) | no direction inconsistent with such written request has been given to the trustee during such 60-day period by the holders of a majority in principal amount of the outstanding Notes. |
Notwithstanding any other provision in the indenture, the holder of any Note shall have the right, which is absolute and unconditional, to receive payment of the principal of (and premium, if any, on) and interest, if any, on such Note on the stated maturity or maturity expressed in such Note (or, in the case of redemption, on the redemption date or, in the case of repayment at the option of the holders, on the repayment date) and to institute suit for the enforcement of any such payment, and such rights shall not be impaired without the consent of such holder.
The trustee shall be under no obligation to exercise any of the rights or powers vested in it by the indenture at the request or direction of any of the holders of the Notes unless such holders shall have offered to the trustee reasonable security or indemnity against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction. Subject to the foregoing, the holders of a majority in principal amount of the outstanding Notes shall have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee or exercising any trust or power conferred on the trustee with respect to the Notes, provided that (i) such direction shall not be in conflict with any rule of law or with this indenture, (ii) the trustee may take any other action deemed proper by the trustee that is not inconsistent with such direction and (iii) the trustee need not take any action that it determines in good faith may involve it in personal liability or be unjustly prejudicial to the holders of Notes not consenting.
The holders of not less than a majority in principal amount of the outstanding Notes may on behalf of the holders of all of the Notes waive any past default under the indenture with respect to the Notes and its consequences, except a default (i) in the payment of (or premium, if any, on) or interest, if any, on any Note, or (ii) in respect of a covenant or provision of the indenture which cannot be modified or amended without the
S-30
consent of the holder of each outstanding Note affected. Upon any such waiver, such default shall cease to exist, and any event of default arising therefrom shall be deemed to have been cured, for every purpose, but no such waiver shall extend to any subsequent or other default or event of default or impair any right consequent thereto.
We are required to deliver to the trustee, within 120 days after the end of each fiscal year, an officers’ certificate stating that to the knowledge of the signers whether we are in default in the performance of any of the terms, provisions or conditions of the indenture.
Within 90 days after the occurrence of any default under the indenture with respect to the Notes, the trustee shall transmit notice of such default known to the trustee, unless such default shall have been cured or waived; provided, however, that, except in the case of a default in the payment of the principal of (or premium, if any, on) or interest, if any, on any Note, the trustee shall be protected in withholding such notice if and so long as the Board of Directors, the executive committee or a trust committee of directors of the trustee in good faith determines that withholding of such notice is in the interest of the holders of the Notes.
Satisfaction and Discharge; Defeasance
We may satisfy and discharge our obligations under the indenture by delivering to the securities registrar for cancellation all outstanding Notes or by depositing with the trustee or delivering to the holders, as applicable, after the Notes have become due and payable, or otherwise, moneys sufficient to pay all of the outstanding Notes and paying all other sums payable under the indenture by us. Such discharge is subject to terms contained in the indenture.
In addition, the Notes are subject to defeasance and covenant defeasance, in each case, in accordance with the terms of the indenture. Defeasance means that, subject to the satisfaction of certain conditions, including, but not limited to, (i) depositing in trust for the benefit of the holders of the Notes a combination of money and/or U.S. government or U.S. government agency notes or bonds that will generate enough cash to make interest, principal and any other payments on the Notes on their various due date and (ii) delivering to the Trustee an opinion of counsel stating that (a) we have received from, or there has been published by, the Internal Revenue Service (the “IRS”) a ruling, or (b) since the date of execution of the indenture, there has been a change in the applicable federal income tax law, in either case to the effect that, and based thereon, the holders and beneficial owners of the Notes and any coupons appertaining thereto will not recognize income, gain or loss for federal income tax purposes as a result of such defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such defeasance had not occurred, we can legally release ourselves from all payment and other obligations on the Notes. Covenant defeasance means that, subject to the satisfaction of certain conditions, including, but not limited to, (i) depositing in trust for the benefit of the holders of the Notes a combination of money and/or U.S. government or U.S. government agency notes or bonds that will generate enough cash to make interest, principal and any other payments on the Notes on their various due dates and (ii) delivering to the Trustee an opinion of counsel to the effect that the holders and beneficial owners of the Notes and any coupons appertaining thereto will not recognize income, gain or loss for federal income tax purposes as a result of such covenant defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such covenant defeasance had not occurred, we will be released from some of the restrictive covenants in the indenture.
Trustee
U.S. Bank National Association is the trustee, security registrar and paying agent. U.S. Bank National Association, in each of its capacities, including without limitation as trustee, security registrar and paying agent, assumes no responsibility for the accuracy or completeness of the information concerning us or our affiliates or any other party contained in this document or the related documents or for any failure by us or any other party to
S-31
disclose events that may have occurred and may affect the significance or accuracy of such information, or for any information provided to it by us, including but not limited to settlement amounts and any other information.
We may maintain banking relationships in the ordinary course of business with the trustee and its affiliates.
Governing Law
The indenture provides that it and the Notes shall be governed by and construed in accordance with the laws of the State of New York, without regard to principles of conflicts of laws that would cause the application of laws of another jurisdiction.
Book-Entry, Settlement and Clearance
Global Notes
The Notes will be initially issued in the form of one or more registered Notes in global form, without interest coupons (the “Global Notes”). Upon issuance, each of the Global Notes will be deposited with the trustee as custodian for DTC and registered in the name of Cede & Co., as nominee of DTC.
Ownership of beneficial interests in a Global Note will be limited to persons who have accounts with DTC (“DTC participants”) or persons who hold interests through DTC participants. We expect that under procedures established by DTC:
| • | upon deposit of a Global Note with DTC’s custodian, DTC will credit portions of the principal amount of the Global Note to the accounts of the DTC participants designated by the underwriters; and |
| • | ownership of beneficial interests in a Global Note will be shown on, and transfer of ownership of those interests will be effected only through, records maintained by DTC (with respect to interests of DTC participants) and the records of DTC participants (with respect to other owners of beneficial interests in the Global Note). |
Beneficial interests in Global Notes may not be exchanged for Notes in physical, certificated form except in the limited circumstances described below.
Book-Entry Procedures for Global Notes
All interests in the Global Notes will be subject to the operations and procedures of DTC. We provide the following summary of those operations and procedures solely for the convenience of investors. The operations and procedures of DTC are controlled by that settlement system and may be changed at any time. Neither we nor the underwriters are responsible for those operations or procedures.
DTC has advised us that it is:
| • | a limited purpose trust company organized under the laws of the State of New York; |
| • | a “banking organization” within the meaning of the New York State Banking Law; |
| • | a member of the Federal Reserve System; |
| • | a “clearing corporation” within the meaning of the Uniform Commercial Code; and |
| • | a “clearing agency” registered under Section 17A of the Exchange Act. |
DTC was created to hold securities for its participants and to facilitate the clearance and settlement of securities transactions between its participants through electronic book-entry changes to the accounts of its participants. DTC’s participants include securities brokers and dealers, including the underwriters; banks and
S-32
trust companies; clearing corporations and other organizations. Indirect access to DTC’s system is also available to others such as banks, brokers, dealers and trust companies; these indirect participants clear through or maintain a custodial relationship with a DTC participant, either directly or indirectly. Investors who are not DTC participants may beneficially own securities held by or on behalf of DTC only through DTC participants or indirect participants in DTC.
So long as DTC’s nominee is the registered owner of a Global Note, that nominee will be considered the sole owner or holder of the Notes represented by that Global Note for all purposes under the indenture. Except as provided below, owners of beneficial interests in a Global Note:
| • | will not be entitled to have Notes represented by the Global Note registered in their names; |
| • | will not receive or be entitled to receive physical, certificated Notes; and |
| • | will not be considered the owners or holders of the Notes under the indenture for any purpose, including with respect to the giving of any direction, instruction or approval to the trustee under the indenture. |
As a result, each investor who owns a beneficial interest in a Global Note must rely on the procedures of DTC to exercise any rights of a holder of Notes under the indenture (and, if the investor is not a participant or an indirect participant in DTC, on the procedures of the DTC participant through which the investor owns its interest).
Payments of principal and interest with respect to the Notes represented by a Global Note will be made by the trustee to DTC’s nominee as the registered holder of the Global Note. Neither we nor the Trustee will have any responsibility or liability for the payment of amounts to owners of beneficial interests in a Global Note, for any aspect of the records relating to or payments made on account of those interests by DTC, or for maintaining, supervising or reviewing any records of DTC relating to those interests.
Payments by participants and indirect participants in DTC to the owners of beneficial interests in a Global Note will be governed by standing instructions and customary industry practice and will be the responsibility of those participants or indirect participants and DTC.
Transfers between participants in DTC will be effected under DTC’s procedures and will be settled in same-day funds.
Certificated Notes
Notes in physical, certificated form will be issued and delivered to each person that DTC identifies as a beneficial owner of the related Notes only if:
| • | DTC notifies us at any time that it is unwilling or unable to continue as depositary for the Global Notes and a successor depositary is not appointed within 90 days; |
| • | DTC ceases to be registered as a clearing agency under the Exchange Act and a successor depositary is not appointed within 90 days; or |
| • | an event of default with respect to the Notes has occurred and is continuing and such beneficial owner requests that its Notes be issued in physical, certificated form. |
S-33
We are offering the Notes described in this prospectus supplement and the accompanying prospectus through a number of underwriters. Citigroup Global Markets Inc. and Jefferies LLC are acting as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally and not jointly agreed to purchase from us, the aggregate principal amount of Notes listed next to its name in the following table:
| Underwriter |
Principal Amount | |||
| Citigroup Global Markets Inc. |
$ | |||
| Jefferies LLC |
$ | |||
|
|
|
|||
| Total |
$ | |||
|
|
|
|||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the Notes sold under the underwriting agreement if any of these Notes are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated.
We have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the Notes, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The following table shows the total underwriting discounts and commissions that we are to pay to the underwriters in connection with this offering.
| Per Note | Total | |||||||
| Public offering price |
% | $ | ||||||
| Underwriting discount |
% | $ | ||||||
| Proceeds, before expenses, to us |
% | $ | ||||||
The underwriters propose to offer some of the Notes to the public at the public offering price set forth on the cover page of this prospectus supplement and some of the Notes to certain other Financial Industry Regulatory Authority, Inc. (FINRA) members at the public offering price less a concession not in excess of % of the aggregate principal amount of the Notes. The underwriters may allow, and the dealers may reallow, a discount not in excess of % of the aggregate principal amount of the Notes. After the initial offering of the Notes to the public, the public offering price and such concessions may be changed. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus supplement.
No Sales of Similar Securities
We have agreed not to directly or indirectly sell, offer to sell, enter into any agreement to sell, or otherwise dispose of, any debt securities issued by the Company which are substantially similar to the Notes or securities
S-34
convertible into such debt securities which are substantially similar to the Notes for a period of 30 days after the date of this prospectus supplement without first obtaining the written consent of the representatives. This consent may be given at any time without public notice.
Listing
The Notes are a new issue of securities with no established trading market. The Notes will not be listed on any securities exchange or quoted on any automated dealer quotation system.
We have been advised by certain of the underwriters that certain of the underwriters presently intend to make a market in the Notes after completion of this offering as permitted by applicable laws and regulations. Such underwriters are not obligated, however, to make a market in the Notes and any such market-making may be discontinued at any time in the sole discretion of such underwriters without any notice. Accordingly, no assurance can be given that an active and liquid public trading market for the Notes will develop or be maintained. If an active public trading market for the Notes does not develop, the market price and liquidity of the Notes may be adversely affected.
Price Stabilization, Short Positions
In connection with the offering, the underwriters may purchase and sell Notes in the open market. These transactions may include covering transactions and stabilizing transactions. Overallotment involves sales of securities in excess of the aggregate principal amount of securities to be purchased by the underwriters in the offering, which creates a short position for the underwriters. Covering transactions involve purchases of the securities in the open market after the distribution has been completed in order to cover short positions. Stabilizing transactions consist of certain bids or purchases of securities made for the purpose of preventing or retarding a decline in the market price of the securities while the offering is in progress.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased Notes sold by or for the account of such underwriter in stabilizing or short covering transactions.
Any of these activities may cause the price of the Notes to be higher than the price that otherwise would exist in the open market in the absence of such transactions. These transactions may be affected in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time without any notice relating thereto.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the Notes. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Notes
The underwriters may make prospectuses available in electronic (PDF) format. A prospectus in electronic (PDF) format may be made available on a web site maintained by the underwriters, and the underwriters may distribute such prospectuses electronically. The underwriters may allocate a limited principal amount of the Notes for sale to their online brokerage customers.
Other Relationships
The underwriters and their affiliates have provided in the past and may provide from time to time in the future in the ordinary course of their business certain commercial banking, financial advisory, investment
S-35
banking and other services to Hercules or our portfolio companies for which they have received or will be entitled to receive separate fees. In particular, the underwriters or their affiliates may execute transactions with Hercules or on behalf of Hercules or any of our portfolio companies.
The underwriters or their affiliates may also trade in our securities, securities of our portfolio companies or other financial instruments related thereto for their own accounts or for the account of others and may extend loans or financing directly or through derivative transactions to us or any of our portfolio companies.
We may purchase securities of third parties from the underwriters or their affiliates after the offering. However, we have not entered into any agreement or arrangement regarding the acquisition of any such securities, and we may not purchase any such securities. We would only purchase any such securities if—among other things—we identified securities that satisfied our investment needs and completed our due diligence review of such securities.
After the date of this prospectus supplement, the underwriters and their affiliates may from time to time obtain information regarding specific portfolio companies or us that may not be available to the general public. Any such information is obtained by the underwriters and their affiliates in the ordinary course of its business and not in connection with the offering of the Notes. In addition, after the offering period for the sale of the Notes, the underwriters or their affiliates may develop analyses or opinions related to Hercules or our portfolio companies and buy or sell interests in one or more of our portfolio companies on behalf of their proprietary or client accounts and may engage in competitive activities. There is no obligation on behalf of these parties to disclose their respective analyses, opinions or purchase and sale activities regarding any portfolio company or regarding us to our noteholders or any other persons.
In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. Certain of the underwriters and their affiliates that have a lending relationship with us routinely hedge their credit exposure to us consistent with their customary risk management policies. Typically, such underwriters and their affiliates would hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities, including potentially the Notes offered hereby. Any such short positions could adversely affect future trading prices of the Notes offered hereby. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
The principal business address of Citigroup Global Markets Inc. is 388 Greenwich Street, New York, New York 10013. The principal business address of Jefferies LLC is 520 Madison Avenue, New York, New York 10022.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive, each underwriter has represented and agreed that with effect from and including the date on which the Prospectus Directive is implemented in that Member State it has not made and will not make an offer of Notes to the public in that Member State, except that it may, with effect from and including such date, make an offer of Notes to the public in that Member State:
(a) to any legal entity that is a qualified investor as defined in the Prospectus Directive;
(b) to fewer than 100 or, if the Relevant Member State has implemented the relevant provisions of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors, as defined
S-36
in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the underwriters nominated by the Company for any such offer; or
(c) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided, that no such offer of Notes shall require the Company or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
For the purposes of the above, the expression an “offer of Notes to the public” in relation to any Notes in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the Notes to be offered so as to enable an investor to decide to purchase or subscribe the Notes, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, and the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
United Kingdom
Each underwriter has represented and agreed that:
(a) it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act of 2000 (the “FSMA”)) received by it in connection with the issue or sale of the Notes in circumstances in which Section 21(1) of the FSMA does not apply to the Company; and
(b) it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the Notes in, from or otherwise involving the United Kingdom.
Other Jurisdictions
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the Notes offered by this prospectus supplement in any jurisdiction where action for that purpose is required. The Notes offered by this prospectus supplement may not be offered or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements in connection with the offer and sale of any such Notes be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus supplement comes are advised to inform themselves about and to observe any restriction relating to the offering and the distribution of this prospectus supplement. This prospectus supplement and the accompanying prospectus do not constitute an offer to sell or a solicitation of an offer to buy the Notes offered by this prospectus supplement and the accompanying prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
S-37
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a general summary of the material U.S. federal income tax considerations (and, in the case of a non-U.S. holder (as defined below), the material U.S. federal estate tax consequences) applicable to an investment in the Notes. This summary deals only with Notes that are purchased for cash in this offering for a price equal to the “issue price” of the Notes (i.e., the first price at which a substantial amount of the Notes is sold for money to investors, other than to bond houses, brokers or similar persons or organizations acting in the capacity of underwriters, placement agents or wholesalers). This summary does not purport to be a complete description of the income and estate tax considerations applicable to such an investment. The discussion is based upon the Code, Treasury Regulations, and administrative and judicial interpretations, each as of the date of this prospectus supplement and all of which are subject to change, potentially with retroactive effect. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of the tax aspects set forth below. You should consult your own tax advisor with respect to tax considerations that pertain to your acquisition, ownership and disposition of our Notes. For a summary of the U.S. federal income tax considerations applicable to us regarding our election to be treated as a RIC, please refer to “Certain United States Federal Income Tax Considerations—Election to be Taxed as a RIC” and “Taxation as a Regulated Investment Company” in the accompanying prospectus.
This discussion deals only with Notes held as capital assets within the meaning of Section 1221 of the Code (generally, property held for investment purposes) and does not purport to deal with persons in special tax situations, such as financial institutions, insurance companies, regulated investment companies, dealers in securities or currencies, traders in securities, former citizens of the United States, persons holding the Notes as a hedge against currency risks or as a position in a “straddle,” “hedge,” “constructive sale transaction” or “conversion transaction” for tax purposes, entities that are tax-exempt for U.S. federal income tax purposes, retirement plans, individual retirement accounts, tax-deferred accounts, persons subject to the alternative minimum tax, pass-through entities (including partnerships and entities and arrangements classified as partnerships for U.S. federal income tax purposes) and beneficial owners of pass-through entities, or U.S. holders (as defined below) whose functional currency (as defined in Section 985 of the Code) is not the U.S. dollar. In addition, this discussion does not deal with any tax consequences other than U.S. federal income tax consequences (and, in the case of a non-U.S. holder, U.S. federal estate tax consequences). If you are considering acquiring the Notes, you should consult your own tax advisor concerning the application of the U.S. federal income and estate tax laws to you in light of your particular situation, as well as any consequences to you of purchasing, owning and disposing of the Notes under the laws of any other taxing jurisdiction.
For purposes of this discussion, the term “U.S. holder” means a beneficial owner of a Note that is, for U.S. federal income tax purposes, (i) an individual citizen or resident of the United States, (ii) a corporation or other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States or of any state thereof or the District of Columbia, (iii) a trust (a) subject to the control of one or more U.S. persons and the primary supervision of a court in the United States, or (b) that existed on August 20, 1996 and has made a valid election (under applicable Treasury Regulations) to be treated as a domestic trust, or (iv) an estate the income of which is subject to U.S. federal income taxation regardless of its source.
The term “non-U.S. holder” means a beneficial owner of a Note that is neither a U.S. holder nor a partnership (including an entity or arrangement treated as a partnership for U.S. federal income tax purposes). An individual may, subject to certain exceptions, be subject to treatment as a resident alien, as opposed to a non-resident alien, for U.S. federal income tax purposes by, among other ways, being physically present in the U.S. (i) on at least 31 days during a calendar year, and (ii) for an aggregate period of at least 183 days during a three-year period ending in the current calendar year, counting for such purposes all of the days present in the current calendar year, one-third of the days present in the immediate preceding year, and one-sixth of the days present in the second preceding year. Resident aliens are subject to U.S. federal income tax as if they were U.S. citizens.
S-38
If a partnership (including an entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds any Notes, the U.S. federal income tax treatment of a partner of the partnership generally will depend upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Partnerships holding Notes, and the persons holding interests in such partnerships, should consult their own tax advisors as to the consequences of investing in the Notes in their individual circumstances.
Taxation of Note Holders
Taxation of U.S. Holders.
Except as discussed below, payments or accruals of interest on a Note generally will be taxable to a U.S. holder as ordinary interest income at the time they are received (actually or constructively) or accrued, in accordance with the U.S. holder’s regular method of tax accounting.
Upon the sale, exchange, redemption, retirement or other taxable disposition of a Note, a U.S. holder generally will recognize capital gain or loss equal to the difference between the amount realized on the sale, exchange, redemption, retirement or other taxable disposition (excluding amounts representing accrued and unpaid interest, which are treated as ordinary income to the extent not previously included in income) and the U.S. holder’s adjusted tax basis in the Note. A U.S. holder’s adjusted tax basis in a Note generally will equal the U.S. holder’s initial investment in the Note. Capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding period in the Note was more than one year. Long-term capital gains generally are taxed at reduced rates for individuals and certain other non-corporate U.S. holders. The distinction between capital gain and loss and ordinary income and loss also is important for purposes of, among other things, the limitations imposed on a U.S. holder’s ability to offset capital losses against ordinary income.
A tax of 3.8% will be imposed on certain “net investment income” (or “undistributed net investment income,” in the case of estates and trusts) received by U.S. holders with modified adjusted gross income above certain threshold amounts. “Net investment income” as defined for U.S. federal Medicare contribution purposes generally includes interest payments on and gain recognized from the sale, redemption, retirement or other disposition of the Notes. U.S. holders should consult their own tax advisors regarding the effect, if any, of this tax on their ownership and disposition of the Notes.
Under applicable Treasury Regulations, if a U.S. holder recognizes a loss with respect to the Notes or shares of our common stock of $2 million or more for a non-corporate U.S. holder or $10 million or more for a corporate U.S. holder in any single taxable year (or a greater loss over a combination of taxable years), the U.S. holder may be required to file with the IRS a disclosure statement on IRS Form 8886. Direct U.S. holders of portfolio securities are in many cases excepted from this reporting requirement, but, under current guidance, U.S. holders of a RIC are not excepted. Future guidance may extend the current exception from this reporting requirement to U.S. holders of most or all RICs. The fact that a loss is reportable under these regulations does not affect the legal determination of whether the taxpayer’s treatment of the loss is proper. Significant monetary penalties apply to a failure to comply with this reporting requirement. States may also have a similar reporting requirement. U.S. holders of the Notes or our common stock should consult their own tax advisors to determine the applicability of these Treasury Regulations in light of their individual circumstances.
Taxation of Non-U.S. Holders. Except as provided below under “Information Reporting and Backup Withholding” and “FATCA,” a non-U.S. holder generally will not be subject to U.S. federal income or withholding taxes on payments of principal or interest on a Note provided that (i) income on the Note is not effectively connected with the conduct by the non-U.S. holder of a trade or business within the United States, (ii) the non-U.S. holder is not a controlled foreign corporation related to the Company through stock ownership, (iii) the non-U.S. holder is not a bank receiving interest described in Section 881(c)(3)(A) of the Code, (iv) the non-U.S. holder does not own (directly or indirectly, actually or constructively) 10% or more of the total combined voting power of all classes of stock of the Company, and (v)(A) the non-U.S. holder provides the
S-39
applicable withholding agent with a valid certification on an IRS Form W-8BEN, Form W-8BEN-E, or other applicable U.S. nonresident withholding tax certification form, certifying its non-U.S. holder status, or (B) a securities clearing organization, bank, or other financial institution that holds customer securities in the ordinary course of its trade or business (i.e., a “financial institution”) and holds a Note on a non-U.S. holder’s behalf certifies to the applicable withhold agent under penalties of perjury that either it or another financial institution has received the required statement from the non-U.S. holder certifying that it is a non-U.S. person and furnishes the applicable withhold agent with a copy of the statement.
A non-U.S. holder that is not exempt from tax under these rules generally will be subject to U.S. federal income tax withholding on payments of interest on the Notes at a rate of 30% unless (i) the income is effectively connected with the conduct of a U.S. trade or business, so long as the non-U.S. holder has provided the applicable withhold agent with an IRS Form W-8ECI or substantially similar substitute U.S. nonresident withholding tax certification form stating that the interest on the Notes is effectively connected with the non-U.S. holder’s conduct of a trade or business in the U.S. in which case the interest will be subject to U.S. federal income tax on a net income basis as applicable to U.S. holders generally (unless an applicable income tax treaty provides otherwise), or (ii) an applicable income tax treaty or provision in the Code provides for a lower rate of, or exemption from, withholding tax, so long as the non-U.S. holder has provided the applicable withhold agent with an IRS Form W-8BEN or Form W-8BEN-E (or other applicable U.S. nonresident withholding tax certification form) signed under penalties of perjury, claiming such lower rate of, or exemption from, withholding tax under such income tax treaty.
To claim the benefit of an income tax treaty or to claim exemption from withholding because income is effectively connected with a U.S. trade or business, the non-U.S. holder must timely provide the appropriate, properly executed IRS U.S. nonresident withholding tax certification form, signed under penalties of perjury, to the applicable withholding agent. These forms may be required to be updated periodically. Additionally, a non-U.S. holder who is claiming the benefits of an income tax treaty may be required to obtain a U.S. taxpayer identification number and provide certain documentary evidence issued by a non-U.S. governmental authority in order to prove residence in a foreign country.
In the case of a non-U.S. holder that is a corporation and that receives income that is effectively connected with the conduct of a U.S. trade or business, such income may also be subject to a branch profits tax (which is generally imposed on a non-U.S. corporation on the actual or deemed repatriation from the United States of earnings and profits attributable to a U.S. trade or business) at a 30% rate. The branch profits tax may not apply (or may apply at a reduced rate) if the non-U.S. holder is a qualified resident of a country with which the United States has an income tax treaty and provides the applicable withhold agent with an IRS Form W-8BEN or Form W-8BEN-E claiming exemption from, or entitlement to a lower rate of, branch profits tax under such treaty.
Generally, a non-U.S. holder will not be subject to U.S. federal income or withholding taxes on any amount that constitutes capital gain upon the sale, exchange, redemption, retirement or other taxable disposition of a Note, provided that the gain is not effectively connected with the conduct of a trade or business in the United States by the non-U.S. holder (and, if required by an applicable income tax treaty, is not attributable to a United States “permanent establishment” maintained by the non-U.S. holder). Non-U.S. holders should consult their own tax advisors with regard to whether taxes will be imposed on capital gain in their individual circumstances.
A Note that is held by an individual who, at the time of death, is not a citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) generally will not be subject to the U.S. federal estate tax, unless, at the time of death, (i) such individual directly or indirectly, actually or constructively, owns ten percent or more of the total combined voting power of all classes of our stock entitled to vote within the meaning of Section 871(h)(3) of the Code and the Treasury Regulations thereunder or (ii) such individual’s interest in the Notes is effectively connected with the individual’s conduct of a U.S. trade or business.
Information Reporting and Backup Withholding. A U.S. holder (other than an “exempt recipient,” including a corporation and certain other persons who, when required, demonstrate their exempt status) may be
S-40
subject to backup withholding at a rate of 28% on, and to information reporting requirements with respect to, payments of principal and interest on, and proceeds from the sale, exchange, redemption or retirement of, the Notes. In general, if a non-corporate U.S. holder subject to information reporting fails to furnish a correct taxpayer identification number or otherwise fails to comply with applicable backup withholding requirements, backup withholding at the applicable rate may apply.
If you are a non-U.S. holder, generally, the applicable withholding agent must report to the IRS and to you payments of interest on the Notes and the amount of tax, if any, withheld with respect to those payments. Copies of the information returns reporting such interest payments and any withholding may also be made available to the tax authorities in the country in which you reside under the provisions of a treaty or agreement. In general, backup withholding will not apply to payments of interest on your Notes if you have provided to the applicable withholding agent the required certification that you are not a U.S. person and the applicable withholding agent does not have actual knowledge or reason to know that you are a U.S. person. Information reporting and, depending on the circumstances, backup withholding will apply to payment to you of the proceeds of a sale or other disposition (including a retirement or redemption) of the Notes within the U.S. or conducted through certain U.S.-related financial intermediaries, unless you certify under penalties of perjury that you are not a U.S. person or you otherwise establish an exemption, and the applicable withholding agent does not have actual knowledge or reason to know that you are a U.S. person.
In addition, backup withholding tax and certain other information reporting requirements apply to payments of principal and interest on, and proceeds from the sale, exchange, redemption or retirement of, the Notes held by a non-U.S. holder, unless an exemption applies. Backup withholding and information reporting will not apply to payments we make to a non-U.S. holder if such non-U.S. holder has provided to the applicable withholding agent under penalties of perjury the required certification of their non-U.S. person status as discussed above (and the applicable withholding agent does not have actual knowledge or reason to know that they are a U.S. person) or if the non-U.S. holder is an exempt recipient.
If a non-U.S. holder sells or redeems a Note through a U.S. broker or the U.S. office of a foreign broker, the proceeds from such sale or redemption will be subject to information reporting and backup withholding unless such non-U.S. holder provides the applicable withhold agent with a withholding certificate or other appropriate documentary evidence establishing that such non-U.S. holder is not a U.S. person to the broker and such broker does not have actual knowledge or reason to know that such non-U.S. holder is a U.S. person, or the non-U.S. holder is an exempt recipient eligible for an exemption from information reporting and backup withholding. If a non-U.S. holder sells or redeems a Note through the foreign office of a broker who is a U.S. person or has certain enumerated connections with the United States, the proceeds from such sale or redemption will be subject to information reporting unless the non-U.S. holder provides to such broker a withholding certificate or other appropriate documentary evidence establishing that the non-U.S. holder is not a U.S. person and such broker does not have actual knowledge or reason to know that such evidence is false, or the non-U.S. holder is an exempt recipient eligible for an exemption from information reporting. In circumstances where information reporting by the foreign office of such a broker is required, backup withholding will be required only if the broker has actual knowledge that the non-U.S. holder is a U.S. person.
You should consult your tax advisor regarding the qualification for an exemption from backup withholding and information reporting and the procedures for obtaining such an exemption, if applicable. Any amounts withheld under the backup withholding rules from a payment to a beneficial owner generally would be allowed as a refund or a credit against such beneficial owner’s U.S. federal income tax provided the required information is timely furnished to the IRS.
FATCA. Certain provisions of the Code, known as FATCA, generally impose a withholding tax of 30% on certain payments to certain foreign entities (including financial intermediaries) unless various U.S. information reporting and diligence requirements (that are in addition to the requirement to deliver an applicable U.S. nonresident withholding tax certification form (e.g., IRS Form W-8BEN), as discussed above) and certain other
S-41
requirements have been satisfied. FATCA withholding generally applies to payments of interest and, after December 31, 2018, payments of gross proceeds (including principal payments) from the sale, redemption, retirement or other disposition of debt securities that can produce U.S. source interest (such as the Notes) (collectively, “withholdable payments”) to certain non-U.S. entities (including, in some circumstances, where such an entity is acting as an intermediary) that fail to comply with certain certification, identification, withholding and information reporting requirements imposed by FATCA. FATCA withholding taxes generally apply to all withholdable payments without regard to whether the beneficial owner of the payment would otherwise be entitled to an exemption from withholding taxes pursuant to an applicable income tax treaty with the U.S. or under U.S. domestic law. If FATCA withholding taxes are imposed with respect to any payments of interest or proceeds made under the Notes, holders that are otherwise eligible for an exemption from, or reduction of, U.S. federal withholding taxes with respect to such interest or proceeds will be required to seek a credit or refund from the IRS in order to obtain the benefit of such exemption or reduction, if any. Holders of or prospective holders of the Notes may be required to provide additional information to enable the applicable withholding agent to determine whether withholding is required. Persons located in jurisdictions that have entered into an intergovernmental agreement with the U.S. to implement FATCA may be subject to different rules. Non-U.S. holders, and U.S. holders that expect to hold the Notes through non-U.S. entities, should consult their own tax advisors regarding the effect, if any, of these withholding and reporting provisions.
The preceding discussion of material U.S. federal income tax considerations is for general information only and is not tax advice. We urge you to consult your own tax advisor with respect to the particular tax consequences to you of an investment in the Notes, including the possible effect of any pending legislation or proposed regulations.
S-42
Certain legal matters in connection with the securities offered hereby will be passed upon for us by Dechert LLP, Philadelphia, PA. Certain legal matters in connection with the securities offered hereby will be passed upon for the underwriters by Fried, Frank, Harris, Shriver & Jacobson LLP, New York, NY.
The consolidated financial statements as of December 31, 2016 and 2015 and for each of the three years in the period ended December 31, 2016 and management’s assessment of the effectiveness of internal control over financial reporting (which is included in Management’s Report on Internal Control over Financial Reporting) as of December 31, 2016 included in the accompanying prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
We have filed with the SEC a registration statement on Form N-2, together with all amendments and related exhibits, under the Securities Act, with respect to our securities offered by this prospectus supplement and the accompanying prospectus. The registration statement contains additional information about us and our securities being offered by this prospectus supplement and the accompanying prospectus.
We file annual, quarterly and current periodic reports, proxy statements and other information with the SEC under the Exchange Act. You may inspect and copy these reports, proxy statements and other information, as well as the registration statement of which this prospectus supplement and accompanying prospectus form a part and the related exhibits and schedules, at the Public Reference Room of the SEC at 100 F Street, N.E., Washington, D.C. 20549-0102. You may obtain information on the operation of the Public Reference Room by calling the SEC at 202-551-8090. The SEC maintains an Internet website that contains reports, proxy and information statements and other information filed electronically by us with the SEC which are available on the SEC’s Internet website at http://www.sec.gov. Copies of these reports, proxy and information statements and other information may be obtained, after paying a duplicating fee, by electronic request at the following E-mail address: publicinfo@sec.gov, or by writing the SEC’s Public Reference Section, Washington, D.C. 20549-0102.
S-43
$600,000,000

Common Stock
Preferred Stock
Warrants
Subscription Rights
Debt Securities
This prospectus relates to the offer, from time to time, in one or more offerings or series, up to $600,000,000 of shares of our common stock, par value $0.001 per share, preferred stock, par value $0.001 per share, warrants representing rights to purchase shares of our common stock, preferred stock or debt securities, subscription rights or debt securities, which we refer to, collectively, as the “securities.” The preferred stock, debt securities, subscription rights and warrants offered hereby may be convertible or exchangeable into shares of our common stock. We may sell our securities through underwriters or dealers, “at-the-market” to or through a market maker into an existing trading market or otherwise directly to one or more purchasers, including existing stockholders in a rights offering, or through agents or through a combination of methods of sale, including auctions. The identities of such underwriters, dealers, market makers or agents, as the case may be, will be described in one or more supplements to this prospectus. The securities may be offered at prices and on terms to be described in one or more supplements to this prospectus.
In the event we offer common stock, the offering price per share will not be less than the net asset value per share of our common stock at the time we make the offering except (1) in connection with a rights offering to our existing stockholders, (2) with the consent of the holders of the majority of our voting securities and approval of our Board of Directors, or (3) under such circumstances as the Securities and Exchange Commission may permit. See “Risk Factors” for more information.
We are a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences and sustainable and renewable technology industries. We primarily finance privately-held companies backed by leading venture capital and private equity firms and publicly-traded companies that lack access to public capital or are sensitive to equity ownership dilution. We source our investments through our principal office located in Palo Alto, CA, as well as through additional offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT and San Diego, CA. Our goal is to be the leading structured debt financing provider for venture capital-backed companies in technology-related industries requiring sophisticated and customized financing solutions. We invest primarily in structured debt with warrants and, to a lesser extent, in senior debt and equity investments. We use the term “structured debt with warrants” to refer to any debt investment, such as a senior or subordinated secured loan, that is coupled with an equity component, including warrants, options or other rights to purchase common or preferred stock. Our structured debt with warrants investments typically are secured by some or all of the assets of the portfolio company. We invest primarily in private companies but also have investments in public companies.
Our investment objective is to maximize our portfolio total return by generating current income from our debt investments and capital appreciation from our warrant and equity-related investments. We are an internally-managed, non-diversified closed-end investment company that has elected to be regulated as a business development company under the Investment Company Act of 1940, as amended.
Our common stock is traded on the New York Stock Exchange, or NYSE, under the symbol “HTGC.” On August 30, 2017, the last reported sale price of a share of our common stock on the NYSE, was $12.31. The net asset value per share of our common stock at June 30, 2017 (the last date prior to the date of this prospectus on which we determined net asset value) was $9.87.
An investment in our securities may be speculative and involves risks including a heightened risk of total loss of investment. In addition, the companies in which we invest are subject to special risks. See “Risk Factors” beginning on page 14 to read about risks that you should consider before investing in our securities, including the risk of leverage.
Please read this prospectus before investing and keep it for future reference. It contains important information about us that a prospective investor ought to know before investing in our securities. We file annual, quarterly and current reports, proxy statements and other information about us with the Securities and Exchange Commission. The information is available free of charge by contacting us at 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301 or by telephone calling collect at (650) 289-3060 or on our website at www.htgc.com. The SEC also maintains a website at www.sec.gov that contains such information.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
This prospectus may not be used to consummate sales of any securities unless accompanied by a prospectus supplement.
The date of this prospectus is September 7, 2017
You should rely only on the information contained in this prospectus. We have not authorized any dealer, salesperson or other person to provide you with different information or to make representations as to matters not stated in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus is not an offer to sell, or a solicitation of an offer to buy, any securities by any person in any jurisdiction where it is unlawful for that person to make such an offer or solicitation or to any person in any jurisdiction to whom it is unlawful to make such an offer or solicitation. The information in this prospectus is accurate only as of its date, and under no circumstances should the delivery of this prospectus or the sale of any securities imply that the information in this prospectus is accurate as of any later date or that the affairs of Hercules Capital, Inc. have not changed since the date hereof. This prospectus will be updated to reflect material changes.
| Page | ||||
| 1 | ||||
| 10 | ||||
| 12 | ||||
| 14 | ||||
| 61 | ||||
| 62 | ||||
| 63 | ||||
| 66 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
67 | |||
| 125 | ||||
| 138 | ||||
| 162 | ||||
| 165 | ||||
| 176 | ||||
| 181 | ||||
| 201 | ||||
| 203 | ||||
| 204 | ||||
| 214 | ||||
| 220 | ||||
| 224 | ||||
| 229 | ||||
| 230 | ||||
| 237 | ||||
| 239 | ||||
| 241 | ||||
| 243 | ||||
| 256 | ||||
| 258 | ||||
| 258 | ||||
| 258 | ||||
| 258 | ||||
| 259 | ||||
| F-1 | ||||
Hercules Capital, Inc., our logo and other trademarks of Hercules Capital, Inc. mentioned in this prospectus are the property of Hercules Capital, Inc. All other trademarks or trade names referred to in this prospectus are the property of their respective owners.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we have filed with the Securities and Exchange Commission using the “shelf” registration process. Under the shelf registration process, which constitutes a delayed offering in reliance on Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”), we may offer, from time to time, up to $600,000,000 of our common stock, preferred stock, warrants representing rights to purchase shares of our common stock, preferred stock or debt securities, subscription rights or debt securities on the terms to be determined at the time of the offering. We may sell our securities through underwriters or dealers, “at-the-market” to or through a market maker, into an existing trading market or otherwise directly to one or more purchasers, including existing stockholders in a rights offering, or through agents or through a combination of methods of sale. The identities of such underwriters, dealers, market makers or agents, as the case may be, will be described in one or more supplements to this prospectus. The securities may be offered at prices and on terms described in one or more supplements to this prospectus. This prospectus provides you with a general description of the securities that we may offer. Each time we use this prospectus to offer securities, we will provide a prospectus supplement that will contain specific information about the terms of that offering. Please carefully read this prospectus and any such supplements together with the additional information described under “Where You Can Find Additional Information” in the “Summary” and “Risk Factors” sections before you make an investment decision.
A prospectus supplement may also add to, update or change information contained in this prospectus.
This summary highlights some of the information in this prospectus and may not contain all of the information that is important to you. For a more complete understanding of this offering, we encourage you to read this entire prospectus and the documents that are referenced in this prospectus, together with any accompanying supplements. In this prospectus, unless the context otherwise requires, the “Company,” “Hercules,” “HTGC,” “we,” “us” and “our” refer to Hercules Capital, Inc. and its wholly owned subsidiaries and its affiliated securitization trusts on or after February 25, 2016 and “Hercules Technology Growth Capital, Inc.” and its wholly owned subsidiaries and its affiliated securitization trusts prior to February 25, 2016 unless the context otherwise requires.
Our Company
We are a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences and sustainable and renewable technology industries. Our investment objective is to maximize our portfolio’s total return by generating current income from our debt investments and capital appreciation from our warrant and equity-related investments. We are an internally managed, non-diversified, closed-end investment company that has elected to be regulated as a business development company under the Investment Company Act of 1940, as amended, or the 1940 Act. Effective January 1, 2006, we elected to be treated for tax purposes as a regulated investment company, or RIC, under the Internal Revenue Code of 1986, as amended, or the Code.
As of June 30, 2017, our total assets were approximately $1.6 billion, of which our investments comprised $1.4 billion at fair value and $1.5 billion at cost. Since inception through June 30, 2017, we have made debt and equity commitments of approximately $6.9 billion to our portfolio companies.
We also make investments in qualifying small businesses through our two wholly-owned small business investment companies, or SBICs. Our SBIC subsidiaries, Hercules Technology II, L.P., or HT II, and Hercules Technology III, L.P., or HT III, hold approximately $104.8 million and $271.5 million in assets, respectively, and accounted for approximately 5.8% and 14.9% of our total assets, respectively, prior to consolidation at June 30, 2017. At June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries. See “Regulation—Small Business Administration Regulations” for additional information regarding our SBIC subsidiaries. As of June 30, 2017, our investment professionals, including Manuel A. Henriquez, our co-founder, Chairman, President and Chief Executive Officer, are currently comprised of 34 professionals who have, on average, more than 15 years of experience in venture capital, structured finance, commercial lending or acquisition finance with the types of technology-related companies that we are targeting. We believe that we can leverage the experience and relationships of our management team to successfully identify attractive investment opportunities, underwrite prospective portfolio companies and structure customized financing solutions.
1
The following chart shows the ownership structure and relationship of certain entities with us.
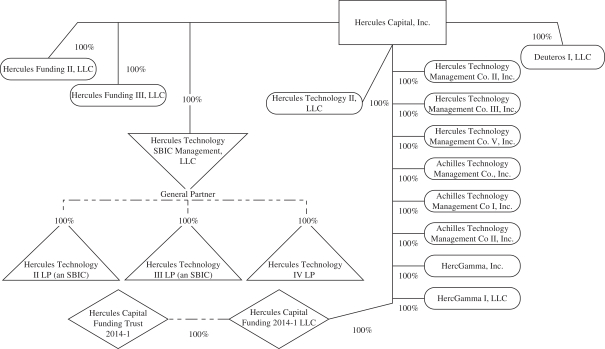
Our Market Opportunity
We believe that technology-related companies compete in one of the largest and most rapidly growing sectors of the U.S. economy and that continued growth is supported by ongoing innovation and performance improvements in technology products as well as the adoption of technology across virtually all industries in response to competitive pressures. We believe that an attractive market opportunity exists for a specialty finance company focused primarily on investments in structured debt with warrants in technology- related companies for the following reasons:
| • | technology-related companies have generally been underserved by traditional lending sources; |
| • | unfulfilled demand exists for structured debt financing to technology-related companies due to the complexity of evaluating risk in these investments; and |
| • | structured debt with warrants products are less dilutive and complement equity financing from venture capital and private equity funds. |
Technology-Related Companies are Underserved by Traditional Lenders. We believe many viable technology-related companies backed by financial sponsors have been unable to obtain sufficient growth financing from traditional lenders, including financial services companies such as commercial banks and finance companies because traditional lenders have continued to consolidate and have adopted a more risk-averse approach to lending. More importantly, we believe traditional lenders are typically unable to underwrite the risk associated with these companies effectively.
The unique cash flow characteristics of many technology-related companies typically include significant research and development expenditures and high projected revenue growth thus often making such companies
2
difficult to evaluate from a credit perspective. In addition, the balance sheets of these companies often include a disproportionately large amount of intellectual property assets, which can be difficult to value. Finally, the speed of innovation in technology and rapid shifts in consumer demand and market share add to the difficulty in evaluating technology-related companies.
Due to the difficulties described above, we believe traditional lenders generally refrain from entering the structured debt financing marketplace, instead preferring the risk-reward profile of asset based lending. Traditional lenders generally do not have flexible product offerings that meet the needs of technology-related companies. The financing products offered by traditional lenders typically impose on borrowers many restrictive covenants and conditions, including limiting cash outflows and requiring a significant depository relationship to facilitate rapid liquidation.
Unfulfilled Demand for Structured Debt Financing to Technology-Related Companies. Private debt capital in the form of structured debt financing from specialty finance companies continues to be an important source of funding for technology-related companies. We believe that the level of demand for structured debt financing is a function of the level of annual venture equity investment activity.
We believe that demand for structured debt financing is currently underserved. The venture capital market for the technology-related companies in which we invest has been active. Therefore, to the extent we have capital available, we believe this is an opportune time to be active in the structured lending market for technology-related companies.
Structured Debt with Warrants Products Complement Equity Financing From Venture Capital and Private Equity Funds. We believe that technology-related companies and their financial sponsors will continue to view structured debt securities as an attractive source of capital because it augments the capital provided by venture capital and private equity funds. We believe that our structured debt with warrants products provide access to growth capital that otherwise may only be available through incremental investments by existing equity investors. As such, we provide portfolio companies and their financial sponsors with an opportunity to diversify their capital sources. Generally, we believe many technology-related companies at all stages of development target a portion of their capital to be debt in an attempt to achieve a higher valuation through internal growth. In addition, because financial sponsor-backed companies have reached a more mature stage prior to reaching a liquidity event, we believe our investments could provide the debt capital needed to grow or recapitalize during the extended period sometimes required prior to liquidity events.
Our Business Strategy
Our strategy to achieve our investment objective includes the following key elements:
Leverage the Experience and Industry Relationships of Our Management Team and Investment Professionals. We have assembled a team of experienced investment professionals with extensive experience as venture capitalists, commercial lenders, and originators of structured debt and equity investments in technology-related companies. Our investment professionals have, on average, more than 15 years of experience as equity investors in, and/or lenders to, technology-related companies. In addition, our team members have originated structured debt, debt with warrants and equity investments in over 380 technology-related companies, representing approximately $6.9 billion in commitments from inception to June 30, 2017, and have developed a network of industry contacts with investors and other participants within the venture capital and private equity communities. In addition, members of our management team also have operational, research and development and finance experience with technology-related companies. We have established contacts with leading venture capital and private equity fund sponsors, public and private companies, research institutions and other industry participants, which we believe will enable us to identify and attract well-positioned prospective portfolio companies.
3
We focus our investing activities generally in industries in which our investment professionals have investment experience. We believe that our focus on financing technology-related companies will enable us to leverage our expertise in structuring prospective investments, to assess the value of both tangible and intangible assets, to evaluate the business prospects and operating characteristics of technology-related companies and to identify and originate potentially attractive investments with these types of companies.
Mitigate Risk of Principal Loss and Build a Portfolio of Equity-Related Securities. We expect that our investments have the potential to produce attractive risk-adjusted returns through current income, in the form of interest and fee income, as well as capital appreciation from warrant and equity-related securities. We believe that we can mitigate the risk of loss on our debt investments through the combination of loan principal amortization, cash interest payments, relatively short maturities (typically between 24-48 months), security interests in the assets of our portfolio companies, and on select investment covenants requiring prospective portfolio companies to have certain amounts of available cash at the time of our investment and the continued support from a venture capital or private equity firm at the time we make our investment. Although we do not currently engage in hedging transactions, we may engage in hedging transactions in the future utilizing instruments such as forward contracts, currency options and interest rate swaps, caps, collars, and floors.
Historically our structured debt investments to technology-related companies typically include warrants or other equity interests, giving us the potential to realize equity-like returns on a portion of our investment. In addition, in some cases, we receive the right to make additional equity investments in our portfolio companies, including the right to convert some portion of our debt into equity, in connection with future equity financing rounds. We believe these equity interests will create the potential for meaningful long-term capital gains in connection with the future liquidity events of these technology-related companies.
Provide Customized Financing Complementary to Financial Sponsors’ Capital. We offer a broad range of investment structures and possess expertise and experience to effectively structure and price investments in technology-related companies. Unlike many of our competitors that only invest in companies that fit a specific set of investment parameters, we have the flexibility to structure our investments to suit the particular needs of our portfolio companies. We offer customized financing solutions ranging from senior debt, including below-investment grade debt instruments (also known as “junk bonds”), to equity capital, with a focus on structured debt with warrants.
We use our relationships in the financial sponsor community to originate investment opportunities. Because venture capital and private equity funds typically invest solely in the equity securities of their portfolio companies, we believe that our debt investments will be viewed as an attractive and complimentary source of capital, both by the portfolio company and by the portfolio company’s financial sponsor. In addition, we believe that many venture capital and private equity fund sponsors encourage their portfolio companies to use debt financing for a portion of their capital needs as a means of potentially enhancing equity returns, minimizing equity dilution and increasing valuations prior to a subsequent equity financing round or a liquidity event.
Invest at Various Stages of Development. We provide growth capital to technology-related companies at all stages of development, including select publicly listed companies and select special opportunity lower middle market companies that require additional capital to fund acquisitions, recapitalizations and refinancings and established-stage companies. We believe that this provides us with a broader range of potential investment opportunities than those available to many of our competitors, who generally focus their investments on a particular stage in a company’s development. Because of the flexible structure of our investments and the extensive experience of our investment professionals, we believe we are well positioned to take advantage of these investment opportunities at all stages of prospective portfolio companies’ development.
Benefit from Our Efficient Organizational Structure. We believe that the perpetual nature of our corporate structure enables us to be a long-term partner for our portfolio companies in contrast to traditional investment
4
funds, which typically have a limited life. In addition, because of our access to the equity markets, we believe that we may benefit from a lower cost of capital than that available to private investment funds. We are not subject to requirements to return invested capital to investors nor do we have a finite investment horizon. Capital providers that are subject to such limitations are often required to seek a liquidity event more quickly than they otherwise might, which can result in a lower overall return on an investment.
Deal Sourcing Through Our Proprietary Database. We have developed a proprietary and comprehensive structured query language-based (“SQL”) database system to track various aspects of our investment process including sourcing, originations, transaction monitoring and post-investment performance. As of June 30, 2017, our proprietary SQL-based database system included approximately 48,000 technology-related companies and approximately 9,600 venture capital firms, private equity sponsors/investors, as well as various other industry contacts. This proprietary SQL system allows us to maintain, cultivate and grow our industry relationships while providing us with comprehensive details on companies in the technology-related industries and their financial sponsors.
Dividend Reinvestment Plan
We maintain an “opt-out” dividend reinvestment plan that provides for reinvestment of our distribution on behalf of our stockholders, unless a stockholder elects to receive cash. See “Dividend Reinvestment Plan.” Those stockholders whose shares are held by a broker or other financial intermediary may receive distributions in cash by notifying their broker or other financial intermediary of their election.
Taxation
Effective January 1, 2006, we elected to be treated for tax purposes as a RIC under the Code. As a RIC, we generally will not be subject to corporate-level federal income taxes on any ordinary income or capital gains that we distribute as dividends for U.S. federal income tax purposes to our stockholders, which allows us to reduce or eliminate our corporate level tax. See “Certain United States Federal Income Tax Considerations.” To maintain our ability to be subject to tax as a RIC, we must meet specified source-of-income and asset diversification requirements and distribute each taxable year dividends for U.S. federal income tax purposes of an amount generally at least equal to 90% of the sum of our net ordinary income and realized net short-term capital gains in excess of realized net long-term capital losses, if any, out of assets legally available for distribution. There is no assurance that we will meet these tests and be able to maintain our RIC status. If we do not qualify as a RIC, we would be subject to tax as a C corporation.
Assuming we continue to be treated as a RIC under the Code, distributions from our taxable earnings (including net realized securities gains) paid to our U.S. resident shareholders generally will be subject to U.S. federal income tax at rates applicable to ordinary income or capital gains, as appropriate, and all or a portion of such distributions paid to qualifying shareholders not resident in the U.S. (i.e., foreign shareholders) generally would not be subject U.S. nonresident withholding tax. See “Certain United States Federal Income Tax Considerations.”
Use of Proceeds
We intend to use the net proceeds from selling our securities for general corporate purposes, which includes investing in debt and equity securities, repayment of indebtedness and other general corporate purposes. The supplement to this prospectus relating to an offering will more fully identify the use of proceeds from such offering.
5
Leverage
We borrow funds to make additional investments, and we have granted, and may in the future grant, a security interest in our assets to a lender in connection with any such borrowings, including any borrowings by any of our subsidiaries. We use this practice, which is known as “leverage,” to attempt to increase returns to our common stockholders. However, leverage involves significant risks. See “Risk Factors.” With certain limited exceptions, we are only allowed to borrow amounts such that our asset coverage, as defined in the 1940 Act, equals at least 200% after such borrowing. We received an exemptive order from the Securities and Exchange Commission, or SEC, that allows us to exclude all SBA leverage from our asset coverage ratio. The amount of leverage that we employ will depend on our assessment of market and other factors at the time of any proposed borrowing. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity, and Capital Resources” for additional information related to our outstanding debt.
Distributions
As a RIC, we are required to distribute dividends for U.S. federal income tax purposes each taxable year to our stockholders of an amount at least equal to 90% of the sum of our net ordinary income and realized net short-term capital gains in excess of realized net long-term capital losses, if any. We are not subject to corporate level income taxation on income we timely distribute as dividends for U.S. federal income tax purposes to our stockholders. See “Certain United States Federal Income Tax Considerations.” We pay regular quarterly distributions based upon an estimate of annual taxable income available for distribution to stockholders as well as the amount of any taxable income carried over from the prior taxable year for distribution in the current taxable year.
Principal Risk Factors
Investing in our common stock may be speculative and involves certain risks relating to our structure and our investment objective that you should consider before deciding whether to invest. In addition, we expect that our portfolio will continue to consist primarily of securities issued by privately-held technology-related companies, which generally require additional capital to become profitable. These investments may involve a high degree of business and financial risk, and they are generally illiquid. Our portfolio companies typically will require additional outside capital beyond our investment in order to succeed or to fully repay the amounts owed to us. A large number of entities compete for the same kind of investment opportunities as we seek.
We borrow funds to make our investments in portfolio companies. As a result, we are exposed to the risks of leverage, which may be considered a speculative investment technique. Borrowings magnify the potential for gain and loss on amounts invested and, therefore increase the risks associated with investing in our common stock. Also, we are subject to certain risks associated with valuing our portfolio, changing interest rates, accessing additional capital, fluctuating quarterly results, and operating in a regulated environment. See “Risk Factors” for a discussion of factors you should carefully consider before deciding whether to invest in our securities.
Certain Anti-Takeover Provisions
Our charter and bylaws, as well as certain statutes and regulations, contain provisions that may have the effect of discouraging a third party from making an acquisition proposal for our company. This could delay or prevent a transaction that could give our stockholders the opportunity to realize a premium over the price for their securities.
6
Recent Developments
Evaluation of Alternative Management Structures
On May 3, 2017, we filed preliminary proxy materials with the SEC for a special meeting of stockholders to seek approval for a proposed advisory agreement with Hamilton Advisers LLC. However, after receiving feedback from our stockholders, on May 15, 2017, we decided to postpone the proposed special meeting of stockholders indefinitely and formally withdrew the proxy materials containing our proposal seeking stockholder approval of our plans to externalize our management structure to expand our ongoing review process. We, along with our professional advisors, are currently evaluating alternatives with respect to our management structure. The evaluation process is still ongoing, and we are continuing to move forward in evaluating various options, but we currently have no definitive timeline for completion. While internal management has served us well since our formation, our board of directors (the “Board of Directors”) has concluded that there are limitations to that management structure that may operate to our disadvantage. To that end, we are exploring the possibility of externalizing our management structure as a means of addressing those concerns; and, we are also examining various alternatives that could be pursued with respect to externalization if we determine that externalization is the proper course to follow. We and our independent directors are working with advisors to assist in these efforts. This program will result in us incurring additional and unusual expense until this project is concluded. Should we determine to pursue externalization, which would be subject to approval by our stockholders, it could involve some disruption (at least on a temporary basis) and expense during the period of transition.
Distribution Declaration
On July 26, 2017, our Board of Directors declared a cash distribution of $0.31 per share to be paid on August 21, 2017 to stockholders of record as of August 14, 2017. This distribution represented our forty-eighth consecutive distribution since our initial public offering, bringing the total cumulative distribution to date to $13.40 per share.
Closed and Pending Commitments
As of August 30, 2017, we have:
| • | Closed debt and equity commitments of approximately $93.2 million to new and existing portfolio companies and funded approximately $73.5 million subsequent to June 30, 2017. |
| • | Pending commitments (signed non-binding term sheets) of approximately $20.0 million. The table below summarizes our year-to-date closed and pending commitments as follows: |
| Closed Commitments and Pending Commitments (in millions) |
||||
| January 1—June 30, 2017 Closed Commitments |
$ | 397.0 | ||
| July 1—August 30, 2017 Closed Commitments(a) |
$ | 93.2 | ||
| Pending Commitments (as of August 30, 2017)(b) |
$ | 20.0 | ||
|
|
|
|||
| Closed and Pending Commitments as of August 30, 2017 |
$ | 510.2 | ||
|
|
|
| a. | Closed Commitments may include renewals of existing credit facilities. Not all Closed Commitments result in future cash requirements. Commitments generally fund over the two succeeding quarters from close. |
| b. | Not all pending commitments (signed non-binding term sheets) are expected to close and they do not necessarily represent any future cash requirements. |
Portfolio Company Developments
As of August 30, 2017, we held warrants or equity positions in six companies that have filed registration statements on Form S-1 with the SEC in contemplation of potential initial public offerings. All six companies
7
filed confidentially under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). There can be no assurance that these companies will complete their initial public offerings in a timely manner or at all. In addition, subsequent to June 30, 2017, our portfolio companies announced the following liquidity events:
| 1. | JumpStart Games, Inc. was acquired by NetDragon Websoft Holdings Limited, a global leader in building internet communities. The acquisition was completed by NetDragon in Hong Kong on July 4, 2017. Terms of the transaction were not disclosed. Hercules initially committed $13.0 million in venture debt financing to JumpStart in March 2014. Hercules is scheduled to receive quarterly interest payments through June 30, 2018; and the potential to receive principal repayment of a portion of its outstanding obligation at maturity on June 30, 2018, subject to adjustments of JumpStart. |
| 2. | Nasty Gal, a Los Angeles, CA-based fashion retail website for girls that sells vintage clothing, shoes and accessories, was acquired in February 2017 by Boohoo.com, a Manchester, England-based online fashion retailer, for $20.0 million in consideration for Nasty Gal’s intellectual property assets and customer databases. Hercules initially committed $20.0 million in venture debt financing. On February 28, 2017, Hercules received a partial payment of $12.6 million from the sale of Nasty Gal assets, with full repayment expected upon close of escrow. In July 2017, Hercules received final payment. The Company realized an IRR of approximately 19.1% from its loan repayments and equity/warrant gains. |
| 3. | Jaguar Animal Health, Inc. (NASDAQ: JAGX) entered a binding merger agreement on May 26, 2017 with Napo Pharmaceuticals, a company that focuses on the development and commercialization of proprietary pharmaceuticals for the global marketplace in collaboration with local partners. In addition, Jaguar Animal Health and Napo Pharmaceuticals announced the filing of two Orphan Drug Designation Applications with U.S. Food & Drug Administration (FDA) for Mytesi for serious unmet medical needs. |
The merger became effective on July 31, 2017, at which point Jaguar Animal Health’s name changed to Jaguar Health, Inc. and Napo began operating as a wholly-owned subsidiary of Jaguar. Although Jaguar’s name has changed, the public company will continue to trade under the same Nasdaq ticker symbol: JAGX.
Departure of Officer
On June 26, 2017, Andrew Olson announced his resignation, effective July 21, 2017, from his position as Vice President of Finance and Senior Controller. Gerard R. Waldt, Jr., the Company’s current Assistant Controller, will assume the position of Controller.
General Information
Our principal executive offices are located at 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301, and our telephone number is (650) 289-3060. We also have offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT and San Diego, CA. We maintain a website on the Internet at www.htgc.com. We make available, free of charge, on our website our proxy statement, annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider that information to be part of this prospectus.
8
We file annual, quarterly and current periodic reports, proxy statements and other information with the SEC, under the Securities Exchange Act of 1934, as amended, or the Exchange Act. This information is available at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information about the operation of the SEC’s public reference room by calling the SEC at (202) 551-8090. In addition, the SEC maintains an Internet website, at www.sec.gov, that contains reports, proxy and information statements, and other information regarding issuers, including us, who file documents electronically with the SEC.
9
The following table is intended to assist you in understanding the various costs and expenses that an investor in our common stock will bear directly or indirectly. However, we caution you that some of the percentages indicated in the table below are estimates and may vary. The footnotes to the fee table state which items are estimates. Except where the context suggests otherwise, whenever this prospectus contains a reference to fees or expenses paid by “you” or “us” or that “we” will pay fees or expenses, stockholders will indirectly bear such fees or expenses as investors in Hercules Capital, Inc.
| Stockholder Transaction Expenses (as a percentage of the public offering price): |
||||
| Sales load (as a percentage of offering price)(1) |
— | % | ||
| Offering expenses |
— | %(2) | ||
| Dividend reinvestment plan fees |
— | %(3) | ||
|
|
|
|||
| Total stockholder transaction expenses (as a percentage of the public offering price) |
— | %(4) | ||
|
|
|
|||
| Annual Expenses (as a percentage of net assets attributable to common stock):(5) |
||||
| Operating expenses |
5.71 | %(6)(7) | ||
| Interest and fees paid in connection with borrowed funds |
5.53 | %(8) | ||
|
|
|
|||
| Total annual expenses |
11.24 | %(9) | ||
|
|
|
| (1) | In the event that our securities are sold to or through underwriters, a corresponding prospectus supplement to this prospectus will disclose the applicable sales load. |
| (2) | In the event that we conduct an offering of our securities, a corresponding prospectus supplement to this prospectus will disclose the estimated offering expenses. |
| (3) | The expenses associated with the administration of our dividend reinvestment plan are included in “Operating expenses.” We pay all brokerage commissions incurred with respect to open market purchases, if any, made by the administrator under the plan. For more details about the plan, see “Dividend Reinvestment Plan.” |
| (4) | Total stockholder transaction expenses may include sales load and will be disclosed in a future prospectus supplement, if any. |
| (5) | “Net assets attributable to common stock” equals the weighted average net assets for the six-months ended June 30, 2017, which is approximately $834.2 million. |
| (6) | “Operating expenses” represent our estimated operating expenses by annualizing our actual operating expenses incurred for the six-months ended June 30, 2017, including all fees and expenses of our consolidated subsidiaries and excluding interests and fees on indebtedness. This percentage for the year ended December 31, 2016 was 6.21%. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Management.” |
| (7) | We do not have an investment adviser and are internally managed by our executive officers under the supervision of our Board of Directors. As a result, we do not pay investment advisory fees, but instead we pay the operating costs associated with employing investment management professionals. |
| (8) | “Interest and fees paid in connection with borrowed funds” represents our estimated interest, fees and credit facility expenses by annualizing our actual interest fees, and credit facility expenses incurred for the six-months ended June 30, 2017, including our Wells Facility, Union Bank Facility, the 2024 Notes, the 2022 Convertible Notes, the 2021 Asset-Backed Notes and the SBA debentures, each of which is defined herein. This percentage for the year ended December 31, 2016 was 5.04%. |
| (9) | “Total annual expenses” is the sum of “operating expenses,” and “interest and fees paid in connection with borrowed funds.” This percentage for the year ended December 31, 2016 was 11.25%. “Total annual expenses” is presented as a percentage of weighted average net assets attributable to common stockholders, because the holders of shares of our common stock (and not the holders of our debt securities or preferred stock, if any) bear all of our fees and expenses, including the fees and expenses of our wholly-owned consolidated subsidiaries, all of which are included in this fee table presentation. |
10
Example
The following example demonstrates the projected dollar amount of total cumulative expenses that would be incurred over various periods with respect to a hypothetical investment in our common stock. These amounts are based upon our payment of annual operating expenses at the levels set forth in the table above and assume no additional leverage.
| 1 Year | 3 Years | 5 Years | 10 Years | |||||||||||||
| You would pay the following expenses on a $1,000 common stock investment, assuming a 5% annual return |
$ | 109 | $ | 307 | $ | 483 | $ | 836 | ||||||||
The example and the expenses in the tables above should not be considered a representation of our future expenses, and actual expenses may be greater or lesser than those shown. Moreover, while the example assumes, as required by the applicable rules of the SEC, a 5% annual return, our performance will vary and may result in a return greater or lesser than 5%. In addition, while the example assumes reinvestment of all distributions at net asset value (“NAV”), participants in our dividend reinvestment plan may receive shares valued at the market price in effect at that time. This price may be at, above or below NAV. See “Dividend Reinvestment Plan” for additional information regarding our dividend reinvestment plan.
11
SELECTED CONSOLIDATED FINANCIAL DATA
The selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Senior Securities” and the consolidated financial statements and related notes included elsewhere herein. The selected balance sheet data as of the end of fiscal year 2016, 2015, 2014, 2013, and 2012 and the financial statement of operations data for fiscal 2016, 2015, 2014, 2013, and 2012 has been derived from our audited financial statements, which have been audited by PricewaterhouseCoopers LLP, our independent registered public accounting firm, but not all of which are presented in this Form N-2.” The historical data are not necessarily indicative of results to be expected for any future period. The selected financial and other data for the six months ended June 30, 2017 and other quarterly financial information is derived from our unaudited financial statements, but in the opinion of management, reflects all adjustments (consisting only of normal recurring adjustments) that are necessary to present fairly the results of such interim periods. Interim results as of and for the six months ended June 30, 2017 are not necessarily indicative of the results that may be expected for the year ending December 31, 2017.
| For the Six-Months Ended June 30, (unaudited) |
For the Year Ended December 31, | |||||||||||||||||||||||||||
| (in thousands, except per share amounts) |
2017 | 2016 | 2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||||||||
| Investment income: |
||||||||||||||||||||||||||||
| Interest |
$ | 83,367 | $ | 76,095 | $ | 158,727 | $ | 140,266 | $ | 126,618 | $ | 123,671 | $ | 87,603 | ||||||||||||||
| Fees |
11,450 | 6,382 | 16,324 | 16,866 | 17,047 | 16,042 | 9,917 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total investment income |
94,817 | 82,477 | 175,051 | 157,132 | 143,665 | 139,713 | 97,520 | |||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||
| Interest |
18,861 | 14,589 | 32,016 | 30,834 | 28,041 | 30,334 | 19,835 | |||||||||||||||||||||
| Loan fees |
4,186 | 2,267 | 5,042 | 6,055 | 5,919 | 4,807 | 3,917 | |||||||||||||||||||||
| General and administrative: |
||||||||||||||||||||||||||||
| Legal expenses |
2,867 | 2,677 | 4,823 | 3,079 | 1,366 | 1,440 | 799 | |||||||||||||||||||||
| Other expenses |
5,947 | 5,303 | 11,283 | 13,579 | 8,843 | 7,914 | 7,309 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total general and administrative |
8,814 | 7,980 | 16,106 | 16,658 | 10,209 | 9,354 | 8,108 | |||||||||||||||||||||
| Employee Compensation: |
||||||||||||||||||||||||||||
| Compensation and benefits |
11,262 | 10,016 | 22,500 | 20,713 | 16,604 | 16,179 | 13,326 | |||||||||||||||||||||
| Stock-based compensation |
3,742 | 4,174 | 7,043 | 9,370 | 9,561 | 5,974 | 4,227 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total employee compensation |
15,004 | 14,190 | 29,543 | 30,083 | 26,165 | 22,153 | 17,553 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
46,865 | 39,026 | 82,707 | 83,630 | 70,334 | 66,648 | 49,413 | |||||||||||||||||||||
| Other income (loss) |
— | — | 8,000 | (1 | ) | (1,581 | ) | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net investment income |
47,952 | 43,451 | 100,344 | 73,501 | 71,750 | 73,065 | 48,107 | |||||||||||||||||||||
| Net realized gain (loss) on investments |
(2,475 | ) | (4,443 | ) | 4,576 | 5,147 | 20,112 | 14,836 | 3,168 | |||||||||||||||||||
| Net change in unrealized appreciation (depreciation) on investments |
(17,916 | ) | (15,238 | ) | (36,217 | ) | (35,732 | ) | (20,674 | ) | 11,545 | (4,516 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total net realized and unrealized gain (loss) |
(20,391 | ) | (19,681 | ) | (31,641 | ) | (30,585 | ) | (562 | ) | 26,381 | (1,348 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net increase (decrease) in net assets resulting from operations |
$ | 27,561 | $ | 23,770 | $ | 68,703 | $ | 42,916 | $ | 71,188 | $ | 99,446 | $ | 46,759 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Change in net assets per common share (basic) |
$ | 0.33 | $ | 0.32 | $ | 0.91 | $ | 0.60 | $ | 1.12 | $ | 1.67 | $ | 0.93 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Distributions declared per common share: |
$ | 0.62 | $ | 0.62 | $ | 1.24 | $ | 1.24 | $ | 1.24 | $ | 1.11 | $ | 0.95 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
12
| For the Six-Months Ended June 30, (unaudited) |
For the Year Ended December 31, | |||||||||||||||||||||||||||
| (in thousands, except per share amounts) |
2017 | 2016 | 2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||||||||
| Balance sheet data: |
||||||||||||||||||||||||||||
| Investments, at value |
$ | 1,395,469 | $ | 1,291,310 | $ | 1,423,942 | $ | 1,200,638 | $ | 1,020,737 | $ | 910,295 | $ | 906,300 | ||||||||||||||
| Cash and cash equivalents |
160,412 | 13,478 | 13,044 | 95,196 | 227,116 | 268,368 | 182,994 | |||||||||||||||||||||
| Total assets |
1,588,709 | 1,331,815 | 1,464,204 | 1,334,761 | 1,299,223 | 1,221,715 | 1,123,643 | |||||||||||||||||||||
| Total liabilities |
771,258 | 613,435 | 676,260 | 617,627 | 640,359 | 571,708 | 607,675 | |||||||||||||||||||||
| Total net assets |
817,451 | 718,380 | 787,943 | 717,134 | 658,864 | 650,007 | 515,968 | |||||||||||||||||||||
| Other Data: |
||||||||||||||||||||||||||||
| Total return (3) |
(2.04 | %) | 7.24 | % | 26.87 | % | (9.70 | %) | (1.75 | %) | 58.49 | % | 28.28 | % | ||||||||||||||
| Total debt investments, at value |
1,287,623 | 1,211,782 | 1,328,803 | 1,110,209 | 923,906 | 821,988 | 827,540 | |||||||||||||||||||||
| Total warrant investments, at value |
32,530 | 25,091 | 27,485 | 22,987 | 25,098 | 35,637 | 29,550 | |||||||||||||||||||||
| Total equity investments, at value |
75,316 | 65,905 | 67,654 | 67,442 | 71,733 | 52,670 | 49,210 | |||||||||||||||||||||
| Unfunded Commitments (2) |
57,595 | 71,157 | 59,683 | 75,402 | 147,689 | 69,091 | 19,265 | |||||||||||||||||||||
| Net asset value per share (1) |
$ | 9.87 | $ | 9.66 | $ | 9.90 | $ | 9.94 | $ | 10.18 | $ | 10.51 | $ | 9.75 | ||||||||||||||
| (1) | Based on common shares outstanding at period end |
| (2) | Amount represents unfunded commitments, including undrawn revolving facilities, which are available at the request of the portfolio company. Amount excludes unfunded commitments which are unavailable due to the borrower having not met certain milestones. |
| (3) | The total return equals the change in the ending market value over the beginning of the period price per share plus distributions paid per share during the period, divided by the beginning price assuming the distribution is reinvested on the date of the issuance. The total return does not reflect any sales load that must be paid by investors. |
The following tables set forth certain quarterly financial information for each of the last eight quarters ended December 31, 2016 and the quarters ending March 31, 2017 and June 30, 2017. This information was derived from the Company’s unaudited consolidated financial statements. Results for any quarter are not necessarily indicative of results for the full year or for any further quarter.
| For the Quarter Ended (unaudited) |
||||||||
| (in thousands, except per share data) |
June 30, 2017 |
March 31, 2017 |
||||||
| Total investment income |
$ | 48,452 | $ | 46,365 | ||||
| Net investment income |
25,275 | 22,678 | ||||||
| Net increase (decrease) in net assets resulting from operations |
33,149 | (5,588 | ) | |||||
| Change in net assets resulting from operations per common share (basic) |
$ | 0.40 | $ | (0.07 | ) | |||
| Quarter Ended | ||||||||||||||||
| (in thousands, except per share data) |
March 31, 2016 |
June 30, 2016 |
September 30, 2016 |
December 31, 2016 |
||||||||||||
| Total investment income |
$ | 38,939 | $ | 43,538 | $ | 45,102 | $ | 47,472 | ||||||||
| Net investment income |
20,097 | 23,354 | 23,776 | 33,117 | ||||||||||||
| Net increase in net assets resulting from operations |
14,295 | 9,475 | 30,812 | 14,121 | ||||||||||||
| Change in net assets resulting from operations per common share (basic) |
$ | 0.20 | $ | 0.13 | $ | 0.41 | $ | 0.18 | ||||||||
| Quarter Ended | ||||||||||||||||
| March 31, 2015 |
June 30, 2015 |
September 30, 2015 |
December 31, 2015 |
|||||||||||||
| Total investment income |
$ | 32,494 | $ | 38,126 | $ | 47,132 | $ | 39,380 | ||||||||
| Net investment income |
12,993 | 16,781 | 23,590 | 20,137 | ||||||||||||
| Net increase in net assets resulting from operations |
21,919 | 2,752 | 4,075 | 14,170 | ||||||||||||
| Change in net assets resulting from operations per common share (basic) |
$ | 0.33 | $ | 0.03 | $ | 0.05 | $ | 0.20 | ||||||||
13
Investing in our securities may be speculative and involves a high degree of risk. You should consider carefully the risks described below and all other information contained in this prospectus, including our financial statements and the related notes and the schedules and exhibits to this prospectus. The risks set forth below are not the only risks we face. Additional risks and uncertainties not presently known to us or not presently deemed material by us may also impair our operations and performance. If any of the following risks occur, our business, financial condition and results of operations could be materially adversely affected. In such case, our NAV and the trading price of our securities could decline, and you may lose all or part of your investment.
Risks Related to our Business Structure
We are evaluating alternative management structures.
We, along with our professional advisors, are currently evaluating alternatives with respect to our management structure. While internal management has served us well since our formation, the Board of Directors has concluded that there are limitations to that management structure that may operate to our disadvantage. As an internally managed business development company, the size and categories of our assets under management is limited. Assuming we remain internally managed, we are and will be unable to offer as wide a variety of financial products to prospective portfolio companies and sponsors as an externally managed business development company, which has a greater ability to spread its operating costs over a larger, more diversified asset base, enabling the funds it advises to benefit from cost savings and greater management resources. Additionally, as an internally managed business development company, our ability to offer more competitive and flexible compensation structures, such as offering both a profit sharing plan and an equity incentive plan, is subject to the limitations imposed by the 1940 Act, which limits our ability to attract and retain talented investment management professionals. As such, if we remain an internally managed business development company, these limitations could inhibit our ability to grow, pursue our business plan and attract and retain professional talent, any or all of which may have a negative impact on our business, financial condition and results of operations.
To that end, we are exploring the possibility of externalizing our management structure as a means of addressing those concerns; and, we are also examining various alternatives that could be pursued with respect to externalization if we determine that externalization is the proper course to follow. We and our independent directors are working with advisors to assist in these efforts. This program will result in us incurring additional and unusual expense until this project is concluded. Should we determine to pursue externalization, which would be subject to approval by our stockholders, it could involve some disruption (at least on a temporary basis) and expense during the period of transition including, among other things, those arising from the transition of our current employees and investment professionals, along with the transition for the responsibility of the provision of certain key services for our business moving from the Company to an external investment adviser and/or administrator. There can be no assurance regarding the outcome of our examination of alternatives, including with respect to whether the Board of Directors recommends externalization to our stockholders, the terms of any external management agreement (including with respect to fees) or the identity of any external manager that may be recommended by the Board of Directors.
As an internally managed business development company, we are subject to certain restrictions that may adversely affect our business.
We are currently evaluating alternatives with respect to our management structure, including externalizing our management structure. We have not decided on any course of action, and there can be no assurance regarding the outcome of our examination of alternatives, including with respect to whether we decide to recommend externalization of our management to our stockholders. If we remain an internally managed business development company, we will continue to be subject to certain restrictions that may place us at a competitive disadvantage to other similar business development companies that are externally managed. As an internally
14
managed business development company, the size and categories of our assets under management is limited, and we are unable to offer as wide a variety of financial products to prospective portfolio companies and sponsors (potentially limiting the size and diversification of our asset base). We therefore may not achieve efficiencies of scale and greater management resources available to externally managed business development companies. Additionally, as an internally managed business development company, our ability to offer more competitive and flexible compensation structures, such as offering both a profit sharing plan and an equity incentive plan, is subject to the limitations imposed by the 1940 Act, which limits our ability to attract and retain talented investment management professionals. As such, if we remain an internally managed business development company, these limitations could inhibit our ability to grow, pursue our business plan and attract and retain professional talent, any or all of which may have a negative impact on our business, financial condition and results of operations.
If we externalize our management structure, we will be dependent upon key personnel of the external adviser.
If we externalize our management structure, the external adviser will depend on the efforts, skills, reputations and business contacts of its key personnel, the information and deal flow they and others generate during the normal course of their activities and the synergies among the diverse fields of expertise and knowledge held by the external adviser’s professionals. The loss of the services of any of them could have a material adverse effect on us and could harm the external adviser’s ability to manage our business.
If we externalize our management structure, our external adviser may experience conflicts of interest in connection with the management of the Company.
If we externalize our management structure, our external adviser may experience conflicts of interest in connection with the management of the Company, including, but not limited to, the following:
| • | The members, officers and other personnel of the external adviser allocate their time, resources and other services between the Company and other investment and business activities in which they may be involved. This may include providing a broad range of financial services to companies in which we invest, in compliance with applicable law, and generally being paid fees for such services. Accordingly, they may have obligations to such other entities, the fulfillment of which obligations may not be in the best interests of us or our stockholders; |
| • | The external adviser may compete with certain of its affiliates, including other entities it manages, for investments for us, subjecting the external adviser to potential conflicts of interest in evaluating the suitability of investment opportunities and making or recommending acquisitions on our behalf; |
| • | The compensation payable by us to the external adviser will be approved by the Board of Directors consistent with the exercise of the requisite standard of care applicable to trustees under state law. Such compensation is payable, in most cases, regardless of the quality of the assets acquired, the services provided to us or whether we make distributions to stockholders. There is the possibility that if we are managed by an external investment adviser, we will be subject to higher fees and expenses, but such arrangements will not be determined until the specific fee arrangement entered into with the external adviser is finalized; |
| • | Affiliated investment vehicles formed in the future and managed by the external adviser or its affiliates may have overlapping investment objectives with our own and, accordingly, may invest in asset classes similar to those targeted by us. As a result, the external adviser may face conflicts in allocating investment opportunities between us and such other entities; |
| • | The external adviser and its affiliates may not be not restricted from forming additional investment funds, from entering into other investment advisory relationships (including, among others, relationships with clients that are employee benefit plans subject to ERISA and related regulations), or from engaging in other business activities without the prior approval of our stockholders or our Board |
15
| of Directors, even though such activities may be in competition with us and/or may involve substantial time and resources of the external adviser, which could detract from the external adviser’s time spent on our business; |
| • | The external adviser and its affiliates may give advice and recommend securities to other clients which may differ from, or be contrary to, advice given to, or securities recommended or bought for, us even though their investment objectives may be similar to ours; and |
| • | To the extent not restricted by confidentiality requirements or applicable law, the external adviser may apply experience and information gained in providing services to our portfolio companies in providing services to competing companies invested in by affiliates’ other clients. |
As an internally managed business development company, we are dependent upon key management personnel for their time availability and for our future success, particularly Manuel A. Henriquez, and if we are not able to hire and retain qualified personnel, or if we lose any member of our senior management team, our ability to implement our business strategy could be significantly harmed.
As an internally managed business development company, our ability to achieve our investment objectives and to make distributions to our stockholders depends upon the performance of our senior management. We depend upon the members of our senior management, particularly Mr. Henriquez, as well as other key personnel for the identification, final selection, structuring, closing and monitoring of our investments. These employees have critical industry experience and relationships on which we rely to implement our business plan. If we lose the services of Mr. Henriquez or any senior management members, we may not be able to operate the business as we expect, and our ability to compete could be harmed, which could cause our operating results to suffer. Furthermore, we do not have an employment agreement with Mr. Henriquez or our senior management that restricts them from creating new investment vehicles subject to compliance with applicable law. We believe our future success will depend, in part, on our ability to identify, attract and retain sufficient numbers of highly skilled employees. If we do not succeed in identifying, attracting and retaining such personnel, we may not be able to operate our business as we expect.
As an internally managed business development company, our compensation structure is determined and set by our Board of Directors. This structure currently includes salary and bonus and incentive compensation, which is issued through grants and subsequent vesting of restricted stock. We are not generally permitted by the 1940 Act to employ an incentive compensation structure that directly ties performance of our investment portfolio and results of operations to compensation owing to our granting of restricted stock as incentive compensation.
Members of our senior management may receive offers of more flexible and attractive compensation arrangements from other companies, particularly from investment advisers to externally managed business development companies that are not subject to the same limitations on incentive-based compensation that we, as an internally managed business development company are subject to. We do not currently have agreements with our senior management that prohibit them from leaving and competing with our business. In addition, the evaluation of alternative management structures discussed above may lead to changes in our management structure. A departure by one or more members of our senior management could have a negative impact on our business, financial condition and results of operations.
Our business model depends to a significant extent upon strong referral relationships with venture capital and private equity fund sponsors, and our inability to develop or maintain these relationships, or the failure of these relationships to generate investment opportunities, could adversely affect our business.
We expect that members of our management team will maintain their relationships with venture capital and private equity firms, and we will rely to a significant extent upon these relationships to provide us with our deal flow. If we fail to maintain our existing relationships, our relationships become strained as a result of enforcing our rights with respect to non-performing portfolio companies in protecting our investments or we fail to develop
16
new relationships with other firms or sources of investment opportunities, then we will not be able to grow our investment portfolio. In addition, persons with whom members of our management team have relationships are not obligated to provide us with investment opportunities and, therefore, there is no assurance that such relationships will lead to the origination of debt or other investments.
We may be the target of litigation.
We may be the target of securities litigation in the future, particularly if the trading price of our common stock and our debt securities fluctuates significantly. We could also generally be subject to litigation, including derivative actions by our stockholders. Any litigation could result in substantial costs and divert management’s attention and resources from our business and cause a material adverse effect on our business, financial condition and results of operations.
We operate in a highly competitive market for investment opportunities, and we may not be able to compete effectively.
A number of entities compete with us to make the types of investments that we plan to make in prospective portfolio companies. We compete with a large number of venture capital and private equity firms, as well as with other investment funds, business development companies, investment banks and other sources of financing, including traditional financial services companies such as commercial banks and finance companies. Many of our competitors are substantially larger and have considerably greater financial, technical, marketing and other resources than we do. For example, some competitors may have a lower cost of funds and/or access to funding sources that are not available to us. This may enable some competitors to make loans with interest rates that are comparable to or lower than the rates that we typically offer.
A significant increase in the number and/or the size of our competitors, including traditional commercial lenders and other financing sources, in technology-related industries could force us to accept less attractive investment terms. We may be unable to capitalize on certain opportunities if we do not match competitors’ pricing, terms and structure. If we do match competitors’ pricing, terms or structure, we may experience decreased net interest income and increased risk of credit losses. In addition, some of our competitors may have higher risk tolerances or different risk assessments, which could allow them to consider a wider variety of investments, establish more relationships and build their market shares. An increasing number of competitors may also have the effect of compressing our margins, which could harm our ability to retain employees, increase our operating costs, and decrease the amount and frequency of future distributions. Furthermore, many potential competitors are not subject to the regulatory restrictions that the 1940 Act imposes on us as a business development company or that the Code imposes on us as a RIC. If we are not able to compete effectively, our business, financial condition, and results of operations will be adversely affected. As a result of this competition, there can be no assurance that we will be able to identify and take advantage of attractive investment opportunities, or that we will be able to fully invest our available capital.
If we are unable to manage our future growth effectively, we may be unable to achieve our investment objective, which could adversely affect our financial condition and results of operations and cause the value of your investment to decline.
Our ability to achieve our investment objective will depend on our ability to sustain growth. Sustaining growth will depend, in turn, on our senior management team’s ability to identify, evaluate, finance and invest in suitable companies that meet our investment criteria. Accomplishing this result on a cost-effective basis is largely a function of our marketing capabilities, our management of the investment process, our ability to provide efficient services and our access to financing sources on acceptable terms. Organizational growth and scale-up of our investments could strain our existing managerial, investment, financial and other resources. Management of the Company’s growth divert financial resources from other projects. Failure to manage our future growth effectively could lead to a decrease in our future distributions and have a material adverse effect on our business, financial condition and results of operations.
17
Because we intend to distribute substantially all of our income to our stockholders in order to qualify as a RIC, we will continue to need additional capital to finance our growth. If additional funds are unavailable or not available on favorable terms, our ability to grow will be impaired.
In order to satisfy the tax requirements applicable to a RIC, to avoid being subject to excise taxes and to minimize or avoid being subject to income taxes, we intend to make distributions to our stockholders treated as dividends for U.S. federal income tax purposes generally of an amount at least equal to substantially all of our net ordinary income and realized net capital gains except for certain realized net capital gains, which we may retain, pay applicable income taxes with respect thereto and elect to treat as deemed distributions to our stockholders. As a business development company, we generally are required to meet a coverage ratio of total assets to total borrowings and other senior securities, which includes all of our borrowings and any preferred stock that we may issue in the future, of at least 200%. This requirement limits the amount that we may borrow. This limitation may prevent us from incurring debt and require us to raise additional equity at a time when it may be disadvantageous to do so. We cannot assure you that debt and equity financing will be available to us on favorable terms, or at all, and debt financings may be restricted by the terms of any of our outstanding borrowings. If we are unable to incur additional debt, we may be required to raise additional equity at a time when it may be disadvantageous to do so. In addition, shares of closed-end investment companies have recently traded at discounts to their NAV. This characteristic of closed-end investment companies is separate and distinct from the risk that our NAV per share may decline. We cannot predict whether shares of our common stock will trade above, at or below our NAV. If our common stock trades below its NAV, we generally will not be able to issue additional shares of our common stock at its market price without first obtaining the approval for such issuance from our stockholders and our independent directors. If additional funds are not available to us, we could be forced to curtail or cease new lending and investment activities, and our NAV could decline. In addition, our results of operations and financial condition could be adversely affected.
Because most of our investments typically are not in publicly-traded securities, there is uncertainty regarding the value of our investments, which could adversely affect the determination of our NAV.
At June 30, 2017, portfolio investments, whose fair value is determined in good faith by the Board of Directors, were approximately 87.8% of our total assets. We expect our investments to continue to consist primarily of securities issued by privately-held companies, the fair value of which is not readily determinable. In addition, we are not permitted to maintain a general reserve for anticipated loan losses. Instead, we are required by the 1940 Act to specifically value each investment and record an unrealized gain or loss for any asset that we believe has increased or decreased in value.
There is no single standard for determining fair value in good faith. We value these securities at fair value as determined in good faith by our Board of Directors, based on the recommendations of our Audit Committee. In making a good faith determination of the value of these securities, we generally start with the cost basis of each security, which includes the amortized original issue discount, or OID, and payment-in-kind, or PIK, interest, if any. The Audit Committee uses its best judgment in arriving at the fair value of these securities. As a result, determining fair value requires that judgment be applied to the specific facts and circumstances of each portfolio investment while applying a valuation process for the types of investments we make, which includes but is not limited to deriving a hypothetical exit price.
However, the Board of Directors retains ultimate authority as to the appropriate valuation of each investment. Because such valuations are inherently uncertain and may be based on estimates, our determinations of fair value may differ materially from the values that would be assessed if a ready market for these securities existed. We adjust quarterly the valuation of our portfolio to reflect the Board of Directors’ determination of the fair value of each investment in our portfolio. Any changes in fair value are recorded in our statement of operations as net change in unrealized appreciation or depreciation. Our NAV could be adversely affected if our determinations regarding the fair value of our investments were materially higher than the values that we ultimately realize upon the disposal of such securities.
18
Because we have substantial indebtedness, there could be increased risk in investing in our company.
Lenders have fixed dollar claims on our assets that are superior to the claims of stockholders, and we have granted, and may in the future grant, lenders a security interest in our assets in connection with borrowings. In the case of a liquidation event, those lenders would receive proceeds before our stockholders. In addition, borrowings, also known as leverage, magnify the potential for gain or loss on amounts invested and, therefore, increase the risks associated with investing in our securities. Leverage is generally considered a speculative investment technique. If the value of our assets increases, then leverage would cause the NAV attributable to our common stock to increase more than it otherwise would have had we not leveraged. Conversely, if the value of our assets decreases, leverage would cause the NAV attributable to our common stock to decline more than it otherwise would have had we not used leverage. Similarly, any increase in our revenue in excess of interest expense on our borrowed funds would cause our net income to increase more than it would without the leverage. Any decrease in our revenue would cause our net income to decline more than it would have had we not borrowed funds and could negatively affect our ability to make distributions on common stock. Our ability to service any debt that we incur will depend largely on our financial performance and will be subject to prevailing economic conditions and competitive pressures. We and, indirectly, our stockholders will bear the cost associated with our leverage activity. If we are not able to service our substantial indebtedness, our business could be harmed materially.
Our secured credit facilities with Wells Fargo Capital Finance LLC (the “Wells Facility”) and MUFG Union Bank, N.A. (the “Union Bank Facility,” and together with the Wells Facility, our “Credit Facilities”), our 2024 Notes and our 2021 Asset-Backed Notes (as each term is defined below) contain financial and operating covenants that could restrict our business activities, including our ability to declare dividend distributions if we default under certain provisions.
As of June 30, 2017, we had no borrowings outstanding under the Wells Facility and the Union Bank Facility. In addition, as of June 30, 2017, we had approximately $190.2 million of indebtedness outstanding incurred by our SBIC subsidiaries, approximately $258.5 million in aggregate principal amount of 6.25% notes due 2024 (the “2024 Notes”), approximately $230.0 million in aggregate principal amount of 4.375% convertible notes due 2022 (the “2022 Convertible Notes”) and approximately $87.7 million in aggregate principal amount of fixed rate asset-backed notes issued in November 2014 (the “2021 Asset-Backed Notes”) in connection with our $237.4 million debt securitization (the “2014 Debt Securitization”).
There can be no assurance that we will be successful in obtaining any additional debt capital on terms acceptable to us or at all. If we are unable to obtain debt capital, then our equity investors will not benefit from the potential for increased returns on equity resulting from leverage to the extent that our investment strategy is successful and we may be limited in our ability to make new commitments or fundings to our portfolio companies.
As a business development company, generally, we are not permitted to incur indebtedness unless immediately after such borrowing we have an asset coverage for total borrowings of at least 200% (i.e., the amount of debt may not exceed 50% of the value of our assets). In addition, we may not be permitted to declare any cash distribution on our outstanding common shares, or purchase any such shares, unless, at the time of such declaration or purchase, we have asset coverage of at least 200% after deducting the amount of such distribution or purchase price. If this ratio declines below 200%, we may not be able to incur additional debt and may need to sell a portion of our investments to repay some debt when it is disadvantageous to do so, and we may not be able to make distributions. As of June 30, 2017 our asset coverage ratio under our regulatory requirements as a business development company was 241.9% excluding our SBIC debentures as a result of our exemptive order from the SEC that allows us to exclude all SBA leverage from our asset coverage ratio and was 206.7% when including all SBA leverage.
Based on assumed leverage equal to 93.8% of our net assets as of June 30, 2017, our investment portfolio would have been required to experience an annual return of at least 3.1% to cover annual interest payments on our additional indebtedness.
19
Illustration. The following table illustrates the effect of leverage on returns from an investment in our common stock assuming various annual returns, net of expenses. The calculations in the table below are hypothetical and actual returns may be higher or lower than those appearing below:
| Annual Return on Our Portfolio | ||||||||||||||||||||
| (Net of Expenses) | ||||||||||||||||||||
| -10% | -5% | 0% | 5% | 10% | ||||||||||||||||
| Corresponding return to stockholder(1) |
(25.54 | %) | (15.83 | %) | (6.11 | %) | 3.61 | % | (13.33 | %) | ||||||||||
| (1) | Assumes $1.6 billion in total assets, $766.4 million in debt outstanding, $817.5 million in stockholders’ equity, and an average cost of funds of 6.5%, which is the approximate average cost of borrowed funds, including our Credit Facilities, 2022 Convertible Notes, 2024 Notes, our SBA debentures and our 2021 Asset-Backed Notes for the period ended June 30, 2017. Actual interest payments may be different. |
It is likely that the terms of any current or future long-term or revolving credit or warehouse facility we may enter into in the future could constrain our ability to grow our business.
Under our borrowings and our Credit Facilities, current lenders have, and any future lender or lenders may have, fixed dollar claims on our assets that are senior to the claims of our stockholders and, thus, will have a preference over our stockholders with respect to our assets pledged as collateral under the Credit Facilities. Our Credit Facilities and borrowings also subject us to various financial and operating covenants, including, but not limited to, maintaining certain financial ratios and minimum tangible net worth amounts. Future credit facilities and borrowings will likely subject us to similar or additional covenants. In addition, we may grant a security interest in our assets in connection with any such credit facilities and borrowings.
Our Credit Facilities generally contain customary default provisions such as a minimum net worth amount, a profitability test, and a restriction on changing our business and loan quality standards. In addition, our Credit Facilities require or are expected to require the repayment of all outstanding debt on the maturity which may disrupt our business and potentially the business of our portfolio companies that are financed through the facilities. An event of default under these facilities would likely result, among other things, in termination of the availability of further funds under the facilities and accelerated maturity dates for all amounts outstanding under the facilities, which would likely disrupt our business and, potentially, the business of the portfolio companies whose loans we finance through the facilities. This could reduce our revenues and, by delaying any cash payment allowed to us under our facilities until the lender has been paid in full, reduce our liquidity and cash flow and impair our ability to grow our business and our ability to make distributions sufficient to maintain our ability to be subject to tax as a RIC.
The terms of future available financing may place limits on our financial and operation flexibility. If we are unable to obtain sufficient capital in the future, we may be forced to reduce or discontinue our operations, not be able to make new investments, or otherwise respond to changing business conditions or competitive pressures.
In addition to regulatory requirements that restrict our ability to raise capital, our Credit Facilities and the 2024 Notes contain various covenants which, if not complied with, could require accelerated repayment under the facility or require us to repurchase the 2024 Notes thereby materially and adversely affecting our liquidity, financial condition, results of operations and ability to pay distributions.
The credit agreements governing our Credit Facilities and the 2024 Notes require us to comply with certain financial and operational covenants. These covenants require us to, among other things, maintain certain financial ratios, including asset coverage, debt to equity and interest coverage. Our ability to continue to comply with these covenants in the future depends on many factors, some of which are beyond our control. There are no assurances that we will be able to comply with these covenants. Failure to comply with these covenants would result in a default which, if we were unable to obtain a waiver from the lenders under our Credit Facilities and could accelerate repayment under the facilities or the 2024 Notes and thereby have a material adverse impact on our liquidity, financial condition, results of operations and ability to pay a sufficient amount of distributions and
20
maintain our ability to be subject to tax as a RIC. We may not have enough available cash or be able to obtain financing at the time we are required to make repurchases. See “Management’s Discussion and Analysis of Financial Condition of Results of Operations—Borrowings.”
We may be unable to obtain debt capital on favorable terms or at all, in which case we would not be able to use leverage to increase the return on our investments.
If we are unable to obtain debt capital, then our equity investors will not benefit from the potential for increased returns on equity resulting from leverage to the extent that our investment strategy is successful and we may be limited in our ability to make new commitments or fundings to our portfolio companies. An inability to obtain debt capital may also limit our ability to refinance existing indebtedness, particularly during periods of adverse credit market conditions when refinancing indebtedness may not be available under interest rates and other terms acceptable to us or at all.
We are subject to certain risks as a result of our interests in connection with the 2014 Debt Securitization and our equity interest in the 2014 Securitization Issuer.
On November 13, 2014, in connection with the 2014 Debt Securitization and the offering of the 2021 Asset-Backed Notes by Hercules Capital Funding Trust 2014-1 (the “2014 Securitization Issuer”), we sold and/or contributed to Hercules Capital Funding 2014-1 LLC, as trust depositor (the “2014 Trust Depositor”), certain senior loans made to certain of our portfolio companies (the “2014 Loans”), which the 2014 Trust Depositor in turn sold and/or contributed to the 2014 Securitization Issuer in exchange for 100% of the equity interest in the 2014 Securitization Issuer, cash proceeds and other consideration. Following these transfers, the 2014 Securitization Issuer, and not the 2014 Trust Depositor or us, held all of the ownership interest in the 2014 Loans.
As a result of the 2014 Debt Securitization, we hold, indirectly through the 2014 Trust Depositor, 100% of the equity interests in the 2014 Securitization Issuer. As a result, we consolidate the financial statements of the 2014 Trust Depositor and the 2014 Securitization Issuer, as well as our other subsidiaries, in our consolidated financial statements. Because the 2014 Trust Depositor and the 2014 Securitization Issuer is disregarded as an entity separate from its owners for U.S. federal income tax purposes, the sale or contribution by us to the 2014 Trust Depositor, and by the 2014 Trust Depositor to the 2014 Securitization Issuer, as applicable, did not constitute a taxable event for U.S. federal income tax purposes. If the U.S. Internal Revenue Service (“IRS”) were to take a contrary position, there could be a material adverse effect on our business, financial condition, results of operations or cash flows.
Further, a failure of the 2014 Securitization Issuer to be treated as a disregarded entity for U.S. federal income tax purposes would constitute an event of default pursuant to the indenture under the 2014 Debt Securitization, upon which the trustee under the 2014 Debt Securitization (the “2014 Trustee”), may and will at the direction of a supermajority of the holders of the 2021 Asset-Backed Notes (the “2021 Noteholders”), declare the 2021 Asset-Backed Notes, to be immediately due and payable and exercise remedies under the applicable indenture, including (i) to institute proceedings for the collection of all amounts then payable on the 2021 Asset-Backed Notes, or under the applicable indenture, enforce any judgment obtained, and collect from the 2014 Securitization Issuer and any other obligor upon the 2021 Asset-Backed Notes monies adjudged due; (ii) institute proceedings from time to time for the complete or partial foreclosure of the applicable indenture with respect to the property of the 2014 Securitization Issuer; (iii) exercise any remedies as a secured party under the relevant Uniform Commercial Code and take other appropriate action under applicable law to protect and enforce the rights and remedies of the 2014 Trustee and the 2021 Noteholders; or (iv) sell the property of the 2014 Securitization Issuer or any portion thereof or rights or interest therein at one or more public or private sales called and conducted in any matter permitted by law. Any such exercise of remedies could have a material adverse effect on our business, financial condition, results of operations or cash flows.
21
An event of default in connection with the 2014 Debt Securitization could give rise to a cross-default under our other material indebtedness.
The documents governing our other material indebtedness contain customary cross-default provisions that could be triggered if an event of default occurs in connection with the 2014 Debt Securitization. An event of default with respect to our other indebtedness could lead to the acceleration of such indebtedness and the exercise of other remedies as provided in the documents governing such other indebtedness. This could have a material adverse effect on our business, financial condition, results of operations and cash flows and may result in our inability to make distributions sufficient to maintain our ability to be subject to tax as a RIC.
We may not receive cash distributions in respect of our indirect ownership interests in the 2014 Securitization Issuer.
Apart from fees payable to us in connection with our role as servicer of the 2014 Loans and the reimbursement of related amounts under the documents governing the 2014 Debt Securitization, we receive cash in connection with the 2014 Debt Securitization only to the extent that the 2014 Trust Depositor receives payments in respect of its equity interests in the 2014 Securitization Issuer. The respective holders of the equity interests in the 2014 Securitization Issuer are the residual claimants on distributions, if any, made by the 2014 Securitization Issuer after the respective 2021 Noteholders and other claimants have been paid in full on each payment date or upon maturity of the 2021 Asset-Backed Notes, subject to the priority of payments under the 2014 Debt Securitization documents governing the 2014 Debt Securitization. To the extent that the value of a 2014 Securitization Issuer’s portfolio of loans is reduced as a result of conditions in the credit markets (relevant in the event of a liquidation event), other macroeconomic factors, distressed or defaulted loans or the failure of individual portfolio companies to otherwise meet their obligations in respect of the loans, or for any other reason, the ability of the 2014 Securitization Issuer to make cash distributions in respect of the 2014 Trust Depositor’s equity interests would be negatively affected and consequently, the value of the equity interests in the 2014 Securitization Issuer would also be reduced. In the event that we fail to receive cash indirectly from the 2014 Securitization Issuer, we could be unable to make distributions, if at all, in amounts sufficient to maintain our ability to be subject to tax as a RIC.
The interests of the 2021 Noteholders may not be aligned with our interests.
The 2021 Asset-Backed Notes are debt obligations ranking senior in right of payment to the rights of the holder of the equity interests in the 2014 Securitization Issuer, as residual claimants in respect of distributions, if any, made by the 2014 Securitization Issuer. As such, there are circumstances in which the interests of the 2021 Noteholders may not be aligned with the interests of holders of the equity interests in the 2014 Securitization Issuer. For example, under the terms of the documents governing the 2014 Debt Securitization, the 2021 Noteholders have the right to receive payments of principal and interest prior to holders of the equity interests.
For as long as the 2021 Asset-Backed Notes remain outstanding, the respective 2021 Noteholders have the right to act in certain circumstances with respect to the 2014 Loans in ways that may benefit their interests but not the interests of the respective holders of the equity interests in the 2014 Securitization Issuer, including by exercising remedies under the documents governing the 2014 Debt Securitization.
If an event of default occurs, the 2021 Noteholders will be entitled to determine the remedies to be exercised, subject to the terms of the documents governing the 2014 Debt Securitization. For example, upon the occurrence of an event of default with respect to the 2021 Asset-Backed Notes, the 2014 Trustee may and will at the direction of the holders of a supermajority of the applicable 2021 Asset-Backed Notes declare the principal, together with any accrued interest, of the notes to be immediately due and payable. This would have the effect of accelerating the principal on such notes, triggering a repayment obligation on the part of the 2014 Securitization Issuer. The 2021 Asset-Backed Notes then outstanding will be paid in full before any further payment or distribution on the equity interest is made. There can be no assurance that there will be sufficient funds through collections on the 2014 Loans or through the proceeds of the sale of the 2014 Loans in the event of a bankruptcy or insolvency to repay in full the obligations under the 2021 Asset-Backed Notes, or to make any distribution to holders of the equity interests in the 2014 Securitization Issuer.
22
Remedies pursued by the 2021 Noteholders could be adverse to our interests as the indirect holder of the equity interests in the 2014 Securitization Issuer. The 2021 Noteholders have no obligation to consider any possible adverse effect on such other interests. Thus, there can be no assurance that any remedies pursued by the 2021 Noteholders will be consistent with the best interests of the 2014 Trust Depositor or that we will receive, indirectly through the 2014 Trust Depositor, any payments or distributions upon an acceleration of the 2021 Asset-Backed Notes. Any failure of the 2014 Securitization Issuer to make distributions in respect of the equity interests that we indirectly hold, whether as a result of an event of default and the acceleration of payments on the 2021 Asset-Backed Notes or otherwise, could have a material adverse effect on our business, financial condition, results of operations and cash flows and may result in our inability to make distributions sufficient to maintain our ability to be subject to tax as a RIC.
Certain events related to the performance of 2014 Loans could lead to the acceleration of principal payments on the 2021 Asset-Backed Notes.
The following constitute rapid amortization events (“Rapid Amortization Events”) under the documents governing the 2014 Debt Securitization: (i) the aggregate outstanding principal balance of delinquent 2014 Loans, and restructured 2014 Loans that would have been delinquent 2014 Loans had such loans not become restructured loans exceeds 10% of the current aggregate outstanding principal balance of the 2014 Loans for a period of three consecutive months; (ii) the aggregate outstanding principal balance of defaulted 2014 Loans exceeds 5% of the initial outstanding principal balance of the 2014 Loans determined as November 13, 2014 for a period of three consecutive months; (iii) the aggregate outstanding principal balance of the 2021 Asset-Backed Notes exceeds the borrowing base for a period of three consecutive months; (iv) the 2014 Securitization Issuer’s pool of 2014 Loans contains 2014 Loans to ten or fewer obligors; and (v) the occurrence of an event of default under the documents governing the 2014 Debt Securitization. After a Rapid Amortization Event has occurred, subject to the priority of payments under the documents governing the 2014 Debt Securitization, principal collections on the 2014 Loans will be used to make accelerated payments of principal on the 2021 Asset-Backed Notes until the principal balance of the 2021 Asset-Back Notes is reduced to zero. Such an event could delay, reduce or eliminate the ability of the 2014 Securitization Issuer to make distributions in respect of the equity interests that we indirectly hold, which could have a material adverse effect on our business, financial condition, results of operations and cash flows and may result in our inability to make distributions sufficient to maintain our ability to be subject to tax as a RIC.
We have certain repurchase obligations with respect to the 2014 Loans transferred in connection with the 2014 Debt Securitization.
As part of the 2014 Debt Securitization, we entered into a sale and contribution agreement and a sale and servicing agreement under which we would be required to repurchase any 2014 Loan (or participation interest therein) which was sold to the 2014 Securitization Issuer in breach of certain customary representations and warranty made by us or by the 2014 Trust Depositors with respect to such 2014 Loan or the legal structure of the 2014 Debt Securitization. To the extent that there is a breach of such representations and warranties and we fail to satisfy any such repurchase obligation, a 2014 Trustee may, on behalf of the 2014 Securitization Issuer, bring an action against us to enforce these repurchase obligations.
Our investments in a portfolio company, whether debt, equity, or a combination thereof, may lead to our receiving material non-public information (“MNPI”) or obtaining ‘control’ of the target company. Our ability to exit an investment where we have MNPI or control could be limited and could result in a realized loss on the investment.
If we receive MNPI, or a controlling interest in a portfolio company, our ability to divest ourselves from a debt or equity investment could be restricted. Causes of such restriction could include market factors, such as liquidity in a private stock, or limited trading volume in a public company’s securities, or regulatory factors, such as the receipt of MNPI or insider blackout periods, where we are under legal obligation not to sell. Additionally,
23
we may choose not to take certain actions to protect a debt investment in a control investment portfolio company. As a result, we could experience a decrease in the value of our portfolio company holdings and potentially incur a realized loss on the investment.
Regulations governing our operations as a business development company may affect our ability to, and the manner in which, we raise additional capital, which may expose us to risks.
Our business will require a substantial amount of capital. We may acquire additional capital from the issuance of senior securities, including borrowings, securitization transactions or other indebtedness, or the issuance of additional shares of our common stock. However, we may not be able to raise additional capital in the future on favorable terms or at all. We may issue debt securities, other evidences of indebtedness or preferred stock, and we may borrow money from banks or other financial institutions, which we refer to collectively as “senior securities,” up to the maximum amount permitted by the 1940 Act. Under the 1940 Act, we are not permitted to incur indebtedness unless immediately after such borrowing we have an asset coverage for total borrowings of at least 200% (i.e., the amount of debt may not exceed 50% of the value of our assets). In addition, we may not be permitted to declare any cash distribution on our outstanding common shares, or purchase any such shares, unless, at the time of such declaration or purchase, we have asset coverage of at least 200% after deducting the amount of such distribution or purchase price. Our ability to pay distributions or issue additional senior securities would be restricted if our asset coverage ratio were not at least 200%. Legislation introduced in the U.S. House of Representatives during the 114th Congress proposed to modify this section of the 1940 Act and increase the amount of debt that business development companies may incur by modifying the asset coverage percentage from 200% to 150%. If such legislation is passed, we may be able to incur additional indebtedness in the future and, therefore, your risk of an investment in our securities may increase.
If the value of our assets declines, we may be unable to satisfy this test. If that happens, we may be required to liquidate a portion of our investments and repay a portion of our indebtedness at a time when such transaction may be disadvantageous. As a result of issuing senior securities, we would also be exposed to risks associated with leverage, including an increased risk of loss. If we issue preferred stock, the preferred stock would rank “senior” to common stock in our capital structure, preferred stockholders would have separate voting rights and might have rights, preferences, or privileges more favorable than those of our common stockholders and the issuance of preferred stock could have the effect of delaying, deferring, or preventing a transaction or a change of control that might involve a premium price for holders of our common stock or otherwise be in your best interest. It is likely that any senior securities or other indebtedness we issue will be governed by an indenture or other instrument containing covenants restricting our operating flexibility. Additionally, some of these securities or other indebtedness may be rated by rating agencies, and in obtaining a rating for such securities and other indebtedness, we may be required to abide by operating and investment guidelines that further restrict operating and financial flexibility.
To the extent that we are constrained in our ability to issue debt or other senior securities, we will depend on issuances of common stock to finance operations. Other than in certain limited situations such as rights offerings, as a business development company, we are generally not able to issue our common stock at a price below NAV without first obtaining required approvals from our stockholders and our independent directors. If we raise additional funds by issuing more common stock or senior securities convertible into, or exchangeable for, our common stock, then the percentage ownership of our stockholders at that time will decrease, and you might experience dilution. Moreover, we can offer no assurance that we will be able to issue and sell additional equity securities in the future, on favorable terms or at all.
When we are a debt or minority equity investor in a portfolio company, we may not be in a position to control the entity, and management of the company may make decisions that could decrease the value of our portfolio holdings.
We make both debt and minority equity investments; therefore, we are subject to the risk that a portfolio company may make business decisions with which we disagree, and the stockholders and management of such
24
company may take risks or otherwise act in ways that do not serve our interests. As a result, a portfolio company may make decisions that could decrease the value of our portfolio holdings.
If we do not invest a sufficient portion of our assets in qualifying assets, we could fail to qualify as a business development company or be precluded from investing according to our current business strategy.
As a business development company, we may not acquire any assets other than “qualifying assets” as defined under the 1940 Act, unless, at the time of and after giving effect to such acquisition, at least 70% of our total assets are qualifying assets. See “Regulation.”
We believe that most of the senior loans we make will constitute qualifying assets. However, we may be precluded from investing in what we believe are attractive investments if such investments are not qualifying assets for purposes of the 1940 Act. If we do not invest a sufficient portion of our assets in qualifying assets, we could lose our status as a business development company, which would have a material adverse effect on our business, financial condition and results of operations. In addition, a rise in the equity markets may result in increased market valuations of certain of our existing and prospective portfolio companies, which may lead to new investments with such companies being qualified as non-eligible portfolio company assets and would require we invest in qualified assets or risk losing our status as a business development company. Similarly, these rules could prevent us from making follow-on investments in existing portfolio companies (which could result in the dilution of our position) or could require us to dispose of investments at inopportune times in order to comply with the 1940 Act. If we need to dispose of such investments quickly, it would be difficult to dispose of such investments on favorable terms. For example, we may have difficulty in finding a buyer and, even if we do find a buyer, we may have to sell the investments at a substantial loss.
A failure on our part to maintain our qualification as a business development company would significantly reduce our operating flexibility.
If we fail to continuously qualify as a business development company, we might be subject to regulation as a registered closed-end investment company under the 1940 Act, which would significantly decrease our operating flexibility, and lead to situations where we might have to restrict our borrowings, reduce our leverage, sell securities and pursue other activities that we are allowed to engage in as a business development company. In addition, failure to comply with the requirements imposed on business development companies by the 1940 Act could cause the SEC to bring an enforcement action against us. For additional information on the qualification requirements of a business development company, see “Regulation.”
To the extent OID and PIK interest constitute a portion of our income, we will be exposed to risks associated with such income being required to be included in taxable and accounting income prior to receipt of cash representing such income.
Our investments may include OID instruments and contractual PIK interest arrangements, which represents contractual interest added to a loan balance and due at the end of such loan’s term. To the extent OID or PIK interest constitute a portion of our income, we are exposed to risks associated with such income being required to be included in taxable and accounting income prior to receipt of cash, including the following:
| • | The higher interest rates of OID and PIK instruments reflect the payment deferral and increased credit risk associated with these instruments, and OID and PIK instruments generally represent a significantly higher credit risk than coupon loans. |
| • | Even if the accounting conditions for income accrual are met, the borrower could still default when our actual collection is supposed to occur at the maturity of the obligation, which could lead to future losses. |
| • | OID and PIK instruments may have unreliable valuations because their continuing accruals require continuing judgments about the collectability of the deferred payments and the value of any associated collateral. OID and PIK income may also create uncertainty about the source of our cash distributions. |
25
| • | For accounting purposes, any cash distributions to stockholders representing OID and PIK income are not treated as coming from paid-in capital, even though the cash to pay them comes from the offering proceeds. As a result, despite the fact that a distribution representing OID and PIK income could be paid out of amounts invested by our stockholders, the 1940 Act does not require that stockholders be given notice of this fact by reporting it as a return of capital. |
| • | The deferral of PIK interest may have a negative impact on our liquidity as it represents non-cash income that may require cash distributions to our stockholders in order to maintain our ability to be subject to tax as a RIC. |
If we are unable to satisfy Code requirements for qualification as a RIC, then we will be subject to corporate-level income tax, which would adversely affect our results of operations and financial condition.
We elected to be treated as a RIC for U.S. federal income tax purposes with the filing of our federal corporate income tax return for 2006. We will not qualify for the tax treatment allowable to RICs if we are unable to comply with the source of income, asset diversification and distribution requirements contained in Subchapter M of the Code, or if we fail to maintain our election to be regulated as a business development company under the 1940 Act. If we fail to qualify as a RIC for any reason and become subject to a corporate-level income tax, the resulting taxes could substantially reduce our net assets, the amount of income available for distribution to our stockholders and the actual amount of our distributions. Such a failure would have a material adverse effect on us, the NAV of our common stock and the total return, if any, earned from your investment in our common stock.
We may have difficulty paying our required distributions under applicable tax rules if we recognize income before or without receiving cash representing such income.
In accordance with U.S. federal tax requirements, we are required to include in income for tax purposes certain amounts that we have not yet received in cash, such as OID and contractual PIK interest arrangements, which represent contractual interest added to a loan balance and due at the end of such loan’s term. In addition to the cash yields received on our loans, in some instances, our loans generally include one or more of the following: exit fees, balloon payment fees, commitment fees, success fees or prepayment fees. In some cases our loans also include contractual PIK interest arrangements. The increases in loan balances as a result of contractual PIK arrangements are included in income for the period in which such PIK interest was accrued, which is often in advance of receiving cash payment, and are separately identified on our statements of cash flows. We also may be required to include in income for tax purposes certain other amounts prior to receiving the related cash.
Any warrants that we receive in connection with our debt investments will generally be valued as part of the negotiation process with the particular portfolio company. As a result, a portion of the aggregate purchase price for the debt investments and warrants will be allocated to the warrants that we receive. This will generally result in OID for tax purposes, which we must recognize as ordinary income, increasing the amount that we are required to distribute in order to be subject to tax as a RIC. Because these warrants generally will not produce distributable cash for us at the same time as we are required to make distributions in respect of the related OID, if ever, we would need to obtain cash from other sources or to pay a portion of our distributions using shares of newly issued common stock, consistent with IRS guidelines and the Code, to satisfy such distribution requirements.
Other features of the debt instruments that we hold may also cause such instruments to generate OID in excess of current cash interest received. Since in certain cases we may recognize income before or without receiving cash representing such income, we may have difficulty meeting the RIC tax requirement to make distributions each taxable year to our stockholders treated as dividends for U.S. federal income tax purposes generally of an amount equal to at least 90% of our investment company taxable income, determined without regard to any deduction for dividends paid. Under such circumstances, we may have to sell some of our assets, raise additional debt or equity capital or reduce new investment originations to meet these distribution
26
requirements. If we are unable to obtain cash from other sources and are otherwise unable to satisfy such distribution requirements, we may fail to qualify to be subject to tax as a RIC and, thus, become subject to a corporate-level income tax on all our taxable income (including any net realized securities gains).
Furthermore, we may invest in the equity securities of non-U.S. corporations (or other non-U.S. entities classified as corporations for U.S. federal income tax purposes) that could be treated under the Code and U.S. Treasury regulations as “passive foreign investment companies” (“PFICs”) and/or “controlled foreign corporations” (“CFCs”). The rules relating to investment in these types of non-U.S. entities are designed to ensure that U.S. taxpayers are either, in effect, taxed currently (or on an accelerated basis with respect to corporate level events) or taxed at increased tax rates at distribution or disposition. In certain circumstances, these rules also could require us to recognize taxable income or gains where we do not receive a corresponding payment in cash. Furthermore, under recently proposed Treasury Regulations, certain income derived by us either from a PFIC with respect to which we have made a certain U.S. tax election or from a CFC would generally constitute qualifying income for purposes of determining our ability to be subject to tax as a RIC only to the extent the PFIC or CFC respectively makes distributions of that income to us. As such, we may be restricted in our ability to make QEF elections with respect to our holdings in issuers that could either be treated as PFICs or CFCs in order to limit our tax liability or maximize our after-tax return from these investments.
Our portfolio investments may present special tax issues.
Investments in below-investment grade debt instruments and certain equity securities may present special tax issues for us. U.S. federal income tax rules are not entirely clear about issues such as when we may cease to accrue interest, OID or market discount, when and to what extent deductions may be taken for bad debts or worthless debt in equity securities, how payments received on obligations in default should be allocated between principal and interest income, as well as whether exchanges of debt instruments in a bankruptcy or workout context are taxable. Such matters could cause us to recognize taxable income for U.S. federal income tax purposes, even in the absence of cash or economic gain, and require us to make taxable distributions to our stockholders to maintain our RIC status or preclude the imposition of either U.S. federal corporate income or excise taxation. Additionally, because such taxable income may not be matched by corresponding cash received by us, we may be required to borrow money or dispose of other investments to be able to make distributions to our stockholders. These and other issues will be considered by us, to the extent determined necessary, in order that we minimize the level of any U.S. federal income or excise tax that we would otherwise incur. See “Certain United States Federal Income Tax Considerations—Taxation as a Regulated Investment Company.”
Legislative or regulatory tax changes could adversely affect you.
At any time, the U.S. federal income tax laws governing RICs or the administrative interpretations of those laws or regulations may be amended. Any of those new laws, regulations or interpretations may take effect retroactively and could adversely affect the taxation of us or of you as a stockholder. Therefore, changes in tax laws, regulations or administrative interpretations or any amendments thereto could diminish the value of an investment in our shares or the value or the resale potential of our investments.
There is a risk that you may not receive distributions or that our distributions may not grow over time.
We intend to make distributions on a quarterly basis to our stockholders. We cannot assure you that we will achieve investment results, or our business may not perform in a manner that will allow us to make a specified level of distributions or year-to-year increases in cash distributions. In addition, due to the asset coverage test applicable to us as a business development company, we may be limited in our ability to make distributions. Also, our Credit Facilities limit our ability to declare distributions to our stockholders if we default under certain provisions of our Credit Facilities. Furthermore, while we may have undistributed earnings, those earnings may not yield distributions because we may incur unrealized losses or otherwise be unable to distribute such earnings.
27
We have and may in the future choose to pay distributions in our own stock, in which case you may be required to pay tax in excess of the cash you receive.
Under applicable Treasury regulations and other administrative authorities issued by the IRS, RICs are permitted to treat certain distributions payable in their stock, as taxable dividends that will satisfy their annual distribution obligations for U.S. federal income tax and excise tax purposes provided that stockholders have the opportunity to elect to receive all or a portion of such distribution in cash. Taxable stockholders receiving distributions will be required to include the full amount of such distributions as ordinary income (or as long-term capital gain to the extent such distribution is properly designated as a capital gain dividend) to the extent of our current and accumulated earnings and profits for U.S. federal income tax purposes. As a result, a U.S. stockholder may be required to pay tax with respect to such distributions in excess of any cash received. If a U.S. stockholder sells the stock it receives as a distribution in order to pay this tax, the sales proceeds may be less than the amount included in income with respect to the distribution, depending on the market price of our stock at the time of the sale. Furthermore, with respect to non-U.S. stockholders, we may be required to withhold U.S. federal income tax with respect to such distributions, including in respect of all or a portion of such distribution that is payable in stock. In addition, if a significant number of our stockholders determine to sell shares of our stock in order to pay taxes owed on such distributions, then such sales may put downward pressure on the trading price of our stock. We may in the future determine to distribute taxable distributions that are partially payable in our common stock.
We are exposed to risks associated with changes in interest rates, including fluctuations in interest rates which could adversely affect our profitability or the value of our portfolio
General interest rate fluctuations may have a substantial negative impact on our investments and investment opportunities, and, accordingly, may have a material adverse effect on our investment objective and rate of return on investment capital. A portion of our income will depend upon the difference between the rate at which we borrow funds and the interest rate on the debt securities in which we invest. Because we will borrow money to make investments and may issue debt securities, preferred stock or other securities, our net investment income is dependent upon the difference between the rate at which we borrow funds or pay interest or dividends on such debt securities, preferred stock or other securities and the rate at which we invest these funds. Typically, we anticipate that our interest-earning investments will accrue and pay interest at both variable and fixed rates, and that our interest-bearing liabilities will generally accrue interest at fixed rates.
A significant increase in market interest rates could harm our ability to attract new portfolio companies and originate new loans and investments. In addition to potentially increasing the cost of our debt, increasing interest rates may also have a negative impact on our portfolio companies’ ability to repay or service their loans, which could enhance the risk of loan defaults. We expect that most of our current initial investments in debt securities will be at floating rate with a floor. However, in the event that we make investments in debt securities at variable rates, a significant increase in market interest rates could also result in an increase in our non-performing assets and a decrease in the value of our portfolio because our floating-rate loan portfolio companies may be unable to meet higher payment obligations. As of June 30, 2017, approximately 94.5% of our loans were at floating rates or floating rates with a floor and 5.5% of the loans were at fixed rates.
In periods of rising interest rates, our cost of funds would increase, resulting in a decrease in our net investment income. In addition, a decrease in interest rates may reduce net income, because new investments may be made at lower rates despite the increased demand for our capital that the decrease in interest rates may produce. We may, but will not be required to, hedge against the risk of adverse movement in interest rates in our short-term and long-term borrowings relative to our portfolio of assets. If we engage in hedging activities, it may limit our ability to participate in the benefits of lower interest rates with respect to the hedged portfolio. Adverse developments resulting from changes in interest rates or hedging transactions could have a material adverse effect on our business, financial condition, and results of operations.
28
We may expose ourselves to risks if we engage in hedging transactions.
If we engage in hedging transactions, we may expose ourselves to risks associated with such transactions. We may utilize instruments such as forward contracts, currency options and interest rate swaps, caps, collars and floors to seek to hedge against fluctuations in the relative values of our portfolio positions from changes in currency exchange rates and market interest rates. Hedging against a decline in the values of our portfolio positions does not eliminate the possibility of fluctuations in the values of such positions or prevent losses if the values of such positions decline. However, such hedging can establish other positions designed to gain from those same developments, thereby offsetting the decline in the value of such portfolio positions. Such hedging transactions may also limit the opportunity for gain if the values of the underlying portfolio positions should increase. It may not be possible to hedge against an exchange rate or interest rate fluctuation that is so generally anticipated that we are not able to enter into a hedging transaction at an acceptable price. Moreover, for a variety of reasons, we may not seek to establish a perfect correlation between such hedging instruments and there can be no assurance that any such hedging arrangements will achieve the desired effect. During the six-months ended June 30, 2017, we did not engage in any hedging activities.
Legislation may allow us to incur additional leverage.
As a business development company, under the 1940 Act generally we are not permitted to incur indebtedness unless immediately after such borrowing we have an asset coverage for total borrowings of at least 200% (i.e., the amount of debt may not exceed 50% of the value of our assets). If recent legislation introduced in the U.S. House of Representatives is passed, or similar legislation is introduced, it would modify this section of the 1940 Act and increase the amount of debt that business development companies may incur. As a result, we may be able to incur additional indebtedness in the future and therefore your risk of an investment in us may increase. However, the ultimate form and likely outcome of such legislation or any similar legislation cannot be predicted.
Two of our wholly-owned subsidiaries are licensed by the U.S. Small Business Administration, and as a result, we will be subject to SBA regulations, which could limit our capital or investment decisions.
Our wholly-owned subsidiaries HT II and HT III are licensed to act as SBICs and are regulated by the SBA. HT II and HT III hold approximately $104.8 million and $271.5 million in assets, respectively, and they accounted for approximately 5.8% and 14.9% of our total assets, respectively, prior to consolidation at June 30, 2017. The SBIC licenses allow our SBIC subsidiaries to obtain leverage by issuing SBA-guaranteed debentures, subject to the issuance of a capital commitment by the SBA and other customary procedures.
The SBA regulations require that a licensed SBIC be periodically examined and audited by the SBA to determine its compliance with the relevant SBA regulations. The SBA prohibits, without prior SBA approval, a “change of control” of an SBIC or transfers that would result in any person (or a group of persons acting in concert) owning 10.0% or more of a class of capital stock of a licensed SBIC. If either HT II or HT III fail to comply with applicable SBA regulations, the SBA could, depending on the severity of the violation, limit or prohibit HT II’s or HT III’s use of debentures, declare outstanding debentures immediately due and payable, and/ or limit HT II or HT III from making new investments. Such actions by the SBA would, in turn, negatively affect us because HT II and HT III are our wholly owned subsidiaries.
HT II and HT III were in compliance with the terms of the SBIC’s leverage as of June 30, 2017 as a result of having sufficient capital as defined under the SBA regulations. Compliance with SBA requirements may cause HT II and HT III to forego attractive investment opportunities that are not permitted under SBA regulations. See “Regulation—Small Business Administration Regulations.”
29
SBA regulations limit the outstanding dollar amount of SBA guaranteed debentures that may be issued by an SBIC or group of SBICs under common control.
The SBA regulations currently limit the dollar amount of SBA-guaranteed debentures that can be issued by any one SBIC to $150.0 million or to a group of SBICs under common control to $350.0 million.
An SBIC may not borrow an amount in excess of two times (and in certain cases, up to three times) its regulatory capital. As of June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries, which is the maximum combined capacity for our SBIC subsidiaries under our existing licenses. During times that we reach the maximum dollar amount of SBA-guaranteed debentures permitted, and if we require additional capital, our cost of capital is likely to increase, and there is no assurance that we will be able to obtain additional financing on acceptable terms.
Moreover, the current status of our SBIC subsidiaries as SBICs does not automatically assure that our SBIC subsidiaries will continue to receive SBA-guaranteed debenture funding. Receipt of SBA leverage funding is dependent upon our SBIC subsidiaries continuing to be in compliance with SBA regulations and policies and available SBA funding. The amount of SBA leverage funding available to SBICs is dependent upon annual Congressional authorizations and in the future may be subject to annual Congressional appropriations. There can be no assurance that there will be sufficient debenture funding available at the times desired by our SBIC subsidiaries.
The debentures guaranteed by the SBA have a maturity of ten years and require semi-annual payments of interest. Our SBIC subsidiaries will need to generate sufficient cash flow to make required interest payments on the debentures. If our SBIC subsidiaries are unable to meet their financial obligations under the debentures, the SBA, as a creditor, will have a superior claim to our SBIC subsidiaries’ assets over our stockholders in the event we liquidate our SBIC subsidiaries or the SBA exercises its remedies under such debentures as the result of a default by us.
Our wholly-owned SBIC subsidiaries may be unable to make distributions to us that will enable us to maintain RIC status, which could result in the imposition of an entity-level tax.
In order for us to continue to qualify for RIC tax treatment and to minimize corporate-level taxes, we will be required to distribute substantially all of our investment company taxable income, determined without regard to any deduction for dividends paid, and net capital gains, including income from certain of our subsidiaries, which includes the income from our SBIC subsidiaries. We will be partially dependent on our SBIC subsidiaries for cash distributions to enable us to meet the RIC distribution requirements. Our SBIC subsidiaries may be limited by the Small Business Investment Act of 1958, as amended, and SBA regulations governing SBICs, from making certain distributions to us that may be necessary to maintain our ability to be subject to tax as a RIC. We may have to request a waiver of the SBA’s restrictions for our SBIC subsidiaries to make certain distributions to maintain our ability to be subject to tax as a RIC. We cannot assure you that the SBA will grant such waiver. If our SBIC subsidiaries are unable to obtain a waiver, compliance with the SBA regulations may result in loss of RIC tax treatment and a consequent imposition of an entity-level tax on us.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404
30
of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm (when undertaken, as noted below), may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors and lenders to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
Our Board of Directors may change our investment objective, operating policies and strategies without prior notice or stockholder approval, the effects of which may be adverse.
Our Board of Directors has the authority, except as otherwise provided in the 1940 Act, to modify or waive certain of our operating policies and strategies without prior notice and without stockholder approval. However, absent stockholder approval, we may not change the nature of our business so as to cease to be, or withdraw our election as, a business development company. We cannot predict the effect any changes to our current operating policies and strategies would have on our business, operating results and the market price of our common stock. Nevertheless, any such changes could materially and adversely affect our business and impair our ability to make distributions to our stockholders.
Changes in laws or regulations governing our business could negatively affect the profitability of our operations.
Changes in the laws or regulations, or the interpretations of the laws and regulations, which govern business development companies, SBICs, RICs or non-depository commercial lenders could significantly affect our operations and our cost of doing business. We are subject to federal, state and local laws and regulations, in addition to applicable foreign and international laws and regulations, and are subject to judicial and administrative decisions that affect our operations, including our loan originations maximum interest rates, fees and other charges, disclosures to portfolio companies, the terms of secured transactions, collection and foreclosure procedures, and other trade practices. If these laws, regulations or decisions change, or if we expand our business into jurisdictions that have adopted more stringent requirements than those in which we currently conduct business, then we may have to incur significant expenses in order to comply or we may have to restrict our operations. In addition, if we do not comply with applicable laws, regulations and decisions, then we may lose licenses needed for the conduct of our business and be subject to civil fines and criminal penalties, any of which could have a material adverse effect upon our business results of operations or financial condition.
Our business is subject to increasingly complex corporate governance, public disclosure and accounting requirements that could adversely affect our business and financial results.
We are subject to changing rules and regulations of federal and state government as well as the stock exchange on which our common stock is listed. These entities, including the Public Company Accounting Oversight Board, the SEC and the NYSE have issued a significant number of new and increasingly complex requirements and regulations over the course of the last several years and continue to develop additional regulations and requirements in response to laws enacted by Congress. On July 21, 2010, the Dodd-Frank Wall Street Reform and Protection Act, as amended, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation-related provisions in the Dodd-Frank Act, and the SEC has adopted, and will continue to adopt, additional rules and regulations that may impact us. Our efforts to comply with these requirements have resulted in, and are likely to continue to result in, an increase in expenses and a diversion of management’s time from other business activities.
In addition, our failure to maintain compliance with such rules, or for our management to appropriately address issues relating to our compliance with such rules fully and in a timely manner, exposes us to an increasing risk of inadvertent non-compliance. While the Company’s management team takes reasonable efforts to ensure that the Company is in full compliance with all laws applicable to its operations, the increasing rate and
31
extent of regulatory change increases the risk of a failure to comply, which may result in our ability to operate our business in the ordinary course or may subject us to potential fines, regulatory findings or other matters that may materially impact our business.
We incur significant costs as a result of being a publicly traded company.
As a publicly traded company, we incur legal, accounting and other expenses, including costs associated with the periodic reporting requirements applicable to a company whose securities are registered under the Exchange Act as well as additional corporate governance requirements, including requirements under the Sarbanes-Oxley Act and other rules implemented by the SEC.
Results may fluctuate and may not be indicative of future performance.
Our operating results may fluctuate and, therefore, you should not rely on current or historical period results to be indicative of our performance in future reporting periods. Factors that could cause operating results to fluctuate include, but are not limited to, variations in the investment origination volume and fee income earned, changes in the accrual status of our debt investments, variations in timing of prepayments, variations in and the timing of the recognition of net realized gains or losses and changes in unrealized appreciation or depreciation, the level of our expenses, the degree to which we encounter competition in our markets, and general economic conditions.
We face cyber-security risks and the failure in cyber security systems, as well as the occurrence of events unanticipated in our disaster recovery systems and management continuity planning could impair our ability to conduct business effectively.
Our business operations rely upon secure information technology systems for data processing, storage and reporting. Despite careful security and controls design, implementation and updating, our information technology systems could become subject to cyber-attacks. Network, system, application and data breaches could result in operational disruptions or information misappropriation, which could have a material adverse effect on our business, results of operations and financial condition.
The occurrence of a disaster such as a cyber-attack, a natural catastrophe, an industrial accident, a terrorist attack or war, events unanticipated in our disaster recovery systems, or a support failure from external providers, could have an adverse effect on our ability to conduct business and on our results of operations and financial condition, particularly if those events affect our computer-based data processing, transmission, storage, and retrieval systems or destroy data. If a significant number of our managers were unavailable in the event of a disaster, our ability to effectively conduct our business could be severely compromised.
We depend heavily upon computer systems to perform necessary business functions. Despite our implementation of a variety of security measures, our computer systems could be subject to cyber-attacks and unauthorized access, such as physical and electronic break-ins or unauthorized tampering. Like other companies, we may experience threats to our data and systems, including malware and computer virus attacks, unauthorized access, system failures and disruptions. If one or more of these events occurs, it could potentially jeopardize the confidential, proprietary and other information processed and stored in, and transmitted through, our computer systems and networks, or otherwise cause interruptions or malfunctions in our operations, which could result in damage to our reputation, financial losses, litigation, increased costs, regulatory penalties and/or customer dissatisfaction or loss.
Terrorist attacks, acts of war or natural disasters may affect any market for our securities, impact the businesses in which we invest and harm our business, operating results and financial condition.
Terrorist acts, acts of war or natural disasters may disrupt our operations, as well as the operations of the businesses in which we invest. Such acts have created, and continue to create, economic and political
32
uncertainties and have contributed to global economic instability. Future terrorist activities, military or security operations, or natural disasters could further weaken the domestic/global economies and create additional uncertainties, which may negatively impact the businesses in which we invest directly or indirectly and, in turn, could have a material adverse impact on our business, operating results and financial condition. Losses from terrorist attacks and natural disasters are generally uninsurable.
We are dependent on information systems and systems failures could significantly disrupt our business, which may, in turn, negatively affect the market price of our common stock and our ability to pay distributions.
Our business is dependent on our and third parties’ communications and information systems. Any failure or interruption of those systems, including as a result of the termination of an agreement with any third-party service providers, could cause delays or other problems in our activities. Our financial, accounting, data processing, backup or other operating systems and facilities may fail to operate properly or become disabled or damaged as a result of a number of factors including events that are wholly or partially beyond our control and adversely affect our business. There could be:
| • | sudden electrical or telecommunication outages; |
| • | natural disasters such as earthquakes, tornadoes and hurricanes; |
| • | disease pandemics; |
| • | events arising from local or larger scale political or social matters, including terrorist acts; and |
| • | cyber-attacks. |
These events, in turn, could have a material adverse effect on our operating results and negatively affect the market price of our common stock and our ability to pay distributions to our stockholders.
We may be subject to restrictions on our ability to make distributions to our stockholders.
Restrictions imposed on the declaration of dividends or other distributions to holders of our common stock, by both the 1940 Act and by requirements imposed by rating agencies, might impair our ability to be subject to tax as a RIC. While we intend to prepay our Notes and other debt to the extent necessary to enable us to distribute our income as required to maintain our ability to be subject to tax as a RIC, there can be no assurance that such actions can be effected in time or in a manner to satisfy the requirements set forth in the Code.
Further downgrades of the U.S. credit rating, automatic spending cuts or another government shutdown could negatively impact our liquidity, financial condition and earnings.
Recent U.S. debt ceiling and budget deficit concerns have increased the possibility of additional credit-rating downgrades and economic slowdowns, or a recession in the U.S. Although U.S. lawmakers passed legislation to raise the federal debt ceiling on multiple occasions, ratings agencies have lowered or threatened to lower the long-term sovereign credit rating on the United States. The impact of this or any further downgrades to the U.S. government’s sovereign credit rating or its perceived creditworthiness could adversely affect the U.S. and global financial markets and economic conditions. These developments could cause interest rates and borrowing costs to rise, which may negatively impact our ability to access the debt markets on favorable terms. In addition, disagreement over the federal budget has caused the U.S. federal government to shut down for periods of time. Continued adverse political and economic conditions could have a material adverse effect on our business, financial condition and results of operations.
33
Risks Related to Current Economic and Market Conditions
Capital markets may experience periods of disruption and instability and we cannot predict when these conditions will occur. Such market conditions could materially and adversely affect debt and equity capital markets in the United States and abroad, which could have a negative impact on our business, financial condition and results of operations.
The global capital markets have experienced a period of disruption as evidenced by a lack of liquidity in the debt capital markets, write-offs in the financial services sector, the re-pricing of credit risk and the failure of certain major financial institutions. While the capital markets have improved, these conditions could deteriorate again in the future. During such market disruptions, we may have difficulty raising debt or equity capital, especially as a result of regulatory constraints.
Market conditions may in the future make it difficult to extend the maturity of or refinance our existing indebtedness and any failure to do so could have a material adverse effect on our business. The illiquidity of our investments may make it difficult for us to sell such investments if required. As a result, we may realize significantly less than the value at which we have recorded our investments. In addition, significant changes in the capital markets, including the disruption and volatility, have had, and may in the future have, a negative effect on the valuations of our investments and on the potential for liquidity events involving our investments. An inability to raise capital, and any required sale of our investments for liquidity purposes, could have a material adverse impact on our business, financial condition and results of operations.
Various social and political tensions in the United States and around the world, including in the Middle East, Eastern Europe and Russia, may continue to contribute to increased market volatility, may have long-term effects on the United States and worldwide financial markets, and may cause further economic uncertainties or deterioration in the United States and worldwide. Several European Union (“EU”) countries, including Greece, Ireland, Italy, Spain, and Portugal, continue to face budget issues, some of which may have negative long-term effects for the economies of those countries and other EU countries. There is also continued concern about national-level support for the euro and the accompanying coordination of fiscal and wage policy among European Economic and Monetary Union member countries. In July and August 2015, Greece reached agreements with its creditors for bailouts that provide aid in exchange for certain austerity measures. These and similar austerity measures may adversely affect world economic conditions and have an impact on our business and that of our portfolio companies. In the second quarter of 2015, stock prices in China experienced a significant drop, resulting primarily from continued sell-off of trading in Chinese markets. In August 2015, Chinese authorities sharply devalued China’s currency.
The broader fundamentals of the United States economy remain mixed. In the event that the United States economy contracts, it is likely that the financial results of small to mid-sized companies, like many of our portfolio companies, could experience deterioration or limited growth from current levels, which could ultimately lead to difficulty in meeting their debt service requirements and an increase in defaults. In addition, a prolonged continuation of the decline in oil and natural gas prices experienced over the last two years would adversely affect the credit quality of our debt investments and the underlying operating performance of our equity investments in energy-related businesses. Consequently, we can provide no assurance that the performance of certain portfolio companies will not be negatively impacted by economic cycles, industry cycles or other conditions, which could also have a negative impact on our future results.
The government of the United Kingdom (“U.K.”) held an in-or-out referendum on the U.K.’s membership in the EU on June 23, 2016. The referendum resulted in a vote in favor of the exit of the U.K. from the EU (“Brexit”). A process of negotiation will follow that will determine the future terms of the U.K.’s relationship with the EU. The uncertainty in the wake of the referendum could have a negative impact on both the U.K. economy and the economies of other countries in Europe. The Brexit process also may lead to greater volatility in the global currency and financial markets, which could adversely affect us. In connection with investments in non-U.S. issuers, we may engage in foreign currency exchange transactions but is not required to hedge its
34
currency exposure. As such, we make investments that are denominated in British pound sterling or euros. Our assets are generally valued in U.S. dollars, and the depreciation of the British pound sterling and/or the euro in relation to the U.S. dollar in anticipation of Brexit would adversely affect our investments denominated in British pound sterling or euros that are not fully hedged regardless of the performance of their underlying issuers. Global central banks may maintain historically low interest rates longer than was anticipated prior to the Brexit vote, which could adversely affect our income and the level of our distributions.
These market and economic disruptions affect, and these and other similar market and economic disruptions may in the future affect, the U.S. capital markets, which could adversely affect our business and that of our portfolio companies. We cannot predict the duration of the effects related to these or similar events in the future on the United States economy and securities markets or on our investments. We monitor developments and seek to manage our investments in a manner consistent with achieving our investment objective, but there can be no assurance that we will be successful in doing so.
Depending on funding requirements, we may need to raise additional capital to meet our unfunded commitments either through equity offerings or through additional borrowings.
As of June 30, 2017, we had approximately $57.6 million of unfunded commitments, including undrawn revolving facilities, which were available at the request of the portfolio company and unencumbered by milestones.
Our unfunded contractual commitments may be significant from time to time. A portion of these unfunded contractual commitments are dependent upon the portfolio company reaching certain milestones before the debt commitment becomes available. Furthermore, our credit agreements contain customary lending provisions which allow us relief from funding obligations for previously made commitments in instances where the underlying company experiences materially adverse events that affect the financial condition or business outlook for the company. These commitments will be subject to the same underwriting and ongoing portfolio maintenance as are the on-balance sheet financial instruments that we hold. Since these commitments may expire without being drawn upon, the total commitment amount does not necessarily represent future cash requirements. Closed commitments generally fund 70-80% of the committed amount in aggregate over the life of the commitment. We believe that our assets provide adequate cover to satisfy all of our unfunded comments and we intend to use cash flow from normal and early principal repayments and proceeds from borrowings and notes to fund these commitments. However, there can be no assurance that we will have sufficient capital available to fund these commitments as they come due.
Our ability to secure additional financing and satisfy our financial obligations under indebtedness outstanding from time to time will depend upon our future operating performance, which is subject to the prevailing general economic and credit market conditions, including interest rate levels and the availability of credit generally, and financial, business and other factors, many of which are beyond our control. The prolonged continuation or worsening of current economic and capital market conditions could have a material adverse effect on our ability to secure financing on favorable terms, if at all.
Changes relating to the LIBOR calculation process may adversely affect the value of our portfolio of the LIBOR-indexed, floating-rate debt securities.
In the recent past, concerns have been publicized that some of the member banks surveyed by the British Bankers’ Association (“BBA”) in connection with the calculation of the London Interbank Offered Rate, or LIBOR, across a range of maturities and currencies may have been under-reporting or otherwise manipulating the inter-bank lending rate applicable to them in order to profit on their derivatives positions or to avoid an appearance of capital insufficiency or adverse reputational or other consequences that may have resulted from reporting inter-bank lending rates higher than those they actually submitted. A number of BBA member banks entered into settlements with their regulators and law enforcement agencies with respect to alleged manipulation of LIBOR, and investigations by regulators and governmental authorities in various jurisdictions are ongoing.
35
Actions by the BBA, regulators or law enforcement agencies as a result of these or future events, may result in changes to the manner in which LIBOR is determined. Potential changes, or uncertainty related to such potential changes may adversely affect the market for LIBOR-based securities, including our portfolio of LIBOR-indexed, floating-rate debt securities. In addition, any further changes or reforms to the determination or supervision of LIBOR may result in a sudden or prolonged increase or decrease in reported LIBOR, which could have an adverse impact on the market for LIBOR-based securities or the value of our portfolio of LIBOR-indexed, floating-rate debt securities.
Risks Related to Our Investments
Our investments are concentrated in certain industries and in a number of technology-related companies, which subjects us to the risk of significant loss if any of these companies default on their obligations under any of their debt securities that we hold, or if any of the technology-related industry sectors experience a downturn.
We have invested and intend to continue investing in a limited number of technology-related companies and, we have recently seen an increase in the number of investments representing approximately 5% or more of our net asset value. A consequence of this limited number of investments is that the aggregate returns we realize may be significantly adversely affected if a small number of investments perform poorly or if we need to write down the value of any one investment. Beyond the asset diversification requirements to which we are subject as a business development company and a RIC, we do not have fixed guidelines for diversification or limitations on the size of our investments in any one portfolio company and our investments could be concentrated in relatively few issuers. In addition, we have invested in and intend to continue investing, under normal circumstances, at least 80% of the value of our total assets (including the amount of any borrowings for investment purposes) in technology-related companies.
As of June 30, 2017, approximately 76.1% of the fair value of our portfolio was composed of investments in five industries: 31.5% investments in the drug discovery & development industry, 18.2% investments in the software industry, 10.4% investments in the media/content/info industry, 8.8% investments in the drug delivery industry, and 7.2% investments in the internet consumer & business services industry.
As a result, a downturn in technology-related industry sectors and particularly those in which we are heavily concentrated could materially adversely affect our financial condition.
We are a non-diversified investment company within the meaning of the 1940 Act, and therefore we generally are not limited with respect to the proportion of our assets that may be invested in securities of a single issuer.
We are classified as a non-diversified investment company within the meaning of the 1940 Act, which means that we are not limited by the 1940 Act with respect to the proportion of our assets that we may invest in securities of a single issuer, excluding limitations on investments in other investment companies. To the extent that we assume large positions in the securities of a small number of issuers, our NAV may fluctuate to a greater extent than that of a diversified investment company as a result of changes in the financial condition or the market’s assessment of the issuer. We may also be more susceptible to any single economic or regulatory occurrence than a diversified investment company. Beyond the asset diversification requirements to which we are subject as a business development company and a RIC, we do not have fixed guidelines for portfolio diversification, and our investments could be concentrated in relatively few portfolio companies or industries.
36
Our financial results could be negatively affected if a significant portfolio investment fails to perform as expected.
Our total investment in companies may be significant individually or in the aggregate. As a result, if a significant investment in one or more companies fails to perform as expected, our financial results could be more negatively affected and the magnitude of the loss could be more significant than if we had made smaller investments in more companies. The following table shows the fair value of the totals of investments held in portfolio companies at June 30, 2017 that represent greater than 5% of our net assets:
| June 30, 2017 | ||||||||
| (in thousands) |
Fair Value | Percentage of Net Assets |
||||||
| Machine Zone, Inc. |
$ | 108,996 | 13.3 | % | ||||
| Axovant Sciences Ltd. |
56,570 | 6.9 | % | |||||
| Insmed, Incorporated |
56,296 | 6.9 | % | |||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.) |
51,998 | 6.4 | % | |||||
| Fuze, Inc. |
49,990 | 6.1 | % | |||||
| Proterra, Inc. |
42,130 | 5.2 | % | |||||
Machine Zone, Inc. is a technology company that is best known for building mobile Massively Multiplayer Online games with a focus on community-based gameplay.
Axovant Sciences Ltd. is a clinical-stage biopharmaceutical company focused on acquiring, developing and commercializing novel therapeutics for the treatment of dementia.
Insmed, Incorporated is a biopharmaceutical company that focuses on the development of inhaled pharmaceuticals for the site-specific treatment of serious lung diseases.
Paratek Pharmaceuticals, Inc. is a biopharmaceutical company focused on the development and commercialization of innovative therapies based upon its expertise in novel tetracycline chemistry.
Fuze, Inc. is a technology company that provides a cloud-based unified communications-as-a-service platform to server message block, mid-market, and small enterprise customers worldwide.
Proterra, Inc. designs and manufactures zero-emission vehicles that enable bus fleet operators to eliminate the dependency on fossil fuels and significantly reduce operating costs.
Our financial results could be materially adversely affected if these portfolio companies or any of our other significant portfolio companies encounter financial difficulty and fail to repay their obligations or to perform as expected.
Our investments may be in portfolio companies that have limited operating histories and resources.
We expect that our portfolio will continue to consist of investments that may have relatively limited operating histories. These companies may be particularly vulnerable to U.S. and foreign economic downturns may have more limited access to capital and higher funding costs, may have a weaker financial position and may need more capital to expand or compete. These businesses also may experience substantial variations in operating results. They may face intense competition, including from larger, more established companies with greater financial, technical and marketing resources. Furthermore, some of these companies do business in regulated industries and could be affected by changes in government regulation applicable to their given industry. Accordingly, these factors could impair their cash flow or result in other events, such as bankruptcy, which could limit their ability to repay their obligations to us, and may adversely affect the return on, or the recovery of, our investment in these companies. We cannot assure you that any of our investments in our portfolio companies will be successful. We may lose our entire investment in any or all of our portfolio companies.
37
Investing in publicly traded companies can involve a high degree of risk and can be speculative.
We have invested, and expect to continue to invest, a portion of our portfolio in publicly traded companies or companies that are in the process of completing their initial public offering (“IPO”). As publicly traded companies, the securities of these companies may not trade at high volumes, and prices can be volatile, particularly during times of general market volatility, which may restrict our ability to sell our positions and may have a material adverse impact on us.
Our ability to invest in public companies may be limited in certain circumstances.
To maintain our status as a business development company, we are not permitted to acquire any assets other than “qualifying assets” specified in the 1940 Act unless, at the time the acquisition is made, at least 70% of our total assets are qualifying assets (with certain limited exceptions). Subject to certain exceptions for follow-on investments and distressed companies, an investment in an issuer that has outstanding securities listed on a national securities exchange may be treated as a qualifying asset only if such issuer has a market capitalization that is less than $250 million at the time of such investment and meets the other specified requirements.
Our investment strategy focuses on technology-related companies, which are subject to many risks, including volatility, intense competition, shortened product life cycles, changes in regulatory and governmental programs and periodic downturns, and you could lose all or part of your investment.
We have invested and will continue investing primarily in technology-related companies, many of which may have narrow product lines and small market shares, which tend to render them more vulnerable to competitors’ actions and market conditions, as well as to general economic downturns. The revenues, income (or losses), and valuations of technology-related companies can and often do fluctuate suddenly and dramatically. In addition, technology-related industries are generally characterized by abrupt business cycles and intense competition. Overcapacity in technology-related industries, together with cyclical economic downturns, may result in substantial decreases in the market capitalization of many technology-related companies. Such decreases in market capitalization may occur again, and any future decreases in technology-related company valuations may be substantial and may not be temporary in nature. Therefore, our portfolio companies may face considerably more risk of loss than do companies in other industry sectors.
Because of rapid technological change, the average selling prices of products and some services provided by technology-related companies have historically decreased over their productive lives. As a result, the average selling prices of products and services offered by technology-related companies may decrease over time, which could adversely affect their operating results, their ability to meet obligations under their debt securities and the value of their equity securities. This could, in turn, materially adversely affect our business, financial condition and results of operations.
Our investments in sustainable and renewable technology companies are subject to substantial operational risks, such as underestimated cost projections, unanticipated operation and maintenance expenses, loss of government subsidies, and inability to deliver cost-effective alternative energy solutions compared to traditional energy products. In addition, sustainable and renewable technology companies employ a variety of means of increasing cash flow, including increasing utilization of existing facilities, expanding operations through new construction or acquisitions, or securing additional long-term contracts. Thus, some energy companies may be subject to construction risk, acquisition risk or other risks arising from their specific business strategies. Furthermore, production levels for solar, wind and other renewable energies may be dependent upon adequate sunlight, wind, or biogas production, which can vary from market to market and period to period, resulting in volatility in production levels and profitability. Demand for sustainable and renewable technology is also influenced by the available supply and prices for other energy products, such as coal, oil and natural gases. A change in prices in these energy products could reduce demand for alternative energy.
A natural disaster may also impact the operations of our portfolio companies, including our technology-related portfolio companies. The nature and level of natural disasters cannot be predicted and may be exacerbated
38
by global climate change. A portion of our technology-related portfolio companies rely on items assembled or produced in areas susceptible to natural disasters, and may sell finished goods into markets susceptible to natural disasters. A major disaster, such as an earthquake, tsunami, flood or other catastrophic event could result in disruption to the business and operations of our technology-related portfolio companies.
We will invest in technology-related companies that are reliant on U.S. and foreign regulatory and governmental programs. Any material changes or discontinuation, due to change in administration or U.S. Congress or otherwise could have a material adverse effect on the operations of a portfolio company in these industries and, in turn, impair our ability to timely collect principal and interest payments owed to us to the extent applicable.
We have invested in and may continue investing in technology-related companies that do not have venture capital or private equity firms as equity investors, and these companies may entail a higher risk of loss than do companies with institutional equity investors, which could increase the risk of loss of your investment.
Our portfolio companies will often require substantial additional equity financing to satisfy their continuing working capital and other cash requirements and, in most instances, to service the interest and principal payments on our investment. Portfolio companies that do not have venture capital or private equity investors may be unable to raise any additional capital to satisfy their obligations or to raise sufficient additional capital to reach the next stage of development. Portfolio companies that do not have venture capital or private equity investors may be less financially sophisticated and may not have access to independent members to serve on their boards, which means that they may be less successful than portfolio companies sponsored by venture capital or private equity firms. Accordingly, financing these types of companies may entail a higher risk of loss than would financing companies that are sponsored by venture capital or private equity firms.
Sustainable and renewable technology companies are subject to extensive government regulation and certain other risks particular to the sectors in which they operate and our business and growth strategy could be adversely affected if government regulations, priorities and resources impacting such sectors change or if our portfolio companies fail to comply with such regulations.
As part of our investment strategy, we plan to invest in portfolio companies in sustainable and renewable technology sectors that may be subject to extensive regulation by foreign, U.S. federal, state and/or local agencies. Changes in existing laws, rules or regulations, or judicial or administrative interpretations thereof, or new laws, rules or regulations could have an adverse impact on the business and industries of our portfolio companies. In addition, changes in government priorities or limitations on government resources could also adversely impact our portfolio companies. We are unable to predict whether any such changes in laws, rules or regulations will occur and, if they do occur, the impact of these changes on our portfolio companies and our investment returns. Furthermore, if any of our portfolio companies fail to comply with applicable regulations, they could be subject to significant penalties and claims that could materially and adversely affect their operations, which would also impact our ability to realize value since our exit from the investment may be subject to the portfolio company obtaining the necessary regulatory approvals. Our portfolio companies may be subject to the expense, delay and uncertainty of the regulatory approval process for their products and, even if approved, these products may not be accepted in the marketplace.
In addition, there is considerable uncertainty about whether foreign, U.S., state and/or local governmental entities will enact or maintain legislation or regulatory programs that mandate reductions in greenhouse gas emissions or provide incentives for sustainable and renewable technology companies. Without such regulatory policies, investments in sustainable and renewable technology companies may not be economical and financing for sustainable and renewable technology companies may become unavailable, which could materially adversely affect the ability of our portfolio companies to repay the debt they owe to us. Any of these factors could materially and adversely affect the operations and financial condition of a portfolio company and, in turn, the ability of the portfolio company to repay the debt they owe to us.
39
Cyclicality within the energy sector may adversely affect some of our portfolio companies.
Industries within the energy sector are cyclical with fluctuations in commodity prices and demand for, and production of commodities driven by a variety of factors. The highly cyclical nature of the industries within the energy sector may lead to volatile changes in commodity prices, which may adversely affect the earnings of energy companies in which we may invest and the performance and valuation of our portfolio.
Continuation of the decline in oil and natural gas prices for a prolonged period of time could have a material adverse effect on us.
A prolonged continuation of the decline in oil and natural gas prices would adversely affect (i) the credit quality of our debt investments in certain of our portfolio companies and (ii) the underlying operating performance of our portfolio companies’ business that are heavily dependent upon the prices of, and demand for, oil and natural gas. A decrease in credit quality and the operating performance would, in turn, negatively affect the fair value of these investments, which would consequently negatively affect our net asset value. Should the decline in oil and natural gas prices experienced over the last two years persist, it is likely that the ability of these portfolio companies to satisfy financial or operating covenants imposed by us or other lenders will be adversely affected, thereby negatively impacting their financial condition and their ability to satisfy their debt service and other obligations to us. Likewise, should the decline in oil and natural gas prices persist, it is likely that our energy-related portfolio companies’ and other affected companies’ cash flow and profit generating capacities would also be adversely affected thereby negatively impacting their ability to pay us dividends or distributions on our equity investments.
Our investments in the life sciences industry are subject to extensive government regulation, litigation risk and certain other risks particular to that industry.
We have invested and plan to continue investing in companies in the life sciences industry that are subject to extensive regulation by the Food and Drug Administration, or the FDA, and to a lesser extent, other federal, state and other foreign agencies. If any of these portfolio companies fail to comply with applicable regulations, they could be subject to significant penalties and claims that could materially and adversely affect their operations. Portfolio companies that produce medical devices or drugs are subject to the expense, delay and uncertainty of the regulatory approval process for their products and, even if approved, these products may not be accepted in the marketplace. In addition, governmental budgetary constraints effecting the regulatory approval process, new laws, regulations or judicial interpretations of existing laws and regulations might adversely affect a portfolio company in this industry. Portfolio companies in the life sciences industry may also have a limited number of suppliers of necessary components or a limited number of manufacturers for their products, and therefore face a risk of disruption to their manufacturing process if they are unable to find alternative suppliers when needed. Any of these factors could materially and adversely affect the operations of a portfolio company in this industry and, in turn, impair our ability to timely collect principal and interest payments owed to us.
Our investments in the drug discovery industry are subject to numerous risks, including competition, extensive government regulation, product liability and commercial difficulties.
Our investments in the drug discovery industry are subject to numerous risks. The successful and timely implementation of the business model of our drug discovery portfolio companies depends on their ability to adapt to changing technologies and introduce new products. As competitors continue to introduce competitive products, the development and acquisition of innovative products and technologies that improve efficacy, safety, patient’s and clinician’s ease of use and cost-effectiveness are important to the success of such portfolio companies. The success of new product offerings will depend on many factors, including the ability to properly anticipate and satisfy customer needs, obtain regulatory approvals on a timely basis, develop and manufacture products in an economic and timely manner, obtain or maintain advantageous positions with respect to intellectual property, and differentiate products from those of competitors. Failure by our portfolio companies to introduce planned products or other new products or to introduce products on schedule could have a material adverse effect on our business, financial condition and results of operations.
40
Further, the development of products by drug discovery companies requires significant research and development, clinical trials and regulatory approvals. The results of product development efforts may be affected by a number of factors, including the ability to innovate, develop and manufacture new products, complete clinical trials, obtain regulatory approvals and reimbursement in the U.S. and abroad, or gain and maintain market approval of products. In addition, regulatory review processes by U.S. and foreign agencies may extend longer than anticipated as a result of decreased funding and tighter fiscal budgets. Further, patents attained by others can preclude or delay the commercialization of a product. There can be no assurance that any products now in development will achieve technological feasibility, obtain regulatory approval, or gain market acceptance. Failure can occur at any point in the development process, including after significant funds have been invested. Products may fail to reach the market or may have only limited commercial success because of efficacy or safety concerns, failure to achieve positive clinical outcomes, inability to obtain necessary regulatory approvals, failure to achieve market adoption, limited scope of approved uses, excessive costs to manufacture, the failure to establish or maintain intellectual property rights, or the infringement of intellectual property rights of others.
Future legislation, and/or regulations and policies adopted by the FDA or other U.S. or foreign regulatory authorities may increase the time and cost required by some of our portfolio companies to conduct and complete clinical trials for the product candidates that they develop, and there is no assurance that these companies will obtain regulatory approval to market and commercialize their products in the U.S. and in foreign countries.
The FDA has established regulations, guidelines and policies to govern the drug development and approval process, as have foreign regulatory authorities, which affect some of our portfolio companies. Any change in regulatory requirements due to the adoption by the FDA and/or foreign regulatory authorities of new legislation, regulations, or policies may require some of our portfolio companies to amend existing clinical trial protocols or add new clinical trials to comply with these changes. Such amendments to existing protocols and/or clinical trial applications or the need for new ones, may significantly impact the cost, timing and completion of the clinical trials.
In addition, increased scrutiny by the U.S. Congress of the FDA’s and other authorities approval processes may significantly delay or prevent regulatory approval, as well as impose more stringent product labeling and post-marketing testing and other requirements. Foreign regulatory authorities may also increase their scrutiny of approval processes resulting in similar delays. Increased scrutiny and approvals processes may limit the ability of our portfolio companies to market and commercialize their products in the U.S. and in foreign countries.
Life sciences companies, including drug development companies, device manufacturers, service providers and others, are also subject to material pressures when there are changes in the outlook for healthcare insurance markets. The ability for individuals, along with private and public insurers, to account for the costs of paying for healthcare insurance can place strain on the ability of new technology, devices and services to enter those markets, particularly when they are new or untested. As a result, it is not uncommon for changes in the insurance market place to lead to a slower rate of adoption, price pressure and other forces that may materially limit the success of companies bringing such technologies to market. Changes in the health insurance sector might then have an impact on the value of companies in our portfolio or our ability to invest in the sector generally.
Changes in healthcare laws and other regulations, or the enforcement or interpretation of such laws or regulations, applicable to some of our portfolio companies’ businesses may constrain their ability to offer their products and services.
Changes in healthcare or other laws and regulations, or the enforcement or interpretation of such laws or regulations, applicable to the businesses of some of our portfolio companies may occur that could increase their compliance and other costs of doing business, require significant systems enhancements, or render their products or services less profitable or obsolete, any of which could have a material adverse effect on their results of operations. There has also been an increased political and regulatory focus on healthcare laws in recent years, and new legislation could have a material effect on the business and operations of some of our portfolio companies.
41
Additionally, because of the continued uncertainty surrounding the healthcare industry under the Trump Administration, including the potential for further legal challenges or repeal of existing legislation, we cannot quantify or predict with any certainty the likely impact on our portfolio companies, our business model, prospects, financial condition or results of operations. We also anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system. We cannot assure you as to the ultimate content, timing, or effect of changes, nor is it possible at this time to estimate the impact of any such potential legislation on certain of our portfolio companies, our business model, prospects, financial condition or results of operations.
Price declines and illiquidity in the corporate debt markets could adversely affect the fair value of our portfolio investments, reducing our NAV through increased net unrealized depreciation.
As a business development company, we are required to carry our investments at market value or, if no market value is ascertainable, at fair market value as determined in good faith by or under the direction of our Board of Directors. As part of the valuation process, we may take into account the following types of factors, if relevant, in determining the fair value of our investments: the enterprise value of a portfolio company (an estimate of the total fair value of the portfolio company’s debt and equity), the nature and realizable value of any collateral, the portfolio company’s ability to make payments and its earnings and discounted cash flow, the markets in which the portfolio company does business, a comparison of the portfolio company’s securities to similar publicly traded securities, changes in the interest rate environment and the credit markets generally that may affect the price at which similar investments may be made in the future and other relevant factors. When an external event such as a purchase transaction, public offering or subsequent equity sale occurs, we use the pricing indicated by the external event to corroborate our valuation. While most of our investments are not publicly traded, applicable accounting standards require us to assume as part of our valuation process that our investments are sold in a principal market to market participants (even if we plan on holding an investment through its maturity). As a result, volatility in the capital markets can also adversely affect our investment valuations. Decreases in the market values or fair values of our investments are recorded as unrealized depreciation. The effect of all of these factors on our portfolio can reduce our NAV by increasing net unrealized depreciation in our portfolio.
Depending on market conditions, we could incur substantial realized losses and may suffer substantial unrealized depreciation in future periods, which could have a material adverse impact on our business, financial condition and results of operations.
Economic recessions or slowdowns could impair the ability of our portfolio companies to repay loans, which, in turn, could increase our non-performing assets, decrease the value of our portfolio, reduce our volume of new loans and have a material adverse effect on our results of operations.
Many of our portfolio companies may be susceptible to economic slowdowns or recessions in both the U.S. and foreign countries, and may be unable to repay our loans during such periods. Therefore, during such periods, our non-performing assets are likely to increase and the value of our portfolio is likely to decrease. Adverse economic conditions also may decrease the value of collateral securing some of our loans and the value of our equity investments. Economic slowdowns or recessions could lead to financial losses in our portfolio and a decrease in revenues, net income and assets. Unfavorable economic conditions also could increase our funding costs, limit our access to the capital markets or result in a decision by lenders not to extend credit to us. These events could prevent us from increasing investments and harm our operating results.
In particular, intellectual property owned or controlled by our portfolio companies may constitute an important portion of the value of the collateral of our loans to our portfolio companies. Adverse economic conditions may decrease the demand for our portfolio companies’ intellectual property and consequently its value in the event of a bankruptcy or required sale through a foreclosure proceeding. As a result, our ability to fully recover the amounts owed to us under the terms of the loans may be impaired by such events.
42
A portfolio company’s failure to satisfy financial or operating covenants imposed by us or other lenders could lead to defaults and, potentially, termination of the portfolio company’s loans and foreclosure on its secured assets, which could trigger cross-defaults under other agreements and jeopardize the portfolio company’s ability to meet its obligations under the debt securities that we hold. We may incur expenses to the extent necessary to seek recovery upon default or to negotiate new terms with a defaulting portfolio company.
Our portfolio companies may be unable to repay or refinance outstanding principal on their loans at or prior to maturity, and rising interests rates may make it more difficult for portfolio companies to make periodic payments on their loans.
Our portfolio companies may be unable to repay or refinance outstanding principal on their loans at or prior to maturity. This risk and the risk of default is increased to the extent that the loan documents do not require the portfolio companies to pay down the outstanding principal of such debt prior to maturity. In addition, if general interest rates rise, there is a risk that our portfolio companies will be unable to pay escalating interest amounts, which could result in a default under their loan documents with us. Any failure of one or more portfolio companies to repay or refinance its debt at or prior to maturity or the inability of one or more portfolio companies to make ongoing payments following an increase in contractual interest rates could have a material adverse effect on our business, financial condition, results of operations and cash flows.
The disposition of our investments may result in contingent liabilities.
We currently expect that a portion of our investments will involve private securities. In connection with the disposition of an investment in private securities, we may be required to make representations about the business and financial affairs of the portfolio company typical of those made in connection with the sale of a business. We may also be required to indemnify the purchasers of such investment to the extent that any such representations turn out to be inaccurate or with respect to certain potential liabilities. These arrangements may result in contingent liabilities that ultimately yield funding obligations that must be satisfied through our return of certain distributions previously made to us.
The health and performance of our portfolio companies could be adversely affected by political and economic conditions in the countries in which they conduct business.
Some of the products of our portfolio companies are developed, manufactured, assembled, tested or marketed outside the U.S. Any conflict or uncertainty in these countries, including due to natural disasters, public health concerns, political unrest or safety concerns, among other things, could harm their business, financial condition and results of operations. In addition, if the government of any country in which their products are developed, manufactured or sold sets technical or regulatory standards for products developed or manufactured in or imported into their country that are not widely shared, it may lead some of their customers to suspend imports of their products into that country, require manufacturers or developers in that country to manufacture or develop products with different technical or regulatory standards and disrupt cross-border manufacturing, marketing or business relationships which, in each case, could harm their businesses.
Any unrealized depreciation we experience on our investment portfolio may be an indication of future realized losses, which could reduce our income available for distribution and could impair our ability to service our borrowings.
As a business development company, we are required to carry our investments at market value or, if no market value is ascertainable, at fair value as determined in good faith by our Board of Directors. Decreases in the market values or fair values of our investments will be recorded as unrealized depreciation. Any unrealized depreciation in our investment portfolio could be an indication of a portfolio company’s inability to meet its repayment obligations to us with respect to the affected investments. This could result in realized losses in the future and ultimately in reductions of our income available for distribution in future periods and could materially adversely affect our ability to service our outstanding borrowings.
43
A lack of IPO or merger and acquisition opportunities may cause companies to stay in our portfolio longer, leading to lower returns, unrealized depreciation, or realized losses.
A lack of IPO or merger and acquisition (“M&A”) opportunities for venture capital-backed companies could lead to companies staying longer in our portfolio as private entities still requiring funding. This situation may adversely affect the amount of available funding for early-stage companies in particular as, in general, venture-capital firms are being forced to provide additional financing to late-stage companies that cannot complete an IPO or M&A transaction. In the best case, such stagnation would dampen returns, and in the worst case, could lead to unrealized depreciation and realized losses as some companies run short of cash and have to accept lower valuations in private fundings or are not able to access additional capital at all. A lack of IPO or M&A opportunities for venture capital-backed companies can also cause some venture capital firms to change their strategies, leading some of them to reduce funding of their portfolio companies and making it more difficult for such companies to access capital and to fulfill their potential, which can result in unrealized depreciation and realized losses in such companies by other companies such as ourselves who are co-investors in such companies.
The majority of our portfolio companies will need multiple rounds of additional financing to repay their debts to us and continue operations. Our portfolio companies may not be able to raise additional financing, which could harm our investment returns.
The majority of our portfolio companies will often require substantial additional equity financing to satisfy their continuing working capital and other cash requirements and, in most instances, to service the interest and principal payments on our investment. Each round of venture financing is typically intended to provide a company with only enough capital to reach the next stage of development. We cannot predict the circumstances or market conditions under which our portfolio companies will seek additional capital. It is possible that one or more of our portfolio companies will not be able to raise additional financing or may be able to do so only at a price or on terms unfavorable to us, either of which would negatively impact our investment returns. Some of these companies may be unable to obtain sufficient financing from private investors, public capital markets or traditional lenders. This may have a significant impact if the companies are unable to obtain certain federal, state or foreign agency approval for their products or the marketing thereof, of if regulatory review processes extend longer than anticipated, and the companies need continued funding for their operations during these times. Accordingly, financing these types of companies may entail a higher risk of loss than would financing companies that are able to utilize traditional credit sources.
If the assets securing the loans that we make decrease in value, then we may lack sufficient collateral to cover losses.
To attempt to mitigate credit risks, we will typically take a security interest in the available assets of our portfolio companies. There is no assurance that we will obtain or properly perfect our liens.
There is a risk that the collateral securing our loans may decrease in value over time, may be difficult to sell in a timely manner, may be difficult to appraise and may fluctuate in value based upon the success of the business and market conditions, including as a result of the inability of a portfolio company to raise additional capital. In some circumstances, our lien could be subordinated to claims of other creditors. Consequently, the fact that a loan is secured does not guarantee that we will receive principal and interest payments according to the loan’s terms, or that we will be able to collect on the loan should we be forced to enforce our remedies.
In addition, because we invest in technology-related companies, a substantial portion of the assets securing our investment may be in the form of intellectual property, if any, inventory and equipment and, to a lesser extent, cash and accounts receivable. Intellectual property, if any, that is securing our loan could lose value if, among other things, the company’s rights to the intellectual property are challenged or if the company’s license to the intellectual property is revoked or expires, the technology fails to achieve its intended results or a new technology makes the intellectual property functionally obsolete. Inventory may not be adequate to secure our loan if our valuation of the inventory at the time that we made the loan was not accurate or if there is a reduction in the demand for the inventory.
44
Similarly, any equipment securing our loan may not provide us with the anticipated security if there are changes in technology or advances in new equipment that render the particular equipment obsolete or of limited value, or if the company fails to adequately maintain or repair the equipment. Any one or more of the preceding factors could materially impair our ability to recover earned interest and principal in a foreclosure.
At June 30, 2017, approximately 40.2% of our portfolio company debt investments were secured by a first priority security in all of the assets of the portfolio company, including their intellectual property, 45.7% of the debt investments were to portfolio companies that were prohibited from pledging or encumbering their intellectual property, or subject to a negative pledge and, 14.1% of the our portfolio company debt investments were secured by a second priority security interest in all of the portfolio company’s assets, other than intellectual property. At June 30, 2017 we had no equipment only liens on any of our portfolio companies.
We may suffer a loss if a portfolio company defaults on a loan and the underlying collateral is not sufficient.
In the event of a default by a portfolio company on a secured loan, we will only have recourse to the assets collateralizing the loan. If the underlying collateral value is less than the loan amount, we will suffer a loss. In addition, we sometimes make loans that are unsecured, which are subject to the risk that other lenders may be directly secured by the assets of the portfolio company. In the event of a default, those collateralized lenders would have priority over us with respect to the proceeds of a sale of the underlying assets. In cases described above, we may lack control over the underlying asset collateralizing our loan or the underlying assets of the portfolio company prior to a default, and as a result the value of the collateral may be reduced by acts or omissions by owners or managers of the assets.
In the event of bankruptcy of a portfolio company, we may not have full recourse to its assets in order to satisfy our loan, or our loan may be subject to “equitable subordination.” This means that depending on the facts and circumstances, including the extent to which we actually provided significant “managerial assistance,” if any, to that portfolio company, a bankruptcy court might re-characterize our debt holding and subordinate all or a portion of our claim to that of other creditors. In addition, certain of our loans are subordinate to other debt of the portfolio company. If a portfolio company defaults on our loan or on debt senior to our loan, or in the event of a portfolio company bankruptcy, our loan will be satisfied only after the senior debt receives payment. Where debt senior to our loan exists, the presence of intercreditor arrangements may limit our ability to amend our loan documents, assign our loans, accept prepayments, exercise our remedies (through “standstill” periods) and control decisions made in bankruptcy proceedings relating to the portfolio company. Bankruptcy and portfolio company litigation can significantly increase collection losses and the time needed for us to acquire the underlying collateral in the event of a default, during which time the collateral may decline in value, causing us to suffer losses.
If the value of collateral underlying our loan declines or interest rates increase during the term of our loan, a portfolio company may not be able to obtain the necessary funds to repay our loan at maturity through refinancing. Decreasing collateral value and/or increasing interest rates may hinder a portfolio company’s ability to refinance our loan because the underlying collateral cannot satisfy the debt service coverage requirements necessary to obtain new financing. If a borrower is unable to repay our loan at maturity, we could suffer a loss which may adversely impact our financial performance.
The inability of our portfolio companies to commercialize their technologies or create or develop commercially viable products or businesses would have a negative impact on our investment returns.
The possibility that our portfolio companies will not be able to commercialize their technology, products or business concepts presents significant risks to the value of our investment. Additionally, although some of our portfolio companies may already have a commercially successful product or product line when we invest, technology-related products and services often have a more limited market- or life-span than have products in
45
other industries. Thus, the ultimate success of these companies often depends on their ability to continually innovate, or raise additional capital, in increasingly competitive markets. Their inability to do so could affect our investment return. In addition, the intellectual property held by our portfolio companies often represents a substantial portion of the collateral, if any, securing our investments. We cannot assure you that any of our portfolio companies will successfully acquire or develop any new technologies, or that the intellectual property the companies currently hold will remain viable. Even if our portfolio companies are able to develop commercially viable products, the market for new products and services is highly competitive and rapidly changing. Neither our portfolio companies nor we have any control over the pace of technology development. Commercial success is difficult to predict, and the marketing efforts of our portfolio companies may not be successful.
An investment strategy focused on privately-held companies presents certain challenges, including the lack of available information about these companies, a dependence on the talents and efforts of only a few key portfolio company personnel and a greater vulnerability to economic downturns.
We invest primarily in privately-held companies. Generally, very little public information exists about these companies, and we are required to rely on the ability of our management and investment teams to obtain adequate information to evaluate the potential returns from investing in these companies. Such small, privately held companies as we routinely invest in may also lack quality infrastructures, thus leading to poor disclosure standards or control environments. If we are unable to uncover all material information about these companies, then we may not make a fully informed investment decision, and we may not receive the expected return on our investment or lose some or all of the money invested in these companies.
Also, privately-held companies frequently have less diverse product lines and a smaller market presence than do larger competitors. Privately-held companies are, thus, generally more vulnerable to economic downturns and may experience more substantial variations in operating results than do larger competitors. These factors could affect our investment returns and our results of operations and financial condition.
In addition, our success depends, in large part, upon the abilities of the key management personnel of our portfolio companies, who are responsible for the day-to-day operations of our portfolio companies. Competition for qualified personnel is intense at any stage of a company’s development, and high turnover of personnel is common in technology-related companies. The loss of one or more key managers can hinder or delay a company’s implementation of its business plan and harm its financial condition. Our portfolio companies may not be able to attract and retain qualified managers and personnel. Any inability to do so may negatively impact our investment returns and our results of operations and financial condition.
If our portfolio companies are unable to protect their intellectual property rights, or are required to devote significant resources to protecting their intellectual property rights, then our investments could be harmed.
Our future success and competitive position depend in part upon the ability of our portfolio companies to obtain and maintain proprietary technology used in their products and services, which will often represent a significant portion of the collateral, if any, securing our investment. The portfolio companies will rely, in part, on patent, trade secret and trademark law to protect that technology, but competitors may misappropriate their intellectual property, and disputes as to ownership of intellectual property may arise. Portfolio companies may, from time to time, be required to institute litigation in order to enforce their patents, copyrights or other intellectual property rights, to protect their trade secrets, to determine the validity and scope of the proprietary rights of others or to defend against claims of infringement. Such litigation could result in substantial costs and diversion of resources. Similarly, if a portfolio company is found to infringe upon or misappropriate a third party’s patent or other proprietary rights, that portfolio company could be required to pay damages to such third party, alter its own products or processes, obtain a license from the third party and/or cease activities utilizing such proprietary rights, including making or selling products utilizing such proprietary rights. Any of the foregoing events could negatively affect both the portfolio company’s ability to service our debt investment and the value of any related debt and equity securities that we own, as well as any collateral securing our investment.
46
We generally will not control our portfolio companies.
In some instances, we may control our portfolio companies or provide our portfolio companies with significant managerial assistance. However, we generally do not, and do not expect to, control the decision making in many of our portfolio companies, even though we may have board representation or board observation rights, and our debt agreements may contain certain restrictive covenants. As a result, we are subject to the risk that a portfolio company in which we invest will make business decisions with which we disagree and the management of such company, as representatives of the holders of their common equity, will take risks or otherwise act in ways that do not serve our interests as debt investors. Due to the lack of liquidity for our investments in non-traded companies, we may not be able to dispose of our interests in our portfolio companies as readily as we would like or at an appropriate valuation. As a result, a portfolio company may make decisions that would decrease the value of our portfolio holdings.
Our financial condition, results of operations and cash flows could be negatively affected if we are unable to recover our principal investment as a result of a negative pledge or lack of a security interest on the intellectual property of our venture growth stage companies.
In some cases, we collateralize our loans with a secured collateral position in a portfolio company’s assets, which may include a negative pledge or, to a lesser extent, no security on their intellectual property. In the event of a default on a loan, the intellectual property of the portfolio company will most likely be liquidated to provide proceeds to pay the creditors of the company. There can be no assurance that our security interest, if any, in the proceeds of the intellectual property will be enforceable in a court of law or bankruptcy court or that there will not be others with senior or pari passu credit interests.
Our relationship with certain portfolio companies may expose us to our portfolio companies’ trade secrets and confidential information which may require us to be parties to non-disclosure agreements and restrict us from engaging in certain transactions.
Our relationship with some of our portfolio companies may expose us to our portfolio companies’ trade secrets and confidential information (including transactional data and personal data about their employees and clients) which may require us to be parties to non-disclosure agreements and restrict us from engaging in certain transactions. Unauthorized access or disclosure of such information may occur, resulting in theft, loss or other misappropriation. Any theft, loss, improper use, such as insider trading or other misappropriation of confidential information could have a material adverse impact on our competitive positions, our relationship with our portfolio companies and our reputation and could subject us to regulatory inquiries, enforcement and fines, civil litigation (which may cause us to incur significant expense or expose us to losses) and possible financial liability or costs.
Portfolio company litigation could result in additional costs, the diversion of management time and resources and have an adverse impact on the fair value of our investment.
To the extent that litigation arises with respect to any of our portfolio companies, we may be named as a defendant, which could result in additional costs and the diversion of management time and resources. Furthermore, if we are providing managerial assistance to the portfolio company or have representatives on the portfolio company’s board of directors, our costs and diversion of our management’s time and resources in assessing the portfolio company could be substantial in light of any such litigation regardless of whether we are named as a defendant. In addition, litigation involving a portfolio company may be costly and affect the operations of the portfolio company’s business, which could in turn have an adverse impact on the fair value of our investment in such company.
We may not be able to realize our entire investment on equipment-based loans, if any, in the case of default.
We may from time-to-time provide loans that will be collateralized only by equipment of the portfolio company. If the portfolio company defaults on the loan we would take possession of the underlying equipment to
47
satisfy the outstanding debt. The residual value of the equipment at the time we would take possession may not be sufficient to satisfy the outstanding debt and we could experience a loss on the disposition of the equipment. At June 30, 2017, we had no equipment-based loans.
Our investments in foreign securities may involve significant risks in addition to the risks inherent in U.S. investments.
Our investment strategy contemplates that a portion of our investments may be in securities of foreign companies. Our total investments at value in foreign companies were approximately $126.0 million or 8.9% of total investments at June 30, 2017. Investing in foreign companies may expose us to additional risks not typically associated with investing in U.S. companies. These risks include changes in exchange control regulations, political and social instability, expropriation, imposition of foreign taxes, less liquid markets and less available information than is generally the case in the U.S., higher transaction costs, less government supervision of exchanges, brokers and issuers, less developed bankruptcy laws, difficulty in enforcing contractual obligations, lack of uniform accounting and auditing standards and greater price volatility, among other things.
If our investments do not meet our performance expectations, you may not receive distributions.
We intend to make distributions on a quarterly basis to our stockholders. We may not be able to achieve operating results that will allow us to make distributions at a specific level or to increase the amount of these distributions from time to time. In addition, due to the asset coverage test applicable to us as a business development company, we may be limited in our ability to make distributions. Also, restrictions and provisions in any future credit facilities may limit our ability to make distributions. As a RIC, if we do not distribute a certain percentage of our income each taxable year, we will suffer adverse tax consequences, including failure to obtain, or possible loss of, the U.S. federal income tax benefits allowable to RICs. We cannot assure you that you will receive distributions at a particular level or at all.
We may not have sufficient funds to make follow-on investments. Our decision not to make a follow-on investment may have a negative impact on a portfolio company in need of such an investment or may result in a missed opportunity for us.
After our initial investment in a portfolio company, we may be called upon from time to time to provide additional funds to such company or have the opportunity or need to increase our investment in a successful situation or attempt to preserve or enhance the value of our initial investment, for example, the exercise of a warrant to purchase common stock, or a negative situation, to protect an existing investment. We have the discretion to make any follow-on investments, subject to the availability of capital resources and regulatory considerations. We may elect not to make follow-on investments or otherwise lack sufficient funds to make those investments. Any decision we make not to make a follow-on investment or any inability on our part to make such an investment may have a negative impact on a portfolio company in need of such an investment or may result in a missed opportunity for us to increase our participation in a successful operation and may dilute our equity interest or otherwise reduce the expected yield on our investment. Moreover, a follow-on investment may limit the number of companies in which we can make initial investments. In determining whether to make a follow-on investment, our management will exercise its business judgment and apply criteria similar to those used when making the initial investment. There is no assurance that we will make, or will have sufficient funds to make, follow-on investments and this could adversely affect our success and result in the loss of a substantial portion or all of our investment in a portfolio company.
The lack of liquidity in our investments may adversely affect our business and, if we need to sell any of our investments, we may not be able to do so at a favorable price. As a result, we may suffer losses.
We generally invest in debt securities with terms of up to seven years and hold such investments until maturity, and we do not expect that our related holdings of equity securities will provide us with liquidity
48
opportunities in the near-term. We invest and expect to continue investing in companies whose securities have no established trading market and whose securities are and will be subject to legal and other restrictions on resale or whose securities are and will be less liquid than are publicly-traded securities. The illiquidity of these investments may make it difficult for us to sell these investments when desired. In addition, if we are required to liquidate all or a portion of our portfolio quickly, we may realize significantly less than the value at which we had previously recorded these investments. As a result, we do not expect to achieve liquidity in our investments in the near-term. However, to maintain our qualification as a business development company and as a RIC, we may have to dispose of investments if we do not satisfy one or more of the applicable criteria under the respective regulatory frameworks.
Our portfolio companies may incur debt or issue equity securities that rank equally with, or senior to, our investments in such companies.
We invest primarily in debt securities issued by our portfolio companies. In some cases, portfolio companies will be permitted to incur other debt, or issue other equity securities, that rank equally with, or senior to, our investment. Such instruments may provide that the holders thereof are entitled to receive payment of distributions, interest or principal on or before the dates on which we are entitled to receive payments in respect of our investments. These debt instruments would usually prohibit the portfolio companies from paying interest on or repaying our investments in the event and during the continuance of a default under such debt. Also, in the event of insolvency, liquidation, dissolution, reorganization or bankruptcy of a portfolio company, holders of securities ranking senior to our investment in that portfolio company would typically be entitled to receive payment in full before we receive any distribution in respect of our investment. After repaying such holders, the portfolio company might not have any remaining assets to use for repaying its obligation to us. In the case of securities ranking equally with our investments, we would have to share on a pari passu basis any distributions with other security holders in the event of an insolvency, liquidation, dissolution, reorganization or bankruptcy of the relevant portfolio company.
The rights we may have with respect to the collateral securing any junior priority loans we make to our portfolio companies may also be limited pursuant to the terms of one or more intercreditor agreements that we enter into with the holders of senior debt. Under such an intercreditor agreement, at any time that senior obligations are outstanding, we may forfeit certain rights with respect to the collateral to the holders of the senior obligations. These rights may include the right to commence enforcement proceedings against the collateral, the right to control the conduct of such enforcement proceedings, the right to approve amendments to collateral documents, the right to release liens on the collateral and the right to waive past defaults under collateral documents. We may not have the ability to control or direct such actions, even if as a result our rights as junior lenders are adversely affected.
Our warrant and equity-related investments are highly speculative, and we may not realize gains from these investments. If our warrant and equity-related investments do not generate gains, then the return on our invested capital will be lower than it would otherwise be, which could result in a decline in the value of shares of our common stock.
When we invest in debt securities, we generally expect to acquire warrants or other equity-related securities as well. Our goal is ultimately to dispose of these equity interests and realize gains upon disposition of such interests. Over time, the gains that we realize on these equity interests may offset, to some extent, losses that we experience on defaults under debt securities that we hold. However, the equity interests that we receive may not appreciate in value and, in fact, may decline in value. Accordingly, we may not be able to realize gains from our equity interests, and any gains that we do realize on the disposition of any equity interests may not be sufficient to offset any other losses that we experience. In addition, we anticipate that approximately 50% of our warrants may not realize and exit or generate any returns. Furthermore, because of the GAAP requirements, of those approximately 50% of warrants that do not realize and exit, the assigned costs to the initial warrants may lead to realized write-offs when the warrants either expire or are not exercised.
49
Prepayments of our debt investments by our portfolio companies could adversely impact our results of operations and reduce our return on equity.
During the six-months ended June 30, 2017, we received debt investment early principal repayments and pay down of working capital debt investments of approximately $338.8 million. We are subject to the risk that the investments we make in our portfolio companies may be repaid prior to maturity. When this occurs, we will generally reinvest these proceeds in temporary investments, pending their future investment in new portfolio companies. These temporary investments will typically have substantially lower yields than the debt being prepaid and we could experience significant delays in reinvesting these amounts. Any future investment in a new portfolio company may also be at lower yields than the debt that was repaid. As a result, our results of operations could be materially adversely affected if one or more of our portfolio companies elect to prepay amounts owed to us. Additionally, prepayments could negatively impact our return on equity, which could result in a decline in the market price of our common stock.
We may choose to waive or defer enforcement of covenants in the debt securities held in our portfolio, which may cause us to lose all or part of our investment in these companies.
We structure the debt investments in our portfolio companies to include business and financial covenants placing affirmative and negative obligations on the operation of the company’s business and its financial condition. However, from time to time we may elect to waive breaches of these covenants, including our right to payment, or waive or defer enforcement of remedies, such as acceleration of obligations or foreclosure on collateral, depending upon the financial condition and prospects of the particular portfolio company. These actions may reduce the likelihood of receiving the full amount of future payments of interest or principal and be accompanied by a deterioration in the value of the underlying collateral as many of these companies may have limited financial resources, may be unable to meet future obligations and may go bankrupt. This could negatively impact our ability to pay distributions, could adversely affect our results of operation and financial condition and cause the loss of all or part of your investment.
We may also be subject to lender liability claims for actions taken by us with respect to a borrower’s business or instances where we exercise control over the borrower. It is possible that we could become subject to a lender’s liability claim, including as a result of actions taken in rendering significant managerial assistance or actions to compel and collect payments from the borrower outside the ordinary course of business.
Our loans could be subject to equitable subordination by a court which would increase our risk of loss with respect to such loans or we could be subject to lender liability claims.
Courts may apply the doctrine of equitable subordination to subordinate the claim or lien of a lender against a borrower to claims or liens of other creditors of the borrower, when the lender or its affiliates is found to have engaged in unfair, inequitable or fraudulent conduct. The courts have also applied the doctrine of equitable subordination when a lender or its affiliates is found to have exerted inappropriate control over a client, including control resulting from the ownership of equity interests in a client or providing of significant managerial assistance. We have made direct equity investments or received warrants in connection with loans. These investments represent approximately 7.7% of the outstanding value of our investment portfolio as of June 30, 2017. Payments on one or more of our loans, particularly a loan to a client in which we also hold an equity interest, may be subject to claims of equitable subordination. If we were deemed to have the ability to control or otherwise exercise influence over the business and affairs of one or more of our portfolio companies resulting in economic hardship to other creditors of that company, this control or influence may constitute grounds for equitable subordination and a court may treat one or more of our loans as if it were unsecured or common equity in the portfolio company. In that case, if the portfolio company were to liquidate, we would be entitled to repayment of our loan on a pro-rata basis with other unsecured debt or, if the effect of subordination was to place us at the level of common equity, then on an equal basis with other holders of the portfolio company’s common equity only after all of its obligations relating to its debt and preferred securities had been satisfied.
50
In addition to these risks, in the event we elect to convert our debt position to equity, or otherwise take control of a portfolio company (such as through placing a member of our management team on its board of directors), as part of a restructuring, we face additional risks acting in that capacity. It is not uncommon for unsecured, or otherwise unsatisfied creditors, to sue parties that elect to use their debt positions to later control a company following a restructuring or bankruptcy. Apart from lawsuits, key customers and suppliers might act in a fashion contrary to the interests of a portfolio company if they were left unsatisfied in a restructuring or bankruptcy. Any combination of these factors might lead to the loss in value of a company subject to such activity and may divert the time and attention of our management team and investment team to help to address such issues in a portfolio company.
The potential inability of our portfolio companies’ in the healthcare industry to charge desired prices with respect to prescription drugs could impact their revenues and in turn their ability to repay us.
Some of our portfolio companies in the healthcare industry are subject to risks associated with the pricing for prescription drugs. It is uncertain whether customers of our healthcare industry portfolio companies will continue to utilize established prescription drug pricing methods, or whether other pricing benchmarks will be adopted for establishing prices within the industry. Legislation may lead to changes in the pricing for Medicare and Medicaid programs. Regulators have conducted investigations into the use of prescription drug pricing methods for federal program payment, and whether such methods have inflated drug expenditures by the Medicare and Medicaid programs. Federal and state proposals have sought to change the basis for calculating payment of certain drugs by the Medicare and Medicaid programs. Additionally, President Trump has taken actions and made statements that suggest he plans to seek repeal of all or portions of the Affordable Care Act, or the ACA. In May 2017, the House of Representatives voted to pass the American Health Care Act, or the AHCA. As proposed, the AHCA would repeal many provisions of the ACA. The Senate is expected to consider an alternative version of the AHCA and it is expected that Congress will continue to consider this or similar legislation to repeal and replace some or all elements of the ACA. There is currently uncertainty with respect to the impact any such repeal may have and any resulting changes may take time to unfold, which could have an impact on coverage and reimbursement for healthcare items and services covered by plans that were authorized by the ACA. We cannot predict the ultimate content, timing or effect of any such legislation or executive action or the impact of potential legislation or executive action on us. Any changes to the method for calculating prescription drug costs may reduce the revenues of our portfolio companies in the healthcare industry which could in turn impair their ability to timely make any principal and interest payments owed to us.
Risks Related to Our Securities
Investing in shares of our common stock involves an above average degree of risk.
The investments we make in accordance with our investment objective may result in a higher amount of risk, volatility or loss of principal than alternative investment options. Our investments in portfolio companies may be highly speculative and aggressive, and therefore, an investment in our common stock may not be suitable for investors with lower risk tolerance.
Our common stock may trade below its NAV per share, which limits our ability to raise additional equity capital.
If our common stock is trading below its NAV per share, we will generally not be able to issue additional shares of our common stock at its market price without first obtaining the approval for such issuance from our stockholders and our independent directors. If our common stock trades below NAV, the higher cost of equity capital may result in it being unattractive to raise new equity, which may limit our ability to grow. The risk of trading below NAV is separate and distinct from the risk that our NAV per share may decline. We cannot predict whether shares of our common stock will trade above, at or below our NAV.
51
Provisions of our charter and bylaws could deter takeover attempts and have an adverse impact on the price of our common stock.
Our charter and bylaws contain provisions that may have the effect of discouraging, delaying, or making difficult a change in control of our company or the removal of our incumbent directors. Under our charter, our Board of Directors is divided into three classes serving staggered terms, which will make it more difficult for a hostile bidder to acquire control of us. In addition, our Board of Directors may, without stockholder action, authorize the issuance of shares of stock in one or more classes or series, including preferred stock. Subject to compliance with the 1940 Act, our Board of Directors may, without stockholder action, amend our charter to increase the number of shares of stock of any class or series that we have authority to issue. The existence of these provisions, among others, may have a negative impact on the price of our common stock and may discourage third party bids for ownership of our company. These provisions may prevent any premiums being offered to you for shares of our common stock in connection with a takeover.
Sales of substantial amounts of our common stock in the public market may have an adverse effect on the market price of our common stock.
Sales of substantial amounts of our common stock, or the availability of such common stock for sale (including as a result of the conversion of our 2022 Convertible Notes, issued in January 2017, into common stock), could adversely affect the prevailing market prices for our common stock, which may also lead to further dilution of our earnings per share resulting from the outstanding shares attributable to the conversion of the 2022 Convertible Notes. If this occurs and continues, it could impair our ability to raise additional capital through the sale of securities should we desire to do so.
We may periodically obtain the approval of our stockholders to issue shares of our common stock at prices below the then current NAV per share of our common stock. If we receive such approval from the stockholders, we may issue shares of our common stock at a price below the then current NAV per share of common stock. Any such issuance could materially dilute your interest in our common stock and reduce our NAV per share.
We may periodically obtain the approval of our stockholders to issue shares of our common stock at prices below the then current NAV per share of our common stock. Such approval has allowed and may again allow us to access the capital markets in a way that we typically are unable to do as a result of restrictions that, absent stockholder approval, apply to business development companies under the 1940 Act. Any decision to sell shares of our common stock below the then current NAV per share of our common stock is subject to the determination by our Board of Directors that such issuance and sale is in our and our stockholders’ best interests.
Any sale or other issuance of shares of our common stock at a price below NAV per share has resulted and will continue to result in an immediate dilution to your interest in our common stock and a reduction of our NAV per share. This dilution would occur as a result of a proportionately greater decrease in a stockholder’s interest in our earnings and assets and voting interest in us than the increase in our assets resulting from such issuance. Because the number of future shares of common stock that may be issued below our NAV per share and the price and timing of such issuances are not currently known, we cannot predict the actual dilutive effect of any such issuance. We also cannot determine the resulting reduction in our NAV per share of any such issuance at this time. We caution you that such effects may be material, and we undertake to describe all the material risks and dilutive effects of any offering that we make at a price below our then current NAV in the future in a prospectus supplement issued in connection with any such offering. We cannot predict whether shares of our common stock will trade above, at or below our NAV.
If we conduct an offering of our common stock at a price below NAV, investors are likely to incur immediate dilution upon the closing of the offering.
We are not generally able to issue and sell our common stock at a price below NAV per share. We may, however, sell our common stock, at a price below the current NAV of the common stock, or sell warrants,
52
options or other rights to acquire such common stock, at a price below the current NAV of the common stock if our Board of Directors determines that such sale is in our best interests and the best interests of our stockholders and our stockholders have approved the practice of making such sales.
In connection with the receipt of such stockholder approval, we will limit the number of shares that it issues at a price below NAV pursuant to this authorization so that the aggregate dilutive effect on our then outstanding shares will not exceed 20%. Our Board of Directors, subject to its fiduciary duties and regulatory requirements, has the discretion to determine the amount of the discount, and as a result, the discount could be up to 100% of NAV per share. If we were to issue shares at a price below NAV, such sales would result in an immediate dilution to existing common stockholders, which would include a reduction in the NAV per share as a result of the issuance. This dilution would also include a proportionately greater decrease in a stockholder’s interest in our earnings and assets and voting interest in us than the increase in our assets resulting from such issuance.
In addition, if we determined to conduct additional offerings in the future there may be even greater dilution if we determine to conduct such offerings at prices below NAV. As a result, investors will experience further dilution and additional discounts to the price of our common stock. Because the number of shares of common stock that could be so issued and the timing of any issuance is not currently known, the actual dilutive effect of an offering cannot be predicted. We did not sell any of our securities at a price below NAV during the six-months ended June 30, 2017.
We may allocate the net proceeds from an offering in ways with which you may not agree.
We have significant flexibility in investing the net proceeds of an offering and may use the net proceeds from an offering in ways with which you may not agree or for purposes other than those contemplated at the time of the offering.
If we issue preferred stock, debt securities or convertible debt securities, the NAV and market value of our common stock may become more volatile.
We cannot assure you that the issuance of preferred stock and/or debt securities would result in a higher yield or return to the holders of our common stock. The issuance of preferred stock, debt securities or convertible debt would likely cause the NAV and market value of our common stock to become more volatile. If the distribution rate on the preferred stock, or the interest rate on the debt securities, were to approach the net rate of return on our investment portfolio, the benefit of leverage to the holders of our common stock would be reduced. If the distribution rate on the preferred stock, or the interest rate on the debt securities, were to exceed the net rate of return on our portfolio, the use of leverage would result in a lower rate of return to the holders of common stock than if we had not issued the preferred stock or debt securities. Any decline in the NAV of our investment would be borne entirely by the holders of our common stock. Therefore, if the market value of our portfolio were to decline, the leverage would result in a greater decrease in NAV to the holders of our common stock than if we were not leveraged through the issuance of preferred stock. This decline in NAV would also tend to cause a greater decline in the market price for our common stock.
There is also a risk that, in the event of a sharp decline in the value of our net assets, we would be in danger of failing to maintain required asset coverage ratios which may be required by the preferred stock, debt securities, convertible debt or units or of a downgrade in the ratings of the preferred stock, debt securities, convertible debt or our current investment income might not be sufficient to meet the distribution requirements on the preferred stock or the interest payments on the debt securities. If we do not maintain our required asset coverage ratios, we may not be permitted to declare dividend distributions. In order to counteract such an event, we might need to liquidate investments in order to fund redemption of some or all of the preferred stock, debt securities or convertible debt. In addition, we would pay (and the holders of our common stock would bear) all costs and expenses relating to the issuance and ongoing maintenance of the preferred stock, debt securities, convertible debt or any combination of these securities. Holders of preferred stock, debt securities or convertible debt may have different interests than holders of common stock and may at times have disproportionate influence over our affairs.
53
Holders of any preferred stock that we may issue will have the right to elect members of the Board of Directors and have class voting rights on certain matters.
The 1940 Act requires that holders of shares of preferred stock must be entitled as a class to elect two directors at all times and to elect a majority of the directors if distributions on such preferred stock are in arrears by two years or more, until such arrearage is eliminated. In addition, certain matters under the 1940 Act require the separate vote of the holders of any issued and outstanding preferred stock, including changes in fundamental investment restrictions and conversion to open-end status and, accordingly, preferred stockholders could veto any such changes. Restrictions imposed on the declarations and payment of dividends or other distributions to the holders of our common stock and preferred stock, both by the 1940 Act and by requirements imposed by rating agencies, might impair our ability to maintain our ability to be subject to tax as a RIC.
Terms relating to redemption may materially adversely affect your return on any debt securities that we may issue.
If your debt securities are redeemable at our option, we may choose to redeem your debt securities at times when prevailing interest rates are lower than the interest rate paid on your debt securities. In addition, if your debt securities are subject to mandatory redemption, we may be required to redeem your debt securities also at times when prevailing interest rates are lower than the interest rate paid on your debt securities. In this circumstance, you may not be able to reinvest the redemption proceeds in a comparable security at an effective interest rate as high as your debt securities being redeemed.
Additionally, we may redeem the 2024 Notes after July 30, 2017 at a redemption price of 100% of the outstanding principal amount thereof plus accrued and unpaid interest payments. If we choose to redeem the 2024 Notes when the fair market value of the 2024 Notes is above par value, you would experience a loss of any potential premium.
Our shares may trade at discounts from NAV or at premiums that are unsustainable over the long term.
Shares of business development companies may trade at a market price that is less than the NAV that is attributable to those shares. Our shares have historically traded above and below our NAV. The possibility that our shares of common stock will trade at a discount from NAV or at a premium that is unsustainable over the long term is separate and distinct from the risk that our NAV may decrease. It is not possible to predict whether our shares will trade at, above or below NAV in the future.
Our credit ratings may not reflect all risks of an investment in our debt securities.
Our credit ratings are an assessment by third parties of our ability to pay our obligations. Consequently, real or anticipated changes in our credit ratings will generally affect the market value of our debt securities. Our credit ratings, however, may not reflect the potential impact of risks related to market conditions generally or other factors discussed herein on the market value of or trading market for the publicly issued debt securities.
A downgrade, suspension or withdrawal of the credit rating assigned by a rating agency to us or our debt securities, if any, or change in the debt markets could cause the liquidity or market value of our debt securities to decline significantly.
Our credit ratings are an assessment by rating agencies of our ability to pay our debts when due. Consequently, real or anticipated changes in our credit ratings will generally affect the market value of our outstanding debt and equity securities. These credit ratings may not reflect the potential impact of risks relating to the structure or marketing of such debt and equity securities. Credit ratings are not a recommendation to buy, sell or hold any security, and may be revised or withdrawn at any time by the issuing organization in its sole discretion.
54
Neither we nor any underwriter undertakes any obligation to maintain our credit ratings or to advise holders of our debt and equity securities of any changes in our credit ratings. There can be no assurance that a credit rating will remain for any given period of time or that such credit ratings will not be lowered or withdrawn entirely if future circumstances relating to the basis of the credit rating, such as adverse changes in our company, so warrant. An increase in the competitive environment, inability to cover distributions, or increase in leverage could lead to a downgrade in our credit ratings and limit our access to the debt and equity markets capability impairing our ability to grow the business. The conditions of the financial markets and prevailing interest rates have fluctuated in the past and are likely to fluctuate in the future.
Investors in offerings of our common stock will likely incur immediate dilution upon the closing of an offering pursuant to this prospectus.
We generally expect the public offering price of any offering of shares of our common stock to be higher than the book value per share of our outstanding common stock (unless we offer shares pursuant to a rights offering or after obtaining prior approval for such issuance from our stockholders and our independent directors). Accordingly, investors purchasing shares of common stock in offerings pursuant to this prospectus may pay a price per share that exceeds the tangible book value per share after such offering.
Our stockholders may experience dilution upon the conversion of our 2022 Convertible Notes.
Our 2022 Convertible Notes, issued in January 2017, are convertible into shares of our common stock beginning on August 1, 2021 or, under certain circumstances, earlier. Upon conversion of the 2022 Convertible Notes, we have the choice to pay or deliver, as the case may be, at our election, cash, shares of our common stock or a combination of cash and shares of our common stock. The initial conversion price of the 2022 Convertible Notes is $16.41, subject to adjustment in certain circumstances. If we elect to deliver shares of common stock upon a conversion at the time our NAV per share exceeds the conversion price in effect at such time, our stockholders may incur dilution. In addition, our stockholders will experience dilution in their ownership percentage of common stock upon our issuance of common stock in connection with the conversion of the 2022 Convertible Notes and any distributions paid on our common stock will also be paid on shares issued in connection with such conversion after such issuance.
Our stockholders will experience dilution in their ownership percentage if they opt out of our dividend reinvestment plan.
All distributions in cash payable to stockholders that are participants in our dividend reinvestment plan are automatically reinvested in shares of our common stock. As a result, our stockholders that opt out of our dividend reinvestment plan will experience dilution in their ownership percentage of our common stock over time.
Our distribution proceeds may exceed our earnings. Therefore, portions of the distributions that we make may represent a return of capital to stockholders, which will lower their tax basis in their shares.
The tax treatment and characterization of our distributions may vary significantly from time to time due to the nature of our investments. The ultimate tax characterization of our distributions made during a taxable year generally will not finally be determined until after the end of that taxable year. We may make distributions during a taxable year that exceed our investment company taxable income, determined without regard to any deduction for dividends paid, and net capital gains for that taxable year. In such a situation, the amount by which our total distributions exceed investment company taxable income, determined without regard to any deduction for dividends paid, and net capital gains generally would be treated as a return of capital up to the amount of a stockholder’s tax basis in the shares, with any amounts exceeding such tax basis generally treated as a gain from the sale or exchange of such shares. A return of capital generally is a return of a stockholder’s investment rather than a return of earnings or gains derived from our investment activities. Moreover, we may pay all or a substantial portion of our distributions from the proceeds of the sale of shares of our common stock or from
55
borrowings in anticipation of future cash flow, which could constitute a return of stockholders’ capital and will lower such stockholders’ tax basis in our shares, which may result in increased tax liability to stockholders when they sell such shares. The tax liability to stockholders upon the sale of shares may increase even if such shares are sold at a loss.
Our common stock price has been and continues to be volatile and may decrease substantially.
As with any company, the price of our common stock will fluctuate with market conditions and other factors, which include, but are not limited to, the following:
| • | price and volume fluctuations in the overall stock market from time to time; |
| • | significant volatility in the market price and trading volume of securities of RICs, business development companies or other financial services companies; |
| • | any inability to deploy or invest our capital; |
| • | fluctuations in interest rates; |
| • | any shortfall in revenue or net income or any increase in losses from levels expected by investors or securities analysts; |
| • | the financial performance of specific industries in which we invest in on a recurring basis; |
| • | announcement of strategic developments, acquisitions, and other material events by us or our competitors, or operating performance of companies comparable to us; |
| • | changes in regulatory policies or tax guidelines with respect to RICs, SBICs or business development companies; |
| • | losing our ability to either qualify or be subject to U.S. federal income tax as a RIC; |
| • | actual or anticipated changes in our earnings or fluctuations in our operating results, or changes in the expectations of securities analysts; |
| • | changes in the value of our portfolio of investments; |
| • | realized losses in investments in our portfolio companies; |
| • | general economic conditions and trends; |
| • | inability to access the capital markets; |
| • | loss of a major funded source; or |
| • | departure of key personnel. |
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Due to the potential volatility of our stock price, we may be the target of securities litigation in the future. Securities litigation could result in substantial costs and could divert management’s attention and resources from our business.
We may be unable to invest a significant portion of the net proceeds from an offering or from exiting an investment or other capital on acceptable terms, which could harm our financial condition and operating results.
Delays in investing the net proceeds raised in an offering or from exiting an investment or other capital may cause our performance to be worse than that of other fully invested business development companies or other lenders or investors pursuing comparable investment strategies. We cannot assure you that we will be able to identify any investments that meet our investment objective or that any investment that we make will produce a
56
positive return. We may be unable to invest the net proceeds of any offering or from exiting an investment or other capital on acceptable terms within the time period that we anticipate or at all, which could harm our financial condition and operating results.
We anticipate that, depending on market conditions and the amount of the capital, it may take us a substantial period of time to invest substantially all the capital in securities meeting our investment objective. During this period, we will invest the capital primarily in cash equivalents, U.S. government securities and other high-quality debt investments that mature in one year or less or use the net proceeds from such offerings to reduce then-outstanding debt obligations, which may produce returns that are significantly lower than the returns which we expect to achieve when our portfolio is fully invested in securities meeting our investment objective. As a result, any distributions that we pay during such period may be substantially lower than the distributions that we may be able to pay when our portfolio is fully invested in securities meeting our investment objective. In addition, until such time as the net proceeds of any offering or from exiting an investment or other capital are invested in new securities meeting our investment objective, the market price for our securities may decline. Thus, the initial return on your investment may be lower than when, if ever, our portfolio is fully invested in securities meeting our investment objective.
Your interest in us may be diluted if you do not fully exercise your subscription rights in any rights offering. In addition, if the subscription price is less than our NAV per share, then you will experience an immediate dilution of the aggregate NAV of your shares.
In the event we issue subscription rights, stockholders who do not fully exercise their subscription rights should expect that they will, at the completion of a rights offering pursuant to this prospectus, own a smaller proportional interest in us than would otherwise be the case if they fully exercised their rights. We cannot state precisely the amount of any such dilution in share ownership because we do not know at this time what proportion of the shares will be purchased as a result of such rights offering.
In addition, if the subscription price is less than the NAV per share of our common stock, then our stockholders would experience an immediate dilution of the aggregate NAV of their shares as a result of the offering. The amount of any decrease in NAV is not predictable because it is not known at this time what the subscription price and NAV per share will be on the expiration date of a rights offering or what proportion of the shares will be purchased as a result of such rights offering. Such dilution could be substantial.
The trading market or market value of our publicly issued debt securities may fluctuate.
Our publicly issued debt securities may or may not have an established trading market. We cannot assure you that a trading market for our publicly issued debt securities will ever develop or be maintained if developed. In addition to our creditworthiness, many factors may materially adversely affect the trading market for, and market value of, our publicly issued debt securities. These factors include, but are not limited to, the following:
| • | the time remaining to the maturity of these debt securities; |
| • | the outstanding principal amount of debt securities with terms identical to these debt securities; |
| • | the ratings assigned by national statistical ratings agencies; |
| • | the general economic environment; |
| • | the supply of debt securities trading in the secondary market, if any; |
| • | the redemption or repayment features, if any, of these debt securities; |
| • | the level, direction and volatility of market interest rates generally; and |
| • | market rates of interest higher or lower than rates borne by the debt securities. You should also be aware that there may be a limited number of buyers when you decide to sell your debt securities. This too may materially adversely affect the market value of the debt securities or the trading market for the debt securities. |
57
The 2024 Notes are unsecured and therefore are effectively subordinated to any secured indebtedness we have currently incurred or may incur in the future.
The 2024 Notes are not secured by any of our assets or any of the assets of our subsidiaries. As a result, while the 2024 Notes remain senior in priority to our equity securities, they are effectively subordinated to any secured indebtedness we or our subsidiaries have currently incurred and may incur in the future (or any indebtedness that is initially unsecured to which we subsequently grant security) to the extent of the value of the assets securing such indebtedness. In any liquidation, dissolution, bankruptcy or other similar proceeding, the holders of any of our existing or future secured indebtedness and the secured indebtedness of our subsidiaries may assert rights against the assets pledged to secure that indebtedness in order to receive full payment of their indebtedness before the assets may be used to pay other creditors, including the holders of the 2024 Notes.
The 2024 Notes are structurally subordinated to the indebtedness and other liabilities of our subsidiaries.
The 2024 Notes are obligations exclusively of Hercules Capital, Inc. (formerly known as Hercules Technology Growth Capital, Inc.) and not of any of our subsidiaries. None of our subsidiaries are or act as guarantors of the 2024 Notes and the 2024 Notes are not required to be guaranteed by any subsidiaries we may acquire or create in the future. Our secured indebtedness with respect to the SBA debentures is held through our SBIC subsidiaries. The assets of any such subsidiaries are not directly available to satisfy the claims of our creditors, including holders of the 2024 Notes.
Except to the extent we are a creditor with recognized claims against our subsidiaries, all claims of creditors (including holders of preferred stock, if any, of our subsidiaries) will have priority over our equity interests in such subsidiaries (and therefore the claims of our creditors, including holders of the 2024 Notes) with respect to the assets of such subsidiaries. Even if we are recognized as a creditor of one or more of our subsidiaries, our claims would still be structurally subordinated to any security interests in the assets of any such subsidiary and to any indebtedness or other liabilities of any such subsidiary senior to our claims. Consequently, the 2024 Notes are structurally subordinated to all indebtedness and other liabilities (including trade payables) of our subsidiaries and any subsidiaries that we may in the future acquire or establish as financing vehicles or otherwise. In addition, our subsidiaries may incur substantial additional indebtedness in the future, all of which would be structurally senior to the 2024 Notes.
The indenture under which the 2024 Notes were issued contains limited protection for the holders of the 2024 Notes.
The indenture under which 2024 Notes were issued offers limited protection to the holders of the 2024 Notes. The terms of the indenture and the 2024 Notes do not restrict our or any of our subsidiaries’ ability to engage in, or otherwise be a party to, a variety of corporate transactions, circumstances or events that could have an adverse impact on an investment in the 2024 Notes. In particular, the terms of the indenture and the 2024 Notes do not place any restrictions on our or our subsidiaries’ ability to:
| • | issue securities or otherwise incur additional indebtedness or other obligations, including (1) any indebtedness or other obligations that would be equal in right of payment to the 2024 Notes, (2) any indebtedness or other obligations that would be secured and therefore rank effectively senior in right of payment to the 2024 Notes to the extent of the values of the assets securing such debt, (3) indebtedness of ours that is guaranteed by one or more of our subsidiaries and which therefore would rank structurally senior to the 2024 Notes and (4) securities, indebtedness or other obligations issued or incurred by our subsidiaries that would be senior in right of payment to our equity interests in our subsidiaries and therefore would rank structurally senior in right of payment to the 2024 Notes with respect to the assets of our subsidiaries, in each case other than an incurrence of indebtedness or other obligation that would cause a violation of Section 18(a)(1)(A) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act or any successor provisions; |
58
| • | pay distributions on, or purchase or redeem or make any payments in respect of, capital stock or other securities ranking junior in right of payment to the 2024 Notes, in each case other than distributions, purchases, redemptions or payments that would cause a violation of Section 18(a)(1)(B) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act or any successor provisions giving effect to any exemptive relief granted to us by the SEC (these provisions generally prohibit us from declaring any cash distributions upon any class of our capital stock, or purchasing any such capital stock if our asset coverage, as defined in the 1940 Act, is below 200% at the time of the declaration of the distribution or the purchase and after deducting the amount of such distribution or purchase); |
| • | sell assets (other than certain limited restrictions on our ability to consolidate, merge or sell all or substantially all of our assets); |
| • | enter into transactions with affiliates; |
| • | create liens (including liens on the shares of our subsidiaries) or enter into sale and leaseback transactions; |
| • | make investments; or |
| • | create restrictions on the payment of distributions or other amounts to us from our subsidiaries. |
The indenture and the 2024 Notes do not require us to offer to purchase the 2024 Notes in connection with a change of control or any other event.
Furthermore, the terms of the indenture and the 2024 Notes do not protect their respective holders in the event that we experience changes (including significant adverse changes) in our financial condition, results of operations or credit ratings, as they do not require that we or our subsidiaries adhere to any financial tests or ratios or specified levels of net worth, revenues, income, cash flow or liquidity, except as required under the 1940 Act.
Our ability to recapitalize, incur additional debt and take a number of other actions that are not limited by the terms of the 2024 Notes may have important consequences for their holders, including making it more difficult for us to satisfy our obligations with respect to the 2024 Notes or negatively affecting their trading value.
Certain of our current debt instruments include more protections for their respective holders than the indenture and the 2024 Notes. In addition, other debt we issue or incur in the future could contain more protections for its holders than the indenture and the 2024 Notes, including additional covenants and events of default. The issuance or incurrence of any such debt with incremental protections could affect the market for and trading levels and prices of the 2024 Notes.
An active trading market for the 2024 Notes may not develop or be sustained, which could limit the market price of the 2024 Notes or your ability to sell them.
Although the 2024 Notes are listed on the NYSE under the symbol “HTGX,” we cannot provide any assurances that an active trading market will develop or be sustained for the 2024 Notes or that the 2024 Notes will be able to be sold. At various times, the 2024 Notes may trade at a discount from their initial offering price depending on prevailing interest rates, the market for similar securities, our credit ratings, general economic conditions, our financial condition, performance and prospects and other factors. To the extent an active trading market is not sustained, the liquidity and trading price for the 2024 Notes may be harmed.
59
If we default on our obligations to pay our other indebtedness, we may not be able to make payments on the 2024 Notes.
Any default under the agreements governing our indebtedness, including a default under the Wells Facility, the Union Bank Facility or other indebtedness to which we may be a party that is not waived by the required lenders or holders, and the remedies sought by the holders of such indebtedness could make us unable to pay principal, premium, if any, and interest on the 2024 Notes and substantially decrease the market value of the 2024 Notes. If we are unable to generate sufficient cash flow and are otherwise unable to obtain funds necessary to meet required payments of principal, premium, if any, and interest on our indebtedness, or if we otherwise fail to comply with the various covenants, including financial and operating covenants, in the instruments governing our indebtedness, we could be in default under the terms of the agreements governing such indebtedness. In the event of such default, the holders of such indebtedness could elect to declare all the funds borrowed thereunder to be due and payable, together with accrued and unpaid interest, the lenders under the Wells Facility and the Union Bank Facility or other debt we may incur in the future could elect to terminate their commitments, cease making further loans and institute foreclosure proceedings against our assets, and we could be forced into bankruptcy or liquidation. If our operating performance declines, we may in the future need to seek to obtain waivers from the required lenders under the Wells Facility or Union Bank Facility or other debt that we may incur in the future to avoid being in default. If we breach our covenants under the Wells Facility or Union Bank Facility or other debt and seek a waiver, we may not be able to obtain a waiver from the required lenders or holders. If this occurs, we would be in default under the Wells Facility or Union Bank Facility or other debt, the lenders or holders could exercise their rights as described above, and we could be forced into bankruptcy or liquidation. If we are unable to repay debt, lenders having secured obligations, including the lenders under the Wells Facility and the Union Bank Facility, could proceed against the collateral securing the debt. Because the Wells Facility and the Union Bank Facility have, and any future credit facilities will likely have, customary cross-default provisions, if the indebtedness under the 2024 Notes, the Wells Facility, Union Bank Facility or under any future credit facility is accelerated, we may be unable to repay or finance the amounts due.
60
The matters discussed in this prospectus, as well as in future oral and written statements by management of Hercules Capital, Inc. that are forward-looking statements are based on current management expectations that involve substantial risks and uncertainties which could cause actual results to differ materially from the results expressed in, or implied by, these forward-looking statements. Forward-looking statements relate to future events or our future financial performance. We generally identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Important assumptions include our ability to originate new investments, achieve certain margins and levels of profitability, the availability of additional capital, and the ability to maintain certain debt to asset ratios. In light of these and other uncertainties, the inclusion of a projection or forward-looking statement in this prospectus should not be regarded as a representation by us that our plans or objectives will be achieved. The forward-looking statements contained in this prospectus include statements as to:
| • | our current and future management structure; |
| • | our future operating results; |
| • | our business prospects and the prospects of our prospective portfolio companies; |
| • | the impact of investments that we expect to make; |
| • | our informal relationships with third parties including in the venture capital industry; |
| • | the expected market for venture capital investments and our addressable market; |
| • | the dependence of our future success on the general economy and its impact on the industries in which we invest; |
| • | our ability to access debt markets and equity markets; |
| • | the ability of our portfolio companies to achieve their objectives; |
| • | our expected financings and investments; |
| • | our regulatory structure and tax status; |
| • | our ability to operate as a business development company, a SBIC and a RIC; |
| • | the adequacy of our cash resources and working capital; |
| • | the timing of cash flows, if any, from the operations of our portfolio companies; |
| • | the timing, form and amount of any distributions; |
| • | the impact of fluctuations in interest rates on our business; |
| • | the valuation of any investments in portfolio companies, particularly those having no liquid trading market; and |
| • | our ability to recover unrealized losses. |
For a discussion of factors that could cause our actual results to differ from forward-looking statements contained in this prospectus, please see the discussion under “Risk Factors.” You should not place undue reliance on these forward-looking statements. The forward-looking statements made in this prospectus relate only to events as of the date on which the statements are made. We undertake no obligation to update any forward-looking statement to reflect events or circumstances occurring after the date of this prospectus.
The following discussion should be read in conjunction with our consolidated financial statements and related notes and other financial information appearing elsewhere in this prospectus. In addition to historical information, the following discussion and other parts of this prospectus contain forward-looking information that involves risks and uncertainties. Our actual results could differ materially from those anticipated by such forward-looking information due to the factors discussed under “Risk Factors” and “Forward-Looking Statements.”
61
We intend to use the net proceeds from selling our securities for funding investments in debt and equity securities in accordance with our investment objectives, retiring certain debt obligations and other general corporate purposes. The supplement to this prospectus relating to an offering will more fully identify the use of proceeds from such offering.
We anticipate that substantially all of the net proceeds from any offering of our securities will be used as described above within twelve months, but in no event longer than two years. Pending such uses and investments, we will invest the net proceeds primarily in cash, cash equivalents, U.S. government securities or high-quality debt securities maturing in one year or less from the time of investment. Our ability to achieve our investment objective may be limited to the extent that the net proceeds of any offering, pending full investment, are held in lower yielding short-term instruments.
62
PRICE RANGE OF COMMON STOCK AND DISTRIBUTIONS
Our common stock is traded on the NYSE under the symbol “HTGC.”
The following table sets forth the range of high and low sales prices of our common stock, the sales price as a percentage of NAV and the distributions declared by us for each fiscal quarter. The stock quotations are interdealer quotations and do not include markups, markdowns or commissions.
|
Price Range |
Premium/ Discount of High Sales Price to NAV |
Premium/ Discount of Low Sales Price to NAV |
Cash Distribution per Share |
|||||||||||||||||||||
| NAV(1) | High | Low | ||||||||||||||||||||||
| 2015 |
||||||||||||||||||||||||
| First quarter |
$ | 10.47 | $ | 15.27 | $ | 13.47 | 45.8 | % | 28.7 | % | $ | 0.310 | ||||||||||||
| Second quarter |
$ | 10.26 | $ | 13.37 | $ | 11.25 | 30.3 | % | 9.6 | % | $ | 0.310 | ||||||||||||
| Third quarter |
$ | 10.02 | $ | 12.23 | $ | 9.99 | 22.1 | % | -0.3 | % | $ | 0.310 | ||||||||||||
| Fourth quarter |
$ | 9.94 | $ | 12.44 | $ | 10.23 | 25.2 | % | 2.9 | % | $ | 0.310 | ||||||||||||
| 2016 |
||||||||||||||||||||||||
| First quarter |
$ | 9.81 | $ | 12.39 | $ | 10.03 | 26.3 | % | 2.2 | % | $ | 0.310 | ||||||||||||
| Second quarter |
$ | 9.66 | $ | 12.43 | $ | 11.74 | 28.7 | % | 21.6 | % | $ | 0.310 | ||||||||||||
| Third quarter |
$ | 9.86 | $ | 14.00 | $ | 12.42 | 41.9 | % | 25.9 | % | $ | 0.310 | ||||||||||||
| Fourth quarter |
$ | 9.90 | $ | 14.25 | $ | 12.90 | 43.9 | % | 30.2 | % | $ | 0.310 | ||||||||||||
| 2017 |
||||||||||||||||||||||||
| First quarter |
$ | 9.76 | $ | 15.43 | $ | 14.12 | 58.1 | % | 44.7 | % | $ | 0.310 | ||||||||||||
| Second quarter |
$ | 9.87 | $ | 15.56 | $ | 12.66 | 57.6 | % | 28.3 | % | $ | 0.310 | ||||||||||||
| Third quarter (through August 30, 2017) |
* | $ | 13.50 | $ | 12.31 | * | * | ** | ||||||||||||||||
| (1) | Net asset value per share is generally determined as of the last day in the relevant quarter and therefore may not reflect the NAV per share on the date of the high and low sales prices. The NAVs shown are based on outstanding shares at the end of each period. |
| * | Net asset value has not yet been calculated for this period. |
| ** | Cash distribution per share has not yet been determined for this period. |
The last reported price for our common stock on August 30, 2017 was $12.31 per share.
Shares of business development companies may trade at a market price that is less than the value of the net assets attributable to those shares. The possibility that our shares of common stock will trade at a discount from NAV or at premiums that are unsustainable over the long term are separate and distinct from the risk that our NAV will decrease. At times, our shares of common stock have traded at a premium to NAV and at times our shares of common stock have traded at a discount to the net assets attributable to those shares. It is not possible to predict whether the shares offered hereby will trade at, above, or below NAV.
63
Distributions
The following table summarizes distributions declared and paid or to be paid or reinvested on all shares, including restricted stock, for the fiscal years ended December 31, 2015, 2016 and 2017:
| Date Declared |
Record Date |
Payment Date |
Amount Per Share | |||||
| February 24, 2015 |
March 12, 2015 | March 19, 2015 | $ | 0.31 | ||||
| May 4, 2015 |
May 18, 2015 | May 25, 2015 | 0.31 | |||||
| July 29, 2015 |
August 17, 2015 | August 24, 2015 | 0.31 | |||||
| October 28, 2015 |
November 16, 2015 | November 23, 2015 | 0.31 | |||||
| February 17, 2016 |
March 7, 2016 | March 14, 2016 | 0.31 | |||||
| April 27, 2016 |
May 16, 2016 | May 23, 2016 | 0.31 | |||||
| July 27, 2016 |
August 15, 2016 | August 22, 2016 | 0.31 | |||||
| October 24, 2016 |
November 14, 2016 | November 21, 2016 | 0.31 | |||||
| February 16, 2017 |
March 6, 2017 |
March 13, 2017 |
0.31 | |||||
| April 26, 2017 |
May 15, 2017 | May 22, 2017 | 0.31 | |||||
| July 26, 2017 |
August 14, 2017 | August 21, 2017 | 0.31 | |||||
|
|
|
|||||||
| $ | 3.41 | |||||||
|
|
|
|||||||
| * | Distribution paid in cash and stock. |
On July 26, 2017, the Board of Directors declared a cash distribution of $0.31 per share to be paid on August 21, 2017 to stockholders of record as of August 14, 2017. This distribution represented our forty-eighth consecutive distribution since our IPO, bringing the total cumulative distribution to date to $13.40 per share.
Our Board of Directors maintains a variable distribution policy with the objective of distributing four quarterly distributions in an amount that approximates 90—100% of our taxable quarterly income or potential annual income for a particular taxable year. In addition, at the end of our taxable year, our Board of Directors may choose to pay an additional special distribution or fifth distribution, so that we may distribute approximately all of our annual taxable income in the taxable year in which it was earned, or may elect to maintain the option to spill over our excess taxable income into the following taxable year as part of any future distribution payments.
Distributions in excess of our current and accumulated earnings and profits would generally be treated first as a return of capital to the extent of a stockholder’s tax basis in our shares, and any remaining distributions would be treated as a capital gain. The determination of the tax attributes of our distributions is made annually as of the end of our taxable year based upon our taxable income for the full taxable year and distributions paid for the full taxable year. Of the distributions declared during the fiscal years ended December 31, 2016, 2015, and 2014, 100% were distributions derived from our current and accumulated earnings and profits. There can be no certainty to stockholders that this determination is representative of the tax attributes of our 2017 distributions to stockholders.
We maintain an “opt out” dividend reinvestment plan that provides for reinvestment of our distribution on behalf of our stockholders, unless a stockholder elects to receive cash. As a result, if our Board of Directors authorizes, and we declare a cash distribution, then our stockholders who have not “opted out” of our dividend reinvestment plan will have their cash distribution automatically reinvested in additional shares of our common stock, rather than receiving the cash distributions.
Shortly after the close of each calendar year information identifying the source of the distribution (i.e., paid from ordinary income, paid from net capital gains on the sale of securities, and/or a return of paid-in-capital surplus which is a nontaxable distribution, if any) will be provided to the IRS and our stockholders subject to information reporting. To the extent our taxable earnings fall below the total amount of our distributions for any taxable year, a portion of those distributions may be deemed a tax return of capital to our stockholders.
We expect to qualify to be subject to tax as a RIC under Subchapter M of the Code. In order to be subject to tax as a RIC, we are required to satisfy certain annual gross income and quarterly asset composition tests, as well
64
as make distributions to our stockholders each taxable year treated as dividends for U.S. federal income tax purposes of an amount at least equal to 90% of the sum of our investment company taxable income, determined without regard to any deduction for dividends paid, plus our net tax-exempt income, if any. Upon being eligible to be subject to tax as a RIC, we would be entitled to deduct such distributions we pay to our stockholders in determining the overall components of our “taxable income.” Components of our taxable income include our taxable interest, dividend and fee income, reduced by certain deductions, as well as taxable net realized securities gains. Taxable income generally differs from net income for financial reporting purposes due to temporary and permanent differences in the recognition of income and expenses and generally excludes net unrealized appreciation or depreciation as such gains or losses are not included in taxable income until they are realized. In connection with maintaining our ability to be subject to tax as a RIC, among other things, we have made and intend to continue to make the requisite distributions to our stockholders each taxable year, which generally should relieve us from corporate-level U.S. federal income taxes.
As a RIC, we will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income and gains unless we make distributions treated as dividends for U.S. federal income tax purposes in a timely manner to our stockholders in respect of each calendar year of an amount generally at least equal to the sum of (1) 98% of our ordinary income for each calendar year, (2) 98.2% of our capital gain net income for the 1-year period ending October 31 in that calendar year and (3) any income realized, but not distributed, in the preceding years (the “Excise Tax Avoidance Requirement”). We will not be subject to this excise tax on any amount on which we incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains).
Depending on the level of taxable income earned in a taxable year, we may choose to carry over taxable income in excess of current taxable year distributions treated as dividends for U.S. federal income tax purposes from such taxable income into the next taxable year and incur a 4% excise tax on such taxable income, as required. The maximum amount of excess taxable income that may be carried over for distribution in the next taxable year under the Code is the total amount of distributions treated as dividends for U.S. federal income tax purposes paid in the following taxable year, subject to certain declaration and payment guidelines. To the extent we choose to carry over taxable income into the next taxable year, distributions declared and paid by us in a taxable year may differ from our taxable income for that taxable year as such distributions may include the distribution of current taxable year taxable income, the distribution of prior taxable year taxable income carried over into and distributed in the current taxable year, or returns of capital.
We can offer no assurance that we will achieve results that will permit the payment of any cash distributions and, if we issue senior securities, we will be prohibited from making distributions if doing so causes us to fail to maintain the asset coverage ratios stipulated by the 1940 Act or if distributions are limited by the terms of any of our borrowings. See “Regulation.” Our ability to make distributions will be limited by the asset coverage requirements under the 1940 Act.
We intend to distribute 100% of our spillover earnings, which consists of ordinary income and long term capital gains, from our taxable year ended December 31, 2016 to our stockholders during 2017.
65
RATIO OF EARNINGS TO FIXED CHARGES
The following contains our ratio of earnings to fixed charges for the periods indicated, computed as set forth below. You should read these ratios of earnings to fixed charges in connection with our consolidated financial statements, including the notes to those statements, included in this prospectus.
| For the six- months ended June 30, 2017 |
For the year ended December 31, 2016 |
For the year ended December 31, 2015 |
For the year ended December 31, 2014 |
For the year ended December 31, 2013 |
For the year ended December 31, 2012 |
|||||||||||||||||||
| Earnings to Fixed Charges(1) |
2.20 | 2.85 | 2.16 | 3.10 | 3.83 | 2.97 | ||||||||||||||||||
For purposes of computing the ratios of earnings to fixed charges, earnings represent net increase in stockholders’ equity resulting from operations plus fixed charges. Fixed charges include interest and credit facility fees expense and amortization of debt issuance costs.
| (1) | Earnings include net realized and unrealized gains or losses. Net realized and unrealized gains or losses can vary substantially from period to period. |
66
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our consolidated financial statements and related notes and other financial information appearing elsewhere in this prospectus. In addition to historical information, the following discussion and other parts of this prospectus contain forward-looking information that involves risks and uncertainties. Our actual results could differ materially from those anticipated by such forward-looking information due to the factors discussed under “Risk Factors” and “Forward-Looking Statements” appearing elsewhere herein.
Overview
We are a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences, and sustainable and renewable technology industries. We source our investments through our principal office located in Palo Alto, CA, as well as through our additional offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT, and San Diego, CA.
Our goal is to be the leading structured debt financing provider for venture capital-backed companies in technology-related industries requiring sophisticated and customized financing solutions. Our strategy is to evaluate and invest in a broad range of technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology and to offer a full suite of growth capital products. We invest primarily in structured debt with warrants and, to a lesser extent, in senior debt and equity investments. We invest primarily in private companies but also have investments in public companies.
We use the term “structured debt with warrants” to refer to any debt investment, such as a senior or subordinated secured loan, that is coupled with an equity component, including warrants, options or other rights to purchase common or preferred stock. Our structured debt with warrants investments typically are secured by some or all of the assets of the portfolio company.
Our investment objective is to maximize our portfolio’s total return by generating current income from our debt investments and capital appreciation from our warrant and equity-related investments. Our primary business objectives are to increase our net income, net operating income and NAV by investing in structured debt with warrants and equity of venture capital-backed companies in technology-related industries with attractive current yields and the potential for equity appreciation and realized gains. Our equity ownership in our portfolio companies may exceed 25% of the voting securities of such companies, which represents a controlling interest under the 1940 Act. In some cases, we receive the right to make additional equity investments in our portfolio companies in connection with future equity financing rounds. Capital that we provide directly to venture capital-backed companies in technology-related industries is generally used for growth and general working capital purposes as well as in select cases for acquisitions or recapitalizations.
We also make investments in qualifying small businesses through our two wholly-owned SBICs. Our SBIC subsidiaries, HT II and HT III, hold approximately $104.8 million and $271.5 million in assets, respectively, and accounted for approximately 5.8% and 14.9% of our total assets, respectively, prior to consolidation at June 30, 2017. In aggregate, at June 30, 2017, with our net investment of $118.5 million, HT II and HT III have the capacity to issue a total of $190.2 million of SBA-guaranteed debentures, subject to SBA approval. At June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries.
We have qualified as and have elected to be treated for tax purposes as a RIC under Subchapter M of the Code. Pursuant to this election, we generally will not be subject to corporate-level taxes on any income and gains that we distribute as dividends for U.S. federal income tax purposes to our stockholders. However, our
67
qualification and election to be treated as a RIC requires that we comply with provisions contained in Subchapter M of the Code. For example, as a RIC we must earn 90% or more of our gross income during each taxable year from qualified sources, typically referred to as “good income,” as well as satisfy certain quarterly asset diversification and annual income distribution requirements.
We are an internally managed, non-diversified, closed-end investment company that has elected to be regulated as a business development company under the 1940 Act. As a business development company, we are required to comply with certain regulatory requirements. For instance, we generally have to invest at least 70% of our total assets in “qualifying assets,” which includes securities of private U.S. companies, cash, cash equivalents and high-quality debt investments that mature in one year or less.
Our portfolio is comprised of, and we anticipate that our portfolio will continue to be comprised of, investments primarily in technology related companies at various stages of their development. Consistent with requirements under the 1940 Act, we invest primarily in United-States based companies and to a lesser extent in foreign companies.
We regularly engage in discussions with third parties with respect to various potential transactions. We may acquire an investment or a portfolio of investments or an entire company or sell a portion of our portfolio on an opportunistic basis. We, our subsidiaries or our affiliates may also agree to manage certain other funds that invest in debt, equity or provide other financing or services to companies in a variety of industries for which we may earn management or other fees for our services. We may also invest in the equity of these funds, along with other third parties, from which we would seek to earn a return and/or future incentive allocations. Some of these transactions could be material to our business. Consummation of any such transaction will be subject to completion of due diligence, finalization of key business and financial terms (including price) and negotiation of final definitive documentation as well as a number of other factors and conditions including, without limitation, the approval of our Board of Directors and required regulatory or third party consents and, in certain cases, the approval of our stockholders. Accordingly, there can be no assurance that any such transaction would be consummated. Any of these transactions or funds may require significant management resources either during the transaction phase or on an ongoing basis depending on the terms of the transaction.
Portfolio and Investment Activity
The total fair value of our investment portfolio was $1.4 billion at both June 30, 2017 and December 31, 2016. The fair value of our debt investment portfolio was approximately $1.3 billion at both June 30, 2017 and December 31, 2016. The fair value of the equity portfolio at June 30, 2017 was approximately $75.4 million, compared to a fair value of approximately $67.6 million at December 31, 2016. The fair value of the warrant portfolio at June 30, 2017 was approximately $32.5 million, compared to a fair value of approximately $27.5 million at December 31, 2016.
Portfolio Activity
Our investments in portfolio companies take a variety of forms, including unfunded contractual commitments and funded investments. From time to time, unfunded contractual commitments depend upon a portfolio company reaching certain milestones before the debt commitment is available to the portfolio company, which is expected to affect our funding levels. These commitments are subject to the same underwriting and ongoing portfolio maintenance as the on-balance sheet financial instruments that we hold. Debt commitments generally fund over the two succeeding quarters from close. Not all debt commitments represent future cash requirements. Similarly, unfunded contractual commitments may expire without being drawn and thus do not represent future cash requirements.
Prior to entering into a contractual commitment, we generally issue a non-binding term sheet to a prospective portfolio company. Non-binding term sheets are subject to completion of our due diligence and final
68
investment committee approval process, as well as the negotiation of definitive documentation with the prospective portfolio companies. These non-binding term sheets generally convert to contractual commitments in approximately 90 days from signing. Not all non-binding term sheets are expected to close and do not necessarily represent future cash requirements.
Our portfolio activity for the six months ended June 30, 2017 and the year ended December 31, 2016 was comprised of the following:
| (in millions) |
June 30, 2017 | December 31, 2016 | ||||||
| Debt Commitments(1) |
||||||||
| New portfolio company |
$ | 336.0 | $ | 624.0 | ||||
| Existing portfolio company |
57.1 | 171.8 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 393.1 | $ | 795.8 | ||||
|
|
|
|
|
|||||
| Funded and Restructured Debt Investments(2) |
||||||||
| New portfolio company |
$ | 243.2 | $ | 479.0 | ||||
| Existing portfolio company |
93.5 | 181.5 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 336.7 | $ | 660.5 | ||||
|
|
|
|
|
|||||
| Funded Equity Investments |
||||||||
| New portfolio company |
$ | 3.7 | $ | 17.1 | ||||
| Existing portfolio company |
0.2 | 3.1 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 3.9 | $ | 20.2 | ||||
|
|
|
|
|
|||||
| Unfunded Contractual Commitments(3) |
||||||||
| Total |
$ | 57.6 | $ | 59.7 | ||||
|
|
|
|
|
|||||
| Non-Binding Term Sheets |
||||||||
| New portfolio company |
$ | 70.0 | $ | 55.0 | ||||
|
|
|
|
|
|||||
| Total |
$ | 70.0 | $ | 55.0 | ||||
|
|
|
|
|
|||||
| (1) | Includes restructured loans and renewals in addition to new commitments. |
| (2) | Funded amounts include borrowings on revolving facilities. |
| (3) | Amount represents unfunded commitments, including undrawn revolving facilities, which are available at the request of the portfolio company. Amount excludes unfunded commitments which are unavailable due to the borrower having not met certain milestones. |
We receive principal payments on our debt investment portfolio based on scheduled amortization of the outstanding balances. In addition, we receive principal repayments for some of our loans prior to their scheduled maturity date. The frequency or volume of these early principal repayments may fluctuate significantly from period to period. During the six months ended June 30, 2017, we received approximately $338.8 million in aggregate principal repayments. Of the approximately $338.8 million of aggregate principal repayments, approximately $72.1 million were scheduled principal payments and approximately $266.7 million were early principal repayments related to 29 portfolio companies. Of the approximately $266.7 million early principal repayments, approximately $33.5 million were early repayments due to M&A transactions for four portfolio companies.
69
Total portfolio investment activity (inclusive of unearned income and excluding activity related to taxes payable, escrow receivables and warrant participation liabilities) as of and for the six months ended June 30, 2017 and the year ended December 31, 2016 was as follows:
| (in millions) |
June 30, 2017 | December 31, 2016 | ||||||
| Beginning portfolio |
$ | 1,423.9 | $ | 1,200.6 | ||||
| New fundings and restructures |
340.6 | 680.7 | ||||||
| Warrants not related to current period fundings |
0.4 | 0.6 | ||||||
| Principal payments received on investments |
(72.1 | ) | (111.2 | ) | ||||
| Early payoffs |
(266.7 | ) | (324.0 | ) | ||||
| Accretion of loan discounts and paid-in-kind principal |
18.7 | 43.6 | ||||||
| Net acceleration of loan discounts and loan fees due to early payoff or restructure |
(5.8 | ) | (6.3 | ) | ||||
| New loan fees |
(4.5 | ) | (10.1 | ) | ||||
| Warrants converted to equity |
— | 0.3 | ||||||
| Sale of investments |
(10.2 | ) | (4.4 | ) | ||||
| Loss on investments due to write offs |
(10.8 | ) | (10.0 | ) | ||||
| Net change in unrealized depreciation |
(18.0 | ) | (35.9 | ) | ||||
|
|
|
|
|
|||||
| Ending portfolio |
$ | 1,395.5 | $ | 1,423.9 | ||||
|
|
|
|
|
|||||
The following table shows the fair value of our investment portfolio by asset class as of June 30, 2017 and December 31, 2016:
| June 30, 2017 | December 31, 2016 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| Senior Secured Debt with Warrants |
$ | 999,813 | 71.6 | % | $ | 1,078,779 | 75.7 | % | ||||||||
| Senior Secured Debt |
320,340 | 23.0 | % | 277,509 | 19.5 | % | ||||||||||
| Preferred Stock |
43,385 | 3.1 | % | 39,418 | 2.8 | % | ||||||||||
| Common Stock |
31,931 | 2.3 | % | 28,236 | 2.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,395,469 | 100.0 | % | $ | 1,423,942 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
A summary of our investment portfolio as of June 30, 2017 and December 31, 2016 at value by geographic location is as follows:
| June 30, 2017 | December 31, 2016 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| United States |
$ | 1,269,476 | 91.0 | % | $ | 1,362,223 | 95.6 | % | ||||||||
| England |
69,884 | 5.0 | % | 18,395 | 1.3 | % | ||||||||||
| Netherlands |
20,352 | 1.4 | % | 20,089 | 1.4 | % | ||||||||||
| Switzerland |
12,607 | 0.9 | % | 12,377 | 0.9 | % | ||||||||||
| Cayman Islands |
12,376 | 0.9 | % | — | 0.0 | % | ||||||||||
| Canada |
10,773 | 0.8 | % | 8,095 | 0.6 | % | ||||||||||
| Israel |
1 | 0.0 | % | 2,763 | 0.2 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,395,469 | 100.0 | % | $ | 1,423,942 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of June 30, 2017, we held warrants or equity positions in six companies that have filed registration statements on Form S-1 with the SEC in contemplation of potential initial public offerings. All six companies filed confidentially under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. There can be no assurance that companies that have yet to complete their initial public offerings will do so in a timely manner or at all.
70
Changes in Portfolio
We generate revenue in the form of interest income, primarily from our investments in debt securities, and commitment and facility fees. Interest income is recognized in accordance with the contractual terms of the loan agreement to the extent that such amounts are expected to be collected. Fees generated in connection with our debt investments are recognized over the life of the loan or, in some cases, recognized as earned. In addition, we generate revenue in the form of capital gains, if any, on warrants or other equity-related securities that we acquire from our portfolio companies. Our investments generally range from $12.0 million to $40.0 million, although we may make investments in amounts above or below that range. As of June 30, 2017, our debt investments have a term of between two and seven years and typically bear interest at a rate ranging from approximately 5.8% to approximately 12.0%. In addition to the cash yields received on our debt investments, in some instances, our debt investments may also include any of the following: exit fees, balloon payment fees, commitment fees, success fees, PIK provisions or prepayment fees which may be required to be included in income prior to receipt.
Interest on debt securities is generally payable monthly, with amortization of principal typically occurring over the term of the investment. In addition, our loans may include an interest-only period ranging from three to eighteen months or longer. In limited instances in which we choose to defer amortization of the loan for a period of time from the date of the initial investment, the principal amount of the debt securities and any accrued but unpaid interest become due at the maturity date.
Loan origination and commitment fees received in full at the inception of a loan are deferred and amortized into fee income as an enhancement to the related loan’s yield over the contractual life of the loan. We recognize nonrecurring fees amortized over the remaining term of the loan commencing in the quarter relating to specific loan modifications. We had approximately $35.3 million of unamortized fees at June 30, 2017, of which approximately $31.8 million was included as an offset to the cost basis of our current debt investments and approximately $3.5 million was deferred contingent upon the occurrence of a funding or milestone. At December 31, 2016 we had approximately $38.2 million of unamortized fees, of which approximately $35.8 million was included as an offset to the cost basis of our current debt investments and approximately $2.4 million was deferred contingent upon the occurrence of a funding or milestone.
Loan exit fees to be paid at the termination of the loan are accreted into interest income over the contractual life of the loan. At June 30, 2017 we had approximately $25.5 million in exit fees receivable, of which approximately $22.9 million was included as a component of the cost basis of our current debt investments and approximately $2.6 million was a deferred receivable related to expired commitments. At December 31, 2016 we had approximately $32.8 million in exit fees receivable, of which approximately $30.3 million was included as a component of the cost basis of our current debt investments and approximately $2.5 million was a deferred receivable related to expired commitments.
We have debt investments in our portfolio that contain a PIK provision. The PIK interest, computed at the contractual rate specified in each loan agreement, is recorded as interest income and added to the principal balance of the loan on specified capitalization dates. To maintain our ability to be subject to tax as a RIC, this non-cash source of income must be distributed to stockholders with other sources of income in the form of dividend distributions even though we have not yet collected the cash. Amounts necessary to pay these distributions may come from available cash or the liquidation of certain investments. We recorded approximately $2.5 million and $1.8 million in PIK income in the three months ended June 30, 2017 and 2016, respectively. We recorded approximately $4.7 million and $3.5 million in PIK income in the six months ended June 30, 2017 and 2016, respectively.
The core yield on our debt investments, which excludes the effects of fee and income accelerations attributed to early payoffs, restructuring, loan modifications and other one-time events and includes income from expired commitments, was 12.1% and 13.4% during the three months ended June 30, 2017 and 2016, respectively. The effective yield on our debt investments, which includes the effects of fee and income accelerations attributed to early payoffs, restructuring, loan modifications and other one-time events, was 14.9% and 14.4% for the three months ended June 30, 2017 and 2016, respectively. The effective yield is derived by
71
dividing total investment income by the weighted average earning investment portfolio assets outstanding during the quarter, excluding non-interest earning assets such as warrants and equity investments. Both the core yield and effective yield may be higher than what our common stockholders may realize as the core yield and effective yield do not reflect our expenses and any sales load paid by our common stockholders.
The total return for our investors was approximately -2.0% and 7.2% during the six months ended June 30, 2017 and 2016, respectively. The total return equals the change in the ending market value over the beginning of the period price per share plus dividend distributions paid per share during the period, divided by the beginning price assuming the distribution is reinvested on the date of the distribution. The total return does not reflect any sales load that must be paid by investors. See “Note 9—Financial Highlights” included in the notes to our consolidated financial statements appearing elsewhere in this prospectus.
Portfolio Composition
Our portfolio companies are primarily privately held companies and public companies which are active in the drug discovery & development, software, media/content/info, drug delivery, internet consumer & business services, sustainable and renewable technology, medical devices & equipment, specialty pharmaceuticals, healthcare services, consumer & business products, information services, surgical devices, semiconductors, communications & networking, electronics & computer hardware, biotechnology tools, and diagnostic industry sectors. These sectors are characterized by high margins, high growth rates, consolidation and product and market extension opportunities. Value for companies in these sectors is often vested in intangible assets and intellectual property.
As of June 30, 2017, approximately 76.1% of the fair value of our portfolio was composed of investments in five industries: 31.5% investments in the drug discovery & development industry, 18.2% investments in the software industry, 10.4% investments in the media/content/info industry, 8.8% investments in the drug delivery industry, and 7.2% investments in the internet consumer & business services industry.
The following table shows the fair value of our portfolio by industry sector at June 30, 2017 and December 31, 2016:
| June 30, 2017 | December 31, 2016 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| Drug Discovery & Development |
$ | 440,099 | 31.5 | % | $ | 422,550 | 29.7 | % | ||||||||
| Software |
254,215 | 18.2 | % | 219,559 | 15.4 | % | ||||||||||
| Media/Content/Info |
144,450 | 10.4 | % | 137,567 | 9.7 | % | ||||||||||
| Drug Delivery |
122,952 | 8.8 | % | 109,834 | 7.7 | % | ||||||||||
| Internet Consumer & Business Services |
100,705 | 7.2 | % | 97,047 | 6.8 | % | ||||||||||
| Sustainable and Renewable Technology |
92,609 | 6.6 | % | 154,406 | 10.9 | % | ||||||||||
| Medical Devices & Equipment |
83,933 | 6.0 | % | 107,695 | 7.6 | % | ||||||||||
| Specialty Pharmaceuticals |
38,803 | 2.8 | % | 38,944 | 2.7 | % | ||||||||||
| Healthcare Services, Other |
30,009 | 2.2 | % | 30,200 | 2.1 | % | ||||||||||
| Consumer & Business Products |
22,147 | 1.6 | % | 42,713 | 3.0 | % | ||||||||||
| Information Services |
14,722 | 1.1 | % | 6,091 | 0.4 | % | ||||||||||
| Surgical Devices |
13,660 | 1.0 | % | 12,553 | 0.9 | % | ||||||||||
| Semiconductors |
12,236 | 0.9 | % | 11,326 | 0.8 | % | ||||||||||
| Communications & Networking |
9,932 | 0.7 | % | 18,019 | 1.3 | % | ||||||||||
| Electronics & Computer Hardware |
7,619 | 0.5 | % | 7,664 | 0.5 | % | ||||||||||
| Biotechnology Tools |
6,723 | 0.5 | % | 7,200 | 0.5 | % | ||||||||||
| Diagnostic |
655 | 0.0 | % | 574 | 0.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,395,469 | 100.0 | % | $ | 1,423,942 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
72
Industry and sector concentrations vary as new loans are recorded and loans pay off. Loan revenue, consisting of interest, fees, and recognition of gains on equity and warrants or other equity-related interests, can fluctuate materially when a loan is paid off or a warrant or equity interest is sold. Revenue recognition in any given year can be highly concentrated in several portfolio companies.
For the six months ended June 30, 2017 and the year ended December 31, 2016, our ten largest portfolio companies represented approximately 36.8% and 34.0% of the total fair value of our investments in portfolio companies, respectively. At June 30, 2017 and December 31, 2016, we had six and seven investments, respectively, that represented 5% or more of our net assets. At June 30, 2017, we had six equity investments representing approximately 47.9% of the total fair value of our equity investments, and each represented 5% or more of the total fair value of our equity investments. At December 31, 2016, we had seven equity investments which represented approximately 54.7% of the total fair value of our equity investments, and each represented 5% or more of the total fair value of our equity investments.
As of June 30, 2017 approximately 94.5% of the debt investment portfolio was priced at floating interest rates or floating interest rates with a Prime or LIBOR-based interest rate floor. As a result, we believe we are well positioned to benefit should market interest rates continue to rise.
As of June 30, 2017, 85.9% of our debt investments were in a senior secured first lien position with the remaining 14.1% secured by a senior second priority security interest in all of the portfolio company’s assets, other than intellectual property. In the majority of cases, we collateralize our investments by obtaining a first priority security interest in a portfolio company’s assets, which may include its intellectual property. In other cases, we may obtain a negative pledge covering a company’s intellectual property.
At June 30, 2017, of the approximately 85.9% of our debt investments in a senior secured first lien position, 40.2% were secured by a first priority security in all of the assets of the portfolio company, including its intellectual property, and 45.7% were secured by a first priority security in all of the assets of the portfolio company and the portfolio company was prohibited from pledging or encumbering its intellectual property. At June 30, 2017 we had no equipment only liens on material investments.
Our investments in senior secured debt with warrants have detachable equity enhancement features, typically in the form of warrants or other equity-related securities designed to provide us with an opportunity for capital appreciation. These features are treated as OID and are accreted into interest income over the term of the loan as a yield enhancement. Our warrant coverage generally ranges from 3% to 20% of the principal amount invested in a portfolio company, with a strike price generally equal to the most recent equity financing round. As of June 30, 2017, we held warrants in 135 portfolio companies, with a fair value of approximately $32.5 million. The fair value of our warrant portfolio increased by approximately $5.0 million, as compared to a fair value of $27.5 million at December 31, 2016 primarily related to the addition of warrants in 9 new and 6 existing portfolio companies during the period.
Our existing warrant holdings would require us to invest approximately $91.7 million to exercise such warrants as of June 30, 2017. Warrants may appreciate or depreciate in value depending largely upon the underlying portfolio company’s performance and overall market conditions. Of the warrants that we have monetized since inception, we have realized multiples in the range of approximately 1.02x to 29.22x based on the historical rate of return on our investments. However, our warrants may not appreciate in value and, in fact, may decline in value. Accordingly, we may experience losses from our warrant portfolio.
As required by the 1940 Act, we classify our investments by level of control. “Control investments” are defined in the 1940 Act as investments in those companies that we are deemed to “control”, which, in general, includes a company in which we own 25% or more of the voting securities of such company or have greater than 50% representation on its board. “Affiliate investments” are investments in those companies that are “affiliated
73
companies” of ours, as defined in the 1940 Act, which are not control investments. We are deemed to be an “affiliate” of a company in which we have invested if we own 5% or more, but generally less than 25%, of the voting securities of such company. “Non-control/non-affiliate investments” are investments that are neither control investments nor affiliate investments.
The following table summarizes our realized gains and losses and changes in our unrealized appreciation and depreciation on control and affiliate investments for the three and six months ended June 30, 2017 and 2016.
| (in thousands) | For the Three Months Ended June 30, 2017 | For the Six Months Ended June 30, 2017 | ||||||||||||||||||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at June 30, 2017 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/ (Loss) |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/ (Loss) |
||||||||||||||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||||||||||||||||||
| SkyCross, Inc. |
Control | $ | — | $ | — | $ | (261 | ) | $ | 394 | $ | (394 | ) | $ | — | $ | 1,842 | $ | 394 | $ | (394 | ) | ||||||||||||||||||
| Achilles Technology Management Co II, Inc. |
Control | 2,116 | 78 | (267 | ) | — | — | 152 | (2,208 | ) | — | — | ||||||||||||||||||||||||||||
| HercGamma, Inc. |
Control | 1,169 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Tectura Corporation |
Control | 19,991 | 454 | — | — | — | 899 | — | 51 | (51 | ) | |||||||||||||||||||||||||||||
| Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.) |
Control | 8,288 | — | (53,215 | ) | — | — | — | (53,214 | ) | — | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control Investments |
|
$ | 31,564 | $ | 532 | $ | (53,743 | ) | $ | 394 | $ | (394 | ) | $ | 1,051 | $ | (53,580 | ) | $ | 445 | $ | (445 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | $ | 5,991 | $ | — | $ | 681 | $ | — | $ | — | $ | — | $ | 1,119 | $ | — | $ | — | |||||||||||||||||||||
| Stion Corporation |
Affiliate | — | — | — | — | — | 2 | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Affiliate Investments |
|
$ | 5,991 | $ | — | $ | 681 | $ | — | $ | — | $ | 2 | $ | 1,119 | $ | — | $ | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control & Affiliate Investments |
|
$ | 37,555 | $ | 532 | $ | (53,062 | ) | $ | 394 | $ | (394 | ) | $ | 1,053 | $ | (52,461 | ) | $ | 445 | $ | (445 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| (in thousands) | For the Three Months Ended June 30, 2016 | For the Six Months Ended June 30, 2016 | ||||||||||||||||||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at June 30, 2016 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/ (Loss) |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/ (Loss) |
||||||||||||||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||||||||||||||||||
| SkyCross, Inc. |
Control | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | |||||||||||||||||||
| Achilles Technology Management Co II, Inc. |
Control | 4,000 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control Investments |
|
$ | 4,000 | $ | — | $ | (3,421 | ) | $ | — | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | $ | 4,549 | $ | 6 | $ | (2,972 | ) | $ | — | $ | — | $ | 12 | $ | (3,386 | ) | $ | — | $ | — | |||||||||||||||||||
| Stion Corporation |
Affiliate | 1,295 | 44 | — | 648 | — | 103 | 539 | 648 | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Affiliate Investments |
|
$ | 5,844 | $ | 50 | $ | (2,972 | ) | $ | 648 | $ | — | $ | 115 | $ | (2,847 | ) | $ | 648 | $ | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control & Affiliate Investments |
|
$ | 9,844 | $ | 50 | $ | (6,393 | ) | $ | 648 | $ | — | $ | 115 | $ | (6,268 | ) | $ | 648 | $ | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| (1) | Represents reversals of prior period net unrealized depreciation upon being realized as a loss due to write off. |
In June 2017, we acquired 100% ownership of the equity in HercGamma, Inc. and classified it as a control investment in accordance with the requirements of the 1940 Act. In June 2017, HercGamma, Inc. acquired the assets of a medical device company that develops advanced digital imaging to detect breast cancer, as part of an article 9 consensual foreclosure and public auction for consideration with an estimated fair value of $1.2 million. Our investment in HercGamma, Inc. is carried on the consolidated statement of assets and liabilities at fair value.
74
In April 2017, our investment in Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.) became classified as a control investment as a result of obtaining more than 25% of the portfolio company’s voting securities. In April 2017, under Section 363 of the Bankruptcy Code, Sungevity, Inc. entered into a $50.0 million asset purchase agreement and DIP financing facility with a group of investors, led by Northern Pacific Group and including us. On April 7, 2017, the U.S. Bankruptcy Court approved the DIP financing facility and on April 17, the U.S. Bankruptcy Court approved the asset purchase agreement. On April 26, 2017, Solar Spectrum Holdings LLC, a new company backed by the investment group, announced that it had acquired certain assets of Sungevity, Inc. as part of the bankruptcy court-approved sale. As a result, the cost basis of our debt investment in Sungevity, Inc. was converted to an equity position in Solar Spectrum Holdings LLC and our warrant and equity positions in Sungevity, Inc. were written off.
In January 2017, our investment in Tectura Corporation became classified as a control investment as a result of obtaining more than 50% representation on the portfolio company’s board. In March 2017, our warrants in Tectura Corporation expired and were written off for a realized loss.
In June 2016, our investments in SkyCross, Inc. became classified as a control investment as a result of obtaining more than 50% representation on the portfolio company’s board. In June 2016, we also acquired 100% ownership of the equity of Achilles Technology Management Co II, Inc. and classified it as a control investment in accordance with the requirements of the 1940 Act. In June 2016, Achilles Technology Management Co II, Inc. acquired the assets of a global antenna company that produces radio frequency system solutions as part of an article 9 consensual foreclosure and public auction for total consideration in the amount of $4.0 million. In September and November 2016, we made a $1.0 million and $250,000 debt investment, respectively, in Achilles Technology Management II to provide working capital under the terms of a loan servicing agreement. Our investments in Achilles Technology Management Co II, Inc. are carried on the consolidated statement of assets and liabilities at fair value.
Portfolio Grading
We use an investment grading system, which grades each debt investment on a scale of 1 to 5 to characterize and monitor our expected level of risk on the debt investments in our portfolio with 1 being the highest quality. The following table shows the distribution of our outstanding debt investments on the 1 to 5 investment grading scale at fair value as of June 30, 2017 and December 31, 2016, respectively:
| (in thousands) |
June 30, 2017 | December 31, 2016 | ||||||||||||||||||||||
| Investment Grading |
Number of Companies |
Debt Investments at Fair Value |
Percentage of Total Portfolio |
Number of Companies |
Debt Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||||||||
| 1 |
14 | $ | 267,135 | 20.7 | % | 15 | $ | 275,832 | 20.8 | % | ||||||||||||||
| 2 |
31 | 613,674 | 47.6 | % | 32 | 590,547 | 44.4 | % | ||||||||||||||||
| 3 |
17 | 315,224 | 24.5 | % | 25 | 329,393 | 24.8 | % | ||||||||||||||||
| 4 |
9 | 87,014 | 6.8 | % | 8 | 58,874 | 4.4 | % | ||||||||||||||||
| 5 |
7 | 4,576 | 0.4 | % | 8 | 74,157 | 5.6 | % | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 78 | $ | 1,287,623 | 100.0 | % | 88 | $ | 1,328,803 | 100.0 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
As of June 30, 2017, our debt investments had a weighted average investment grading of 2.27 on a cost basis, as compared to 2.41 at December 31, 2016. Our policy is to lower the grading on our portfolio companies as they approach the point in time when they will require additional equity capital. Additionally, we may downgrade our portfolio companies if they are not meeting our financing criteria or are underperforming relative to their respective business plans. Various companies in our portfolio will require additional funding in the near term or have not met their business plans and therefore have been downgraded until their funding is complete or their operations improve.
75
The improvement in weighted average investment grading at June 30, 2017 from December 31, 2016 is primarily due to the conversion of our debt investment in Sungevity Inc. to an equity position in Solar Spectrum Holdings LLC during the period. This position was rated 5 and represented $44.6 million of the rated 5 debt investment fair value at December 31, 2016.
At June 30, 2017, we had seven debt investments on non-accrual with a cumulative investment cost and fair value of approximately $43.6 million and $3.6 million, respectively. At December 31, 2016, we had five debt investments on non-accrual with cumulative investment cost and fair value of approximately $43.9 million and $6.2 million, respectively. The decrease in the cumulative cost of debt investments on non-accrual between June 30, 2017 and December 31, 2016 is the result of receipt of some proceeds on two investments, offset by placing two new debt investments on non-accrual status during the period. The decrease in the fair value of debt investments on non-accrual between periods is the result of a reduction in expected proceeds for those investments.
Results of Operations
Comparison of the three and six months ended June 30, 2017 and 2016
Investment Income
Total investment income for the three months ended June 30, 2017 was approximately $48.5 million as compared to approximately $43.5 million for the three months ended June 30, 2016. Total investment income for the six months ended June 30, 2017 was approximately $94.8 million as compared to approximately $82.5 million for the six months ended June 30, 2016.
Interest and PIK interest income for the three months ended June 30, 2017 totaled approximately $40.5 million as compared to approximately $39.6 million for the three months ended June 30, 2016. Interest and PIK interest income for the six months ended June 30, 2017 totaled approximately $83.4 million as compared to approximately $76.1 million for the six months ended June 30, 2016. The increase in interest and PIK interest income for the three and six months ended June 30, 2017 as compared to the same periods ended June 30, 2016 is primarily attributable to an increase in recurring interest and PIK interest income, along with an increase in interest accelerations due to early loan repayments and other one-time events.
Of the $40.5 million in interest and PIK interest income for the three months ended June 30, 2017, approximately $37.9 million represents recurring income from the contractual servicing of our loan portfolio and approximately $2.6 million represents income related to the acceleration of income due to early loan repayments and other one-time events during the period. Income from recurring interest and the acceleration of interest income due to early loan repayments represented $37.8 million and $1.8 million, respectively, of the $39.6 million interest and PIK interest income for the three months ended June 30, 2016.
Of the $83.4 million in interest and PIK interest income for the six months ended June 30, 2017, approximately $77.9 million represents recurring income from the contractual servicing of our loan portfolio and approximately $5.5 million represents income related to the acceleration of income due to early loan repayments and other one-time events during the period. Income from recurring interest and the acceleration of interest income due to early loan repayments represented $73.6 million and $2.5 million, respectively, of the $76.1 million interest and PIK interest income for the six months ended June 30, 2016.
76
The following table shows the PIK-related activity for the six months ended June 30, 2017 and 2016, at cost:
| Six Months Ended June 30, |
||||||||
| (in thousands) |
2017 | 2016 | ||||||
| Beginning PIK interest receivable balance |
$ | 9,930 | $ | 5,149 | ||||
| PIK interest income during the period |
4,666 | 3,544 | ||||||
| PIK accrued (capitalized) to principal but not recorded as income during the period |
— | (2,146 | ) | |||||
| Payments received from PIK loans |
(2,031 | ) | (438 | ) | ||||
| Realized loss |
— | (266 | ) | |||||
|
|
|
|
|
|||||
| Ending PIK interest receivable balance |
$ | 12,565 | $ | 5,843 | ||||
|
|
|
|
|
|||||
The increase in PIK interest income during the six months ended June 30, 2017 as compared to the six months ended June 30, 2016 is due to an increase in the weighted average principal outstanding of loans which bear PIK interest. The increase is primarily due to new originations and compounding interest, along with a decrease in the number of PIK loans which paid off during the period.
Fee income for the three months ended June 30, 2017 totaled approximately $7.9 million as compared to approximately $3.9 million for the three months ended June 30, 2016. Fee income for the six months ended June 30, 2017 totaled approximately $11.5 million as compared to approximately $6.4 million for the six months ended June 30, 2016. The increase in fee income for the three and six months ended June 30, 2017 is primarily attributable to an increase in the acceleration of unamortized fees due to early repayments and one-time fees between periods.
Of the $7.9 million in fee income for the three months ended June 30, 2017, approximately $1.4 million represents income from recurring fee amortization and approximately $6.5 million represents income related to the acceleration of unamortized fees due to early repayments and one-time fees for the period. Income from recurring fee amortization and the acceleration of unamortized fees due to early loan repayments represented $2.5 million and $1.4 million, respectively, of the $3.9 million in income for the three months ended June 30, 2016.
Of the $11.5 million in fee income for the six months ended June 30, 2017, approximately $3.6 million represents income from recurring fee amortization and approximately $7.9 million represents income related to the acceleration of unamortized fees due to early repayments and one-time fees for the period. Income from recurring fee amortization and the acceleration of unamortized fees due to early loan repayments represented $4.7 million and $1.7 million, respectively, of the $6.4 million in income for the six months ended June 30, 2016.
In certain investment transactions, we may earn income from advisory services; however, we had no income from advisory services in the three and six months ended June 30, 2017 or 2016.
Operating Expenses
Our operating expenses are comprised of interest and fees on our borrowings, general and administrative expenses and employee compensation and benefits. Our operating expenses totaled approximately $23.2 million and $20.2 million during the three months ended June 30, 2017 and 2016, respectively. Our operating expenses totaled approximately $46.9 million and $39.0 million during the six months ended June 30, 2017 and 2016, respectively.
77
Interest and Fees on our Borrowings
Interest and fees on our borrowings totaled approximately $10.6 million and $8.9 million for the three months ended June 30, 2017 and 2016, respectively, and approximately $23.0 million and $16.9 million during the six months ended June 30, 2017 and 2016, respectively. Interest and fee expense for the three and six months ended June 30, 2017, as compared to June 30, 2016, increased due to a higher weighted average principal balance outstanding on our 2024 Notes and Convertible Notes, offset by a reduction in interest expense on our 2019 Notes which were fully redeemed in February 2017.
We had a weighted average cost of debt, comprised of interest and fees, of approximately 5.5% and 5.8% for the three months ended June 30, 2017 and 2016, respectively, and a weighted average cost of debt of approximately 6.5% and 5.7% for the six months ended June 30, 2017 and 2016, respectively. The decrease in the weighted average cost of debt for the three months ended June 30, 2017 as compared to the same period ended June 30, 2016 is primarily attributable to the full redemption of our 2019 Notes between periods. The increase between the six months ended June 30, 2017 and June 30, 2016 was due to a higher weighted average principal balance outstanding and the one-time non-cash acceleration of unamortized fees due to the redemption of our 2019 Notes in February 2017.
General and Administrative Expenses
General and administrative expenses include legal fees, consulting fees, accounting fees, printer fees, insurance premiums, rent, expenses associated with the workout of underperforming investments and various other expenses. Our general and administrative expenses increased to $4.7 million from $4.4 million for the three months ended June 30, 2017 and 2016. Our general and administrative expenses increased to $8.8 million from $8.0 million for the six months ended June 30, 2017 and 2016. The increase for the three and six months ended June 30, 2017 was primarily attributable to an increase in charitable contributions, workout expenses and excise tax accruals, offset by a reduction in corporate legal and other expenses between periods.
Employee Compensation
Employee compensation and benefits totaled $5.9 million for the three months ended June 30, 2017 as compared to $5.3 million for the three months ended June 30, 2016 and $11.3 million for the six months ended June 30, 2017 as compared to $10.0 million for the six months ended June 30, 2016. The increase for the three and six month comparative periods was primarily due to changes in variable compensation expenses due to company performance objectives.
Employee stock-based compensation totaled $1.9 million for the three months ended June 30, 2017 as compared to $1.6 million for the three months ended June 30, 2016 and $3.7 million for the six months ended June 30, 2017 as compared to $4.2 million for the six months ended June 30, 2016. The increase for the three month comparative period and the decrease between the six month comparative period were primarily related to restricted stock award vesting.
Net Investment Realized Gains and Losses and Net Unrealized Appreciation and Depreciation
Realized gains or losses are measured by the difference between the net proceeds from the repayment or sale and the cost basis of an investment without regard to unrealized appreciation or depreciation previously recognized, and includes investments written off during the period, net of recoveries. Net change in unrealized appreciation or depreciation primarily reflects the change in portfolio investment values during the reporting period, including the reversal of previously recorded unrealized appreciation or depreciation when gains or losses are realized.
78
A summary of realized gains and losses for the three and six months ended June 30, 2017 and 2016 is as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Realized gains |
$ | 5,083 | $ | 1,423 | $ | 11,553 | $ | 4,212 | ||||||||
| Realized losses |
(10,796 | ) | (1,398 | ) | (14,028 | ) | (8,655 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net realized gains (losses) |
$ | (5,713 | ) | $ | 25 | $ | (2,475 | ) | $ | (4,443 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
During the three and six months ended June 30, 2017 we recognized net realized losses of $5.7 million and $2.5 million, respectively. During the three months ended June 30, 2017, we recorded gross realized gains of $5.1 million primarily from the acquisition of our holdings in one portfolio company, IronPlanet, Inc. ($5.1 million). These gains were offset by gross realized losses of $10.8 million primarily from the liquidation or write off of our warrant and equity investments in ten portfolio companies.
During the six months ended June 30, 2017, we recorded gross realized gains of $11.5 million primarily from the sale or acquisition of our holdings in four portfolio companies, including IronPlanet, Inc. ($5.1 million), Box, Inc. ($4.0 million) TPI Composites, Inc. ($1.2 million) and Edge Therapeutics, Inc. ($708,000). These gains were offset by gross realized losses of $14.0 million primarily from the liquidation or write off of our warrant and equity investments in twelve portfolio companies and our debt investment in one portfolio company.
During the three and six months ended June 30, 2016, we recognized net realized gains of $25,000 and net realized losses of $4.4 million, respectively. During the three months ended June 30, 2016, we recorded gross realized gains of $1.4 million primarily from the acquisition of our holdings in one portfolio company, Ping Identity Corporation ($1.3 million). These gains were offset by gross realized losses of $1.4 million primarily from the liquidation or write off of our warrant and equity investments in two portfolio companies.
During the six months ended June 30, 2016, we recorded gross realized gains of $4.2 million primarily from the sale or acquisition of our holdings in three portfolio companies, including Celator Pharmaceuticals, Inc. ($1.5 million), Ping Identity Corporation ($1.3 million) and the sale of options on Box, Inc. ($1.1 million). These gains were partially offset by gross realized losses of $8.6 million primarily from the liquidation or write off of our warrant and equity investments in five portfolio companies and our debt investment in three portfolio companies, including the settlement of our outstanding debt investment in the Neat Company ($6.2 million).
The following table summarizes the change in net unrealized appreciation (depreciation) of investments for the three and six months ended June 30, 2017 and 2016:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Gross unrealized appreciation on portfolio investments |
$ | 68,389 | $ | 16,208 | $ | 87,867 | $ | 29,525 | ||||||||
| Gross unrealized depreciation on portfolio investments |
(61,292 | ) | (30,607 | ) | (109,562 | ) | (55,492 | ) | ||||||||
| Reversal of prior period net unrealized appreciation (depreciation) upon a realization event |
10,634 | (340 | ) | 14,129 | (340 | ) | ||||||||||
| Reversal of prior period net unrealized appreciation (depreciation) upon a realization event |
(4,619 | ) | 1,137 | (10,519 | ) | 11,333 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net unrealized appreciation (depreciation) on debt, equity, and warrant investments |
13,112 | (13,602 | ) | (18,085 | ) | (14,974 | ) | |||||||||
| Other net unrealized appreciation (depreciation) |
475 | (302 | ) | 169 | (264 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total net unrealized depreciation on investments |
$ | 13,587 | $ | (13,904 | ) | $ | (17,916 | ) | $ | (15,238 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
79
During the three months ended June 30, 2017, we recorded approximately $13.6 million of net unrealized appreciation, of which $13.2 million was net unrealized appreciation from our debt, equity and warrant investments. Approximately $50.9 million was attributed to net unrealized appreciation on our debt investments related to the reversal of prior period collateral based impairments of $48.8 million on two portfolio companies, including the reversal of the cumulative unrealized depreciation on our debt investment in Sungevity, Inc. upon its conversion to an equity position in Solar Spectrum Holdings LLC at cost. Approximately $5.2 million was attributed to net unrealized appreciation on our warrant investments primarily due to $3.2 million and $2.7 million of unrealized appreciation on our private and public warrant portfolios, respectively, related to portfolio company and industry performance. This unrealized appreciation is partially offset by approximately $42.9 million of net unrealized depreciation on our equity investments which was primarily due to $53.5 million of collateral based impairment on three portfolio companies, including the impairment of our converted equity position in Solar Spectrum Holdings LLC from cost, slightly offset by the reversal of $6.8 million of prior period net unrealized depreciation upon being realized as a loss on the write off of our equity investment in Sungevity, Inc.
During the three months ended June 30, 2016, we recorded approximately $13.9 million of net unrealized depreciation, of which $13.6 million was net unrealized depreciation from our debt, equity and warrant investments. Approximately $8.0 million was net unrealized depreciation on our debt investments which primarily relates to $14.0 million of unrealized depreciation for collateral based impairments on ten portfolio companies offset by the reversal of $5.7 million unrealized depreciation for the prior period collateral based impairments on four portfolio companies. Approximately $6.3 million was attributed to net unrealized depreciation on our equity investments which primarily relates to $5.3 million unrealized depreciation on our public equity portfolio with the largest concentration in our investment in Box, Inc. and $1.0 million of unrealized depreciation on our private portfolio companies related to portfolio company performance. This unrealized depreciation was offset by $694,000 of net unrealized appreciation on our warrant investments primarily attributed to the reversal of unrealized depreciation upon being realized as a loss due to the liquidation of our warrant investments in two portfolio companies.
The following table summarizes the change in net unrealized appreciation (depreciation) in the investment portfolio by investment type, excluding other net unrealized appreciation (depreciation) for the three months ended June 30, 2017 and 2016:
| Three Months Ended June 30, 2017 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral Based Impairments |
$ | 1.3 | $ | (53.5 | ) | $ | — | $ | (52.2 | ) | ||||||
| Reversals of Prior Period Collateral Based Impairments |
48.8 | — | — | 48.8 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity Sales |
2.5 | 6.8 | (0.7 | ) | 8.6 | |||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
— | 1.0 | 2.7 | 3.7 | ||||||||||||
| Level 3 Assets |
(1.7 | ) | 2.8 | 3.2 | 4.3 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
(1.7 | ) | 3.8 | 5.9 | 8.0 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Appreciation/(Depreciation) |
$ | 50.9 | $ | (42.9 | ) | $ | 5.2 | $ | 13.2 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Three Months Ended June 30, 2016 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral Based Impairments |
$ | (14.0 | ) | $ | — | $ | (0.1 | ) | $ | (14.1 | ) | |||||
| Reversals of Prior Period Collateral Based Impairments |
5.7 | — | — | 5.7 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity Sales |
— | — | 0.8 | 0.8 | ||||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
0.1 | (5.3 | ) | 0.5 | (4.7 | ) | ||||||||||
| Level 3 Assets |
0.2 | (1.0 | ) | (0.5 | ) | (1.3 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
0.3 | (6.3 | ) | — | (6.0 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Appreciation/(Depreciation) |
$ | (8.0 | ) | $ | (6.3 | ) | $ | 0.7 | $ | (13.6 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
80
| * | Level 1 assets are generally equities listed in active markets and level 2 assets are generally warrants held in a public company. Observable market prices are typically the primary input in valuing level 1 and 2 assets. Level 3 asset valuations require inputs that are both significant and unobservable. Generally, level 3 assets are debt investments and warrants and equities held in a private company. See Note 2 to the financial statements discussing ASC Topic 820 (“Fair Value Measurements”). |
During the six months ended June 30, 2017, we recorded approximately $17.9 million of net unrealized depreciation, of which $18.0 million was net unrealized depreciation from our debt, equity and warrant investments. Approximately $45.7 million is attributed to net unrealized depreciation on our equity investments, which primarily relates to $54.4 million of collateral based impairment on five portfolio companies, including impairment on our converted equity position in Solar Spectrum Holdings LLC, offset by $2.2 million and $4.4 million of unrealized appreciation on our public and private equity portfolio, respectively, related to portfolio company and industry performance. This unrealized depreciation was partially offset by approximately $19.7 million of net unrealized appreciation on our debt investments related to the reversal of prior period collateral based impairments of $52.0 million on three portfolio companies, including the reversal of the cumulative unrealized depreciation on our debt investment in Sungevity, Inc. upon its conversion to equity, partially offset by $38.5 million of unrealized depreciation for collateral based impairments on seven portfolio companies. In addition, approximately $8.0 million of net unrealized appreciation on our warrant investments is primarily due to $5.5 million and $3.6 million of unrealized appreciation on our private and public warrant portfolio related to portfolio company and industry performance.
During the six months ended June 30, 2016, we recorded approximately $15.2 million of net unrealized depreciation, of which $14.9 million was net unrealized depreciation from our debt, equity and warrant investments. Approximately $2.0 million was attributed to net unrealized depreciation on our debt investments which was primarily related to $20.6 million unrealized depreciation for collateral based impairments on ten portfolio companies offset by the reversal of $12.2 million unrealized depreciation upon payoff or settling of our debt investments and the reversal of $5.7 million unrealized depreciation for prior period collateral based impairments on four portfolio companies. Approximately $12.5 million was attributed to net unrealized depreciation on our equity investments which primarily relates to $10.5 million unrealized depreciation on our public equity portfolio with the largest concentration in our investment in Box, Inc. and $2.1 million of unrealized depreciation on our private portfolio companies related to portfolio company performance. Approximately $455,000 was attributed to net unrealized depreciation on our warrant investments primarily related to our public warrant portfolio.
The following table summarizes the change in net unrealized appreciation (depreciation) in the investment portfolio by investment type, excluding other net unrealized appreciation (depreciation) for the six months ended June 30, 2017 and 2016:
| Six Months Ended June 30, 2017 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral Based Impairments |
$ | (38.5 | ) | $ | (54.4 | ) | $ | (0.3 | ) | $ | (93.2 | ) | ||||
| Reversals of Prior Period Collateral Based Impairments |
52.0 | — | — | 52.0 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity Sales |
4.8 | 2.1 | (0.8 | ) | 6.1 | |||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
— | 2.2 | 3.6 | 5.8 | ||||||||||||
| Level 3 Assets |
1.4 | 4.4 | 5.5 | 11.3 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
1.4 | 6.6 | 9.1 | 17.1 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Appreciation/(Depreciation) |
$ | 19.7 | $ | (45.7 | ) | $ | 8.0 | $ | (18.0 | ) | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
81
| Six Months Ended June 30, 2016 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral Based Impairments |
$ | (20.6 | ) | $ | — | $ | (0.1 | ) | $ | (20.7 | ) | |||||
| Reversals of Prior Period Collateral Based Impairments |
5.7 | — | — | 5.7 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity Sales |
12.2 | 0.1 | 0.8 | 13.1 | ||||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
— | (10.5 | ) | (0.7 | ) | (11.2 | ) | |||||||||
| Level 3 Assets |
0.7 | (2.1 | ) | (0.4 | ) | (1.8 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
0.7 | (12.6 | ) | (1.1 | ) | (13.0 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Appreciation/(Depreciation) |
$ | (2.0 | ) | $ | (12.5 | ) | $ | (0.4 | ) | $ | (14.9 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Level 1 assets are generally equities listed in active markets and level 2 assets are generally warrants held in a public company. Observable market prices are typically the primary input in valuing level 1 and 2 assets. Level 3 asset valuations require inputs that are both significant and unobservable. Generally, level 3 assets are debt investments and warrants and equities held in a private company. See Note 2 to the financial statements discussing ASC Topic 820 (“Fair Value Measurements”). |
Income and Excise Taxes
We account for income taxes in accordance with the provisions of Topic 740 of the FASB Accounting Standards Codification, as amended (“ASC”), “Income Taxes”, under which income taxes are provided for amounts currently payable and for amounts deferred based upon the estimated future tax effects of differences between the financial statements and tax basis of assets and liabilities given the provisions of the enacted tax law. Valuation allowances may be used to reduce deferred tax assets to the amount likely to be realized. Based upon our previous election and anticipated continued qualification to be subject to taxation as a RIC, we are typically not subject to a material level of federal income taxes. We intend to distribute 100% of our spillover earnings, which consists of ordinary income and long-term capital gains, from our taxable year ended December 31, 2016 to our stockholders in 2017.
Net Change in Net Assets Resulting from Operations and Earnings Per Share
For the three months ended June 30, 2017 and 2016, we had a net increase in net assets resulting from operations of approximately $33.1 million and a net increase in net assets resulting from operations of approximately $9.5 million, respectively. For the six months ended June 30, 2017 and 2016, we had a net increase in net assets resulting from operations of approximately $27.6 million and a net increase in net assets resulting from operations of approximately $23.8 million, respectively.
The basic and fully diluted net change in net assets per common share were $0.40 per share and $0.40 per share, respectively, for the three months ended June 30, 2017 and $0.33 per share and $0.33 per share, respectively, for the six months ended June 30, 2017. Both the basic and fully diluted net change in net assets per common share for the three and six months ended June 30, 2016 were $0.13 per share and $0.32 per share, respectively.
For the purpose of calculating diluted earnings per share for three and six months ended June 30, 2017 and 2016, the effect of the 2022 Convertible Notes, outstanding options, and restricted stock units under the treasury stock method was considered. The effect of the 2022 Convertible Notes was excluded from these calculation for the three and six months ended June 30, 2017 as our share price was less than the conversion price in effect which results in anti-dilution. The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016, as such there was no potential additional dilutive effect for the three and six months ended June 30, 2016.
82
Comparison of periods ended December 31, 2016 and 2015
Investment Income
Interest Income
Total investment income for the year ended December 31, 2016 was approximately $175.1 million as compared to approximately $157.1 million for the year ended December 31, 2015.
Interest income for the year ended December 31, 2016 totaled approximately $158.7 million as compared to approximately $140.3 million for the year ended December 31, 2015. The increase in interest income for the year ended December 31, 2016 as compared to the year ended December 31, 2015 is primarily attributable to debt investment portfolio growth, specifically an increase in the weighted average principal outstanding between the periods, slightly offset by a reduction in the acceleration of income due to early repayments and other one-time events during the period.
Of the $158.7 million in interest income for the year ended December 31, 2016, approximately $152.1 million represents recurring income from the contractual servicing of our loan portfolio and approximately $6.6 million represents income related to the acceleration of income due to early loan repayments and other one-time events during the period. Income from recurring interest and the acceleration of interest income due to early loan repayments represented $130.4 million and $9.9 million, respectively, of the $140.3 million interest income for the year ended December 31, 2015.
The following table shows the PIK-related activity, for the years ended December 31, 2016 and 2015, at cost:
| Year Ended December 31, |
||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Beginning PIK loan balance |
$ | 5,149 | $ | 6,250 | ||||
| PIK interest income during the period |
7,825 | 4,658 | ||||||
| PIK accrued (capitalized) to principal but not recorded as income during the period |
(2,146 | ) | — | |||||
| Payments received from PIK loans |
(632 | ) | (5,483 | ) | ||||
| Realized loss |
(266 | ) | (276 | ) | ||||
|
|
|
|
|
|||||
| Ending PIK loan balance |
$ | 9,930 | $ | 5,149 | ||||
|
|
|
|
|
|||||
The increase in PIK interest income during the year ended December 31, 2016 as compared to the year ended December 31, 2015 is due to overall portfolio growth, or more specifically, an increase in the weighted average principal outstanding for loans which bear PIK interest and a decrease in the number of PIK loans which paid-off during the period. PIK receivable represents less than 1% of total debt investments as of December 31, 2016 and December 31, 2015, respectively
Fee Income
Income from commitment, facility and loan related fees for the year ended December 31, 2016 totaled approximately $16.3 million as compared to approximately $16.9 million for the year ended December 31, 2015. The decrease in fee income is primarily attributable to a decrease in the acceleration of unamortized fees due to early repayments and one-time fees during the period.
Of the $16.3 million in income from commitment, facility and loan related fees for the year ended December 31, 2016, approximately $9.5 million represents income from recurring fee amortization and approximately $6.8 million represents income related to the acceleration of unamortized fees during the period.
83
Income from recurring fee amortization and the acceleration of unamortized fees due to early loan repayments represented $5.8 million and $11.1 million, respectively, of the $16.9 million income for the year ended December 31, 2015.
In certain investment transactions, we may earn income from advisory services; however, we had no income from advisory services in the years ended December 31, 2016 and 2015, respectively.
Operating Expenses
Our operating expenses are comprised of interest and fees on our borrowings, general and administrative expenses and employee compensation and benefits. Operating expenses totaled approximately $82.7 million and $83.6 million during the years ended December 31, 2016 and 2015, respectively.
Interest and Fees on our Borrowings
Interest and fees on our borrowings totaled approximately $37.1 million and $36.9 million for the years ended December 31, 2016 and 2015, respectively. Interest and fee expense for the year ended December 31, 2016 as compared to December 31, 2015 increased primarily due to higher weighted average principal balances outstanding on our 2024 Notes related to the issuance of $149.9 million of aggregate principal during the period. The increase in interest and fee expense incurred related to our 2024 notes was partially offset by principal pay-offs and paydowns on our 2016 Convertible Notes, Asset Backed Notes and Credit Facilities during the period.
We had a weighted average cost of debt, comprised of interest and fees and loss on debt extinguishment (long-term liabilities—convertible notes), of approximately 5.8% and 6.0% for the years ended December 31, 2016 and 2015, respectively. The decrease between comparative periods was primarily driven by a reduction in the weighted average principal outstanding on our higher yielding debt instruments compared to the prior period, specifically due to the full impact of redemptions on our 2019 Notes and 2016 Convertible Notes which occurred in the prior period, offset by the incremental issuance of our 2024 Notes in fiscal year 2016. Note that we redeemed the remaining 2019 Notes in full on February 24, 2017.
General and Administrative Expenses
General and administrative expenses include legal fees, consulting fees, accounting fees, printer fees, insurance premiums, rent, expenses associated with the workout of underperforming investments and various other expenses. Our general and administrative expenses decreased to $16.1 million from $16.7 million for the years ended December 31, 2016 and 2015, respectively. This decrease was primarily attributable to a reduction in costs related to strategic hiring objectives and travel and entertainment, slightly offset by an increase in corporate legal and other expenses.
Employee Compensation
Employee compensation and benefits totaled approximately $22.5 million for the year ended December 31, 2016 as compared to approximately $20.7 million for the year ended December 31, 2015. The increase between comparative periods was primarily due to changes in variable incentive compensation related to the achievement of origination and strategic corporate objectives.
Employee stock-based compensation totaled approximately $7.0 million for the year ended December 31, 2016 as compared to approximately $9.4 million for the year ended December 31, 2015. The decrease between comparative periods was primarily related to the number and amount of restricted stock award vesting, specifically the vesting of retention grants issued in 2014 which occurred in the first half of 2016.
84
Other Income (Loss)
Other income (loss) generally consists of income or losses generated from sources other than our investment portfolio. For the years ended December 31, 2016 and December 31, 2015 it consists of $8.0 million of litigation settlement proceeds and $1,000 of loss on extinguishment of debt, respectively.
Litigation Settlement Proceeds
On December 19, 2016, we entered into a Confidential Settlement Agreement (the “Settlement Agreement”) with all defendants in connection with a litigation matter (“the Action”) filed in November 2014. In connection with the Settlement Agreement, the Action was settled among the parties and the Company received a settlement payment in the amount of $8.0 million. The Settlement Agreement also provides a mutual release by the Company and the defendants of any and all claims and cross-claims that were asserted in the Action, the circumstances and events underlying the Action and attorney’s fees and costs related thereto. The Settlement Agreement does not constitute an admission of liability, fault, or wrongdoing by any party. The settlement payment was classified as a component of net investment income in our Consolidated Statement of Operations.
Loss on Extinguishment of Convertible Notes
Our 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016. Throughout their life, holders of approximately $74.8 million of our 2016 Convertible Notes exercised their conversion rights. These 2016 Convertible Notes were settled with a combination of cash equal to the outstanding principal amount of the 2016 Convertible Notes and approximately 1.6 million shares of our common stock, or $24.3 million.
We recorded a loss on extinguishment of debt for the proportionate amount of unamortized debt issuance costs and OID. The loss was partially offset by a gain in the amount of the difference between the outstanding principal balance of the converted notes and the fair value of the debt instrument. The net loss on extinguishment of debt we recorded for the year ended December 31, 2015 was approximately $1,000. We did not record a loss on extinguishment of debt for the year ended December 31, 2016. The loss on extinguishment of debt was classified as a component of net investment income in our Consolidated Statements of Operations.
Net Investment Realized Gains and Losses and Net Unrealized Appreciation and Depreciation
Realized gains or losses are measured by the difference between the net proceeds from the repayment or sale and the cost basis of an investment without regard to unrealized appreciation or depreciation previously recognized, and includes investments written off during the period, net of recoveries. Net change in unrealized appreciation or depreciation primarily reflects the change in portfolio investment values during the reporting period, including the reversal of previously recorded unrealized appreciation or depreciation when gains or losses are realized.
A summary of realized gains and losses for the years ended December 31, 2016 and 2015 is as follows:
| Year Ended December 31, |
||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Realized gains |
$ | 15,202 | $ | 12,677 | ||||
| Realized losses |
(10,626 | ) | (7,530 | ) | ||||
|
|
|
|
|
|||||
| Net realized gains |
$ | 4,576 | $ | 5,147 | ||||
|
|
|
|
|
|||||
During the year ended December 31, 2016, we recognized net realized gains of approximately $4.6 million on the portfolio. These net realized gains included gross realized gains of approximately $15.2 million, primarily
85
from the sale of investments in six portfolio companies, including Box, Inc. ($9.3 million), Celator Pharmaceuticals, Inc. ($1.5 million), Touchcommerce, Inc. ($1.5 million), Ping Identity Corporation ($1.3 million), ReachLocal ($610,000) and Hillcrest Laboratories, Inc. ($225,000). These gains were partially offset by gross realized losses of approximately $10.6 million, primarily from the liquidation or write off of our warrant and equity investments in eight portfolio companies and our debt investments in five portfolio companies, including the settlement of our outstanding debt investment in The Neat Company ($6.2 million).
During the year ended December 31, 2015, we recognized net realized gains of approximately $5.1 million on the portfolio. These net realized gains included gross realized gains of approximately $12.6 million from the sale of investments in seven portfolio companies, including Box, Inc. ($3.2 million), Atrenta, Inc. ($2.6 million), Cempra, Inc. ($2.0 million), Celladon Corporation ($1.4 million), Egalet Corporation ($652,000), Everyday Health, Inc. ($387,000) and Identiv, Inc. ($304,000) and $1.5 million from subsequent recoveries on two previously written-off debt investments. These gains were partially offset by gross realized losses of approximately $7.5 million primarily from the liquidation or write off of our investments in sixteen portfolio companies.
The net unrealized appreciation and depreciation of our investments is based on the fair value of each investment determined in good faith by our Board of Directors. The following table summarizes the change in net unrealized appreciation/depreciation of investments for the years ended December 31, 2016 and 2015:
| Year Ended December 31, |
||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Gross unrealized appreciation on portfolio investments |
$ | 75,264 | $ | 78,991 | ||||
| Gross unrealized depreciation on portfolio investments |
(115,867 | ) | (111,926 | ) | ||||
| Reversal of prior period net unrealized appreciation upon a realization event |
(8,525 | ) | (8,707 | ) | ||||
| Reversal of prior period net unrealized depreciation upon a realization event |
13,186 | 4,599 | ||||||
| Net unrealized appreciation (depreciation) attributable to taxes payable |
(259 | ) | 1,322 | |||||
| Citigroup warrant participation |
(16 | ) | (11 | ) | ||||
|
|
|
|
|
|||||
| Net unrealized depreciation on portfolio investments |
$ | (36,217 | ) | $ | (35,732 | ) | ||
|
|
|
|
|
|||||
During the year ended December 31, 2016, we recorded approximately $36.2 million of net unrealized depreciation, of which $35.9 million is net unrealized depreciation from our debt, equity and warrant investments. Of the $35.9 million, approximately $14.0 million is attributed to net unrealized depreciation on our debt investments which primarily relates to $50.0 million unrealized depreciation for collateral based impairments on eight portfolio companies, offset by the reversal of prior period collateral based impairments of $17.3 million on six portfolio companies and the reversal of $13.1 million of prior period unrealized depreciation upon payoff or settling of our debt investments. Approximately $22.2 million is attributed to net unrealized depreciation on our equity investments which primarily relates to approximately $7.4 million of unrealized depreciation for collateral based impairments on two portfolio companies, $6.6 million of unrealized depreciation on our public equity portfolio, with the largest concentration in our investment in Box, Inc. and the reversal of $5.4 million of prior period net unrealized appreciation upon being realized as a gain for our sale of shares of Box, Inc. This unrealized depreciation was partially offset by approximately $245,000 of unrealized appreciation on our warrant investments, which primarily related to $4.8 million of unrealized appreciation on our private portfolio companies, offset by $2.9 million unrealized depreciation on our public portfolio companies related to individual portfolio company performance.
Net unrealized depreciation increased by approximately $259,000 as a result of increased estimated taxes payable on investments held in subsidiaries subject to corporate taxes for the year ended December 31, 2016.
Net unrealized depreciation further increased by approximately $16,000 due to net depreciation on the pool of warrants collateralized under the warrant participation agreement and a decrease in the liability for the
86
acquisition proceeds received on our Ping Identity Corporation equity investment, which had been exercised from warrants that were included in the collateral pool, during the year ended December 31, 2016.
During the year ended December 31, 2015, we recorded approximately $35.7 million of net unrealized depreciation, of which $37.1 million is net unrealized depreciation from our debt, equity and warrant investments. Of the $37.1 million, approximately $14.0 million is attributed to net unrealized depreciation on our debt investments which primarily related to $20.4 million unrealized depreciation for collateral based impairments on ten portfolio companies offset by the reversal of collateral based impairments of $5.6 million on three portfolio companies. Approximately $19.1 million is attributed to net unrealized depreciation on our equity investments which primarily related to $11.4 million unrealized depreciation on our public equity portfolio with the largest concentration in our investment in Box, Inc. and the reversal of $7.8 million of prior period net unrealized appreciation upon being realized as a gain for our sale of shares of Box, Inc., Atrenta, Inc., Cempra, Inc. Celladon Corporation, Egalet Corporation, Everyday Health, and Identiv, Inc. as discussed above. Finally, approximately $4.0 million is attributed to net unrealized depreciation on our warrant investments which primarily related to $6.0 million of unrealized depreciation on our private portfolio companies related to declining industry performance offset by the reversal of $3.2 million of prior period net unrealized depreciation upon being realized as a loss on the liquidation of our investments in thirteen portfolio companies.
Net unrealized depreciation was offset by approximately $1.3 million as a result of estimated taxes payable on investments held in subsidiaries subject to corporate taxes for the year ended December 31, 2015.
Net unrealized depreciation increased by approximately $11,000 as a result of appreciation of fair value on the pool of warrants collateralized under the warrant participation agreement offset by a decrease in the liability for the acquisition proceeds we received on our Atrenta, Inc. equity investment, which had been exercised from warrants that were included in the collateral pool.
The following table summarizes the change in net unrealized appreciation (depreciation) in the investment portfolio by investment type, excluding net unrealized appreciation (depreciation) on taxes payable, escrow receivables and Citigroup warrant participation, for the years ended December 31, 2016 and December 31, 2015.
| Year Ended December 31, 2016 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral Based Impairments |
$ | (50.0 | ) | $ | (7.4 | ) | $ | (1.1 | ) | $ | (58.5 | ) | ||||
| Reversals of Prior Period Collateral Based Impairments |
17.3 | — | 0.5 | 17.8 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity Sales |
13.1 | (5.4 | ) | (1.0 | ) | 6.7 | ||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
(1.3 | ) | (6.6 | ) | (2.9 | ) | (10.8 | ) | ||||||||
| Level 3 Assets |
6.9 | (2.8 | ) | 4.8 | 8.9 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
5.6 | (9.4 | ) | 1.9 | (1.9 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Appreciation (Depreciation) |
$ | (14.0 | ) | $ | (22.2 | ) | $ | 0.3 | $ | (35.9 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Year Ended December 31, 2015 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral Based Impairments |
$ | (20.4 | ) | $ | (0.2 | ) | $ | (0.4 | ) | (21.0 | ) | |||||
| Reversals of Prior Period Collateral Based Impairments |
5.6 | — | 0.4 | 6.0 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity Sales |
6.2 | (7.8 | ) | 3.2 | 1.6 | |||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
(1.1 | ) | (11.4 | ) | (1.2 | ) | (13.7 | ) | ||||||||
| Level 3 Assets |
(4.3 | ) | 0.3 | (6.0 | ) | (10.0 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
(5.4 | ) | (11.1 | ) | (7.2 | ) | (23.7 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Depreciation |
$ | (14.0 | ) | $ | (19.1 | ) | $ | (4.0 | ) | $ | (37.1 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
87
| * | Level 1 assets are generally equities listed in active markets and Level 2 assets are generally warrants held in a public company. Observable market prices are typically the primary input in valuing Level 1 and 2 assets. Level 3 asset valuations require inputs that are both significant and unobservable. Generally, level 3 assets are debt investments and warrants and equities held in a private company. See Note 2 to the financial statements discussing ASC 820. |
Income and Excise Taxes
We account for income taxes in accordance with the provisions of ASC Topic 740, Income Taxes, under which income taxes are provided for amounts currently payable and for amounts deferred based upon the estimated future tax effects of differences between the financial statements carrying amounts and tax basis of assets and liabilities based upon the provisions of currently enacted tax law. Valuation allowances may be used to reduce deferred tax assets to the amount likely to be realized. Based upon our previous election and anticipated continued qualification to be subject to taxation as a RIC, we are typically not subject to a material level of U.S. federal income taxes. We intend to distribute 100% of our spillover earnings, which consists of ordinary income and long term capital gains, from our taxable year ended December 31, 2016 to our shareholders during 2017.
Net Increase in Net Assets Resulting from Operations and Earnings Per Share
For the years ended December 31, 2016 and 2015, the net increase in net assets resulting from operations totaled approximately $68.7 million and approximately $42.9 million, respectively. These changes are made up of the items previously described.
The basic and fully diluted net change in net assets per common share for the year ended December 31, 2016 was $0.91, whereas the basic and fully diluted net change in net assets per common share for the year ended December 31, 2015 were $0.60 and $0.59, respectively.
For the purpose of calculating diluted earnings per share for year ended December 31, 2015, the dilutive effect of the 2016 Convertible Notes under the treasury stock method is included in this calculation as our share price was greater than the conversion price in effect ( $11.03 as of December 31, 2015) for the 2016 Convertible Notes for such period. The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016, as such, there is no potential additional dilutive effect for the year ended December 31, 2016.
Comparison of periods ended December 31, 2015 and 2014
Investment Income
Interest Income
Total investment income for the year ended December 31, 2015 was approximately $157.1 million as compared to approximately $143.7 million for the year ended December 31, 2014.
Interest income for the year ended December 31, 2015 totaled approximately $140.3 million as compared to approximately $126.6 million for the year ended December 31, 2014. The increase in interest income for the year ended December 31, 2015 as compared to the year ended December 31, 2014 is primarily attributable to debt investment portfolio growth, specifically an increase in the weighted average principal outstanding between the periods.
Of the $140.3 million in interest income for the year ended December 31, 2015, approximately $130.4 million represents recurring income from the contractual servicing of our loan portfolio and approximately $9.9 million represents income related to the acceleration of income due to early loan repayments and other one-time events during the period. Income from recurring interest and the acceleration of interest income due to early loan repayments represented $106.8 million and $19.8 million, respectively, of the $126.6 million interest income for the year ended December 31, 2014.
88
The following table shows the PIK-related activity, for the years ended December 31, 2015 and 2014, at cost:
| Year Ended December 31, |
||||||||
| (in thousands) |
2015 | 2014 | ||||||
| Beginning PIK loan balance |
$ | 6,250 | $ | 5,603 | ||||
| PIK interest income during the period |
4,658 | 3,346 | ||||||
| Payments received from PIK loans |
(5,483 | ) | (2,699 | ) | ||||
| Realized loss |
(276 | ) | — | |||||
|
|
|
|
|
|||||
| Ending PIK loan balance |
$ | 5,149 | $ | 6,250 | ||||
|
|
|
|
|
|||||
The increase in payments received from PIK loans and the increase in PIK interest capitalized during the year ended December 31, 2015 as compared to the year ended December 31, 2014 is due to an increase in the weighted average principal outstanding for loans which bear PIK interest and the number of PIK loans which paid-off during the period.
Fee Income
Income from commitment, facility and loan related fees for the year ended December 31, 2015 totaled approximately $16.9 million as compared to approximately $17.0 million for the year ended December 31, 2014. The decrease in fee income is primarily attributable to the acceleration of early loan repayments and restructures, slightly offset by an increase in normal fee amortization due to a higher weighted average debt investment portfolio outstanding during the period.
Of the $16.9 million in income from commitment, facility and loan related fees for the year ended December 31, 2015, approximately $5.8 million represents income from recurring fee amortization and approximately $11.1 million represents income related to the acceleration of unamortized fees for the period. Income from recurring fee amortization and the acceleration of unamortized fees due to early loan repayments represented $5.2 million and $11.8 million, respectively, of the $17.0 million income for the year ended December 31, 2014.
In certain investment transactions, we may earn income from advisory services; however, we had no income from advisory services in the years ended December 31, 2015 and 2014, respectively.
Operating Expenses
Our operating expenses are comprised of interest and fees on our borrowings, general and administrative expenses and employee compensation and benefits. Operating expenses totaled approximately $83.6 million and $70.3 million during the years ended December 31, 2015 and 2014, respectively.
Interest and Fees on our Borrowings
Interest and fees on our borrowings totaled approximately $36.9 million and $34.0 million for the years ended December 31, 2015 and 2014, respectively. Interest and fee expense for the year ended December 31, 2015 as compared to December 31, 2014 increased primarily due to higher weighted average principal balances outstanding on our Asset Backed Notes, Credit Facilities, 2019 Notes and 2024 Notes (together with the 2019 Notes, the “Baby Bonds”), slightly offset by a reduction in weighted average principal balances outstanding on our SBA debentures, 2016 Convertible Notes and lower debt issuance cost amortization related to our 2016 Convertible Notes and Asset Backed Notes.
We had a weighted average cost of debt, comprised of interest and fees and loss on debt extinguishment (long-term liabilities—convertible notes), of approximately 6.0% and 6.6% for the years ended December 31,
89
2015 and 2014, respectively. The decrease between comparative periods was primarily driven by a reduction in the weighted average principal outstanding on our higher yielding debt instruments and a reduction in non-cash acceleration of debt issuance costs related to our SBA debentures, 2016 Convertible Notes and Asset Backed Notes as compared to the prior period, slightly offset by non-cash accelerations of debt issuance costs due to early pay downs on our Baby Bonds.
General and Administrative Expenses
General and administrative expenses include legal fees, consulting fees, accounting fees, printer fees, insurance premiums, rent, expenses associated with the workout of underperforming investments and various other expenses. Our general and administrative expenses increased to $16.7 million from $10.2 million for the years ended December 31, 2015 and 2014, respectively. This increase was primarily due to increased recruiting costs related to strategic hiring objectives, corporate legal expenses and outside consulting services.
Employee Compensation
Employee compensation and benefits totaled approximately $20.7 million for the year ended December 31, 2015 as compared to approximately $16.6 million for the year ended December 31, 2014. The increase between comparative periods was primarily due to changes in variable incentive compensation.
Employee stock-based compensation totaled approximately $9.4 million for the year ended December 31, 2015 as compared to approximately $9.6 million for the year ended December 31, 2014. The decrease between comparative periods was primarily due to new grants issued related to incentive compensation and strategic hiring objectives, slightly offset by vesting and forfeitures.
Loss on Extinguishment of Convertible Notes
Upon meeting the stock trading price conversion requirement during the three months ended June 30, 2014, September 30, 2014 and December 31, 2014, the 2016 Convertible Notes became convertible on July 1, 2014 and continued to be convertible during each of the three months ended September 30, 2014, December 31, 2014 and March 31, 2015, respectively. During this period and as of December 31, 2015, holders of approximately $57.4 million of our 2016 Convertible Notes have exercised their conversion rights and these 2016 Convertible Notes were settled with a combination of cash equal to the outstanding principal amount of the 2016 Convertible Notes and approximately 1.5 million shares of our common stock, or $24.3 million.
We recorded a loss on extinguishment of debt for the proportionate amount of unamortized debt issuance costs and OID. The loss was partially offset by a gain in the amount of the difference between the outstanding principal balance of the converted notes and the fair value of the debt instrument. The net loss on extinguishment of debt we recorded for the years ended December 31, 2015 and 2014 was approximately $1,000 and $1.6 million, respectively. The loss on extinguishment of debt was classified as a component of net investment income in our Consolidated Statements of Operations.
Net Investment Realized Gains and Losses and Net Unrealized Appreciation and Depreciation
Realized gains or losses are measured by the difference between the net proceeds from the repayment or sale and the cost basis of an investment without regard to unrealized appreciation or depreciation previously recognized, and includes investments written off during the period, net of recoveries. Net change in unrealized appreciation or depreciation primarily reflects the change in portfolio investment values during the reporting period, including the reversal of previously recorded unrealized appreciation or depreciation when gains or losses are realized.
90
A summary of realized gains and losses for the years ended December 31, 2015 and 2014 is as follows:
| Year Ended December 31, |
||||||||
| (in thousands) |
2015 | 2014 | ||||||
| Realized gains |
$ | 12,677 | $ | 24,027 | ||||
| Realized losses |
(7,530 | ) | (3,915 | ) | ||||
|
|
|
|
|
|||||
| Net realized gains |
$ | 5,147 | $ | 20,112 | ||||
|
|
|
|
|
|||||
During the year ended December 31, 2015, we recognized net realized gains of approximately $5.1 million on the portfolio. These net realized gains included gross realized gains of approximately $12.6 million from the sale of investments in seven portfolio companies, including Box, Inc. ($3.2 million), Atrenta, Inc. ($2.6 million), Cempra, Inc. ($2.0 million), Celladon Corporation ($1.4 million), Egalet Corporation ($652,000), Everyday Health, Inc. ($387,000) and Identiv, Inc. ($304,000), and $1.5 million from subsequent recoveries received on two previously written-off debt investments. These gains were partially offset by gross realized losses of approximately $7.5 million primarily from the liquidation or write off of our investments in sixteen portfolio companies.
During the year ended December 31, 2014, we recognized net realized gains of approximately $20.1 million on the portfolio. These net realized gains included gross realized gains of approximately $24.0 million primarily from the sale of investments in seven portfolio companies including Acceleron Pharma, Inc., ($7.9 million), Merrimack Pharmaceuticals, Inc., ($4.3 million), Neuralstem, Inc., ($2.7 million), IPA Holdings, LLC., ($1.5 million), Cell Therapeutics, Inc., ($1.3 million), Trulia, Inc. ($1.0 million), and Portola Pharmaceuticals, Inc. ($700,000). These gains were partially offset by gross realized losses of approximately $3.9 million primarily from the liquidation of our investments in fifteen portfolio companies.
The net unrealized appreciation and depreciation of our investments is based on the fair value of each investment determined in good faith by our Board of Directors. The following table summarizes the change in net unrealized appreciation/depreciation of investments for the years ended December 31, 2015 and 2014:
| Year Ended December 31, |
||||||||
| (in thousands) |
2015 | 2014 | ||||||
| Gross unrealized appreciation on portfolio investments |
$ | 78,991 | $ | 72,968 | ||||
| Gross unrealized depreciation on portfolio investments |
(111,926 | ) | (79,412 | ) | ||||
| Reversal of prior period net unrealized appreciation upon a realization event |
(8,707 | ) | (15,335 | ) | ||||
| Reversal of prior period net unrealized depreciation upon a realization event |
4,599 | 3,182 | ||||||
| Net unrealized appreciation (depreciation) attributable to taxes payable |
1,322 | (1,882 | ) | |||||
| Net unrealized depreciation on escrow receivables |
— | (465 | ) | |||||
| Citigroup warrant participation |
(11 | ) | 270 | |||||
|
|
|
|
|
|||||
| Net unrealized depreciation on portfolio investments |
$ | (35,732 | ) | $ | (20,674 | ) | ||
|
|
|
|
|
|||||
During the year ended December 31, 2015, we recorded approximately $35.7 million of net unrealized depreciation, of which $37.1 million is net unrealized depreciation from our debt, equity and warrant investments. Of the $37.1 million, approximately $14.0 million is attributed to net unrealized depreciation on our debt investments which primarily related to $20.4 million unrealized depreciation for collateral based impairments on ten portfolio companies offset by the reversal of collateral based impairments of $5.6 million on three portfolio companies. Approximately $19.1 million is attributed to net unrealized depreciation on our equity investments which primarily relates to approximately $11.4 million unrealized depreciation on our public equity portfolio with the largest concentration in our investment in Box, Inc. and the reversal of $7.8 million of prior period net unrealized appreciation upon being realized as a gain for our sale of shares of Box, Inc., Atrenta, Inc.,
91
Cempra, Inc. Celladon Corporation, Egalet Corporation, Everyday Health, and Identiv, Inc. as discussed above. Finally, approximately $4.0 million is attributed to net unrealized depreciation on our warrant investments which primarily related to $6.0 million of unrealized depreciation on our private portfolio companies related to declining industry performance offset by the reversal of $3.2 million of prior period net unrealized depreciation upon being realized as a loss on the liquidation of our investments in thirteen portfolio companies.
Net unrealized depreciation was offset by approximately $1.3 million as a result of decreased estimated taxes payable on investments held in subsidiaries subject to corporate taxes for the year ended December 31, 2015.
Net unrealized depreciation increased by approximately $11,000 due to appreciation of fair value on the pool of warrants collateralized under the warrant participation agreement offset by a decrease in the liability for the acquisition proceeds we received on our Atrenta, Inc. equity investment, which had been exercised from warrants that were included in the collateral pool.
During the year ended December 31, 2014, we recorded approximately $20.7 million of net unrealized depreciation, of which $18.6 million is net unrealized depreciation from our debt, equity and warrant investments. Of the $18.6 million, approximately $14.2 million is attributed to net unrealized depreciation on our debt investments which primarily related to $23.2 million unrealized depreciation for collateral based impairments on 12 portfolio companies offset by the reversal of collateral based impairments of $4.1 million on two portfolio companies. Approximately $15.8 million is attributed to net unrealized depreciation on our warrant investments which primarily related to $8.3 million of net unrealized depreciation due to the exercise of our warrants in Box, Inc. to equity and $2.4 million of net unrealized depreciation due to the reversal of prior period net unrealized appreciation upon being realized as a gain. This unrealized depreciation was offset by approximately $11.4 million attributed to net unrealized appreciation on our equity investments, including approximately $13.0 million of net unrealized appreciation on Box, Inc., including the exercise of our remaining warrants in Box, Inc. to equity and approximately $7.7 million of net unrealized appreciation on our public equity portfolio. This was offset by approximately $12.7 million unrealized depreciation due to reversal of prior period net unrealized appreciation upon being realized as a gain.
Net unrealized appreciation decreased by approximately $1.9 million as a result of estimated taxes payable on investments held in subsidiaries subject to corporate taxes for the year ended December 31, 2014.
Net unrealized appreciation further decreased by approximately $465,000 as a result of reducing escrow receivables for the year ended December 31, 2014 related to M&A transactions closed on former portfolio companies.
During the year ended December 31, 2014, net unrealized depreciation was offset by approximately $270,000 due to net depreciation of fair value on the pool of warrants collateralized under the Citigroup warrant participation agreement as a result of the sale of shares in Acceleron Pharma, Inc., Merrimack Pharmaceuticals, Inc., Portola Pharmaceuticals, Inc. and Everyday Health, Inc. that were subject to the Citigroup warrant participation agreement.
92
The following table summarizes the change in net unrealized appreciation/(depreciation) in the investment portfolio by investment type, excluding net unrealized appreciation (depreciation) on taxes payable, escrow receivables and Citigroup warrant participation, for the years ended December 31, 2015 and December 31, 2014:
| Year Ended December 31, 2015 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral based impairments |
$ | (20.4 | ) | $ | (0.2 | ) | $ | (0.4 | ) | $ | (21.0 | ) | ||||
| Reversals of Prior Period Collateral based impairments |
5.6 | — | 0.4 | 6.0 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity sales |
6.2 | (7.8 | ) | 3.2 | 1.6 | |||||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
(1.1 | ) | (11.4 | ) | (1.2 | ) | (13.7 | ) | ||||||||
| Level 3 Assets |
(4.3 | ) | 0.3 | (6.0 | ) | (10.0 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
(5.4 | ) | (11.1 | ) | (7.2 | ) | (23.7 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Depreciation |
$ | (14.0 | ) | $ | (19.1 | ) | $ | (4.0 | ) | $ | (37.1 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Year Ended December 31, 2014 | ||||||||||||||||
| (in millions) |
Debt | Equity | Warrants | Total | ||||||||||||
| Collateral based impairments |
$ | (23.2 | ) | $ | (1.2 | ) | $ | (3.3 | ) | (27.7 | ) | |||||
| Reversals of Prior Period Collateral based impairments |
4.1 | 0.6 | — | 4.7 | ||||||||||||
| Reversals due to Debt Payoffs & Warrant/Equity sales |
— | (11.1 | ) | (9.7 | ) | (20.8 | ) | |||||||||
| Fair Value Market/Yield Adjustments* |
||||||||||||||||
| Level 1 & 2 Assets |
— | 7.6 | (2.9 | ) | 4.7 | |||||||||||
| Level 3 Assets |
4.9 | 15.5 | 0.1 | 20.5 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Fair Value Market/Yield Adjustments |
4.9 | 23.1 | (2.8 | ) | 25.2 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Unrealized Appreciation (Depreciation) |
$ | (14.2 | ) | $ | 11.4 | $ | (15.8 | ) | $ | (18.6 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Level 1 assets are generally equities listed in active markets and Level 2 assets are generally warrants held in a public company. Observable market prices are typically the primary input in valuing Level 1 and 2 assets. Level 3 asset valuations require inputs that are both significant and unobservable. Generally, level 3 assets are debt investments and warrants and equities held in a private company. See Note 2 to the financial statements discussing ASC Topic 820. |
Income and Excise Taxes
We account for income taxes in accordance with the applicable provisions of ASC Topic 740, Income Taxes, under which income taxes are provided for amounts currently payable and for amounts deferred based upon the estimated future tax effects of differences between the financial statement carrying amounts and tax basis of assets and liabilities based upon the provisions of currently enacted tax law. Valuation allowances may be used to reduce deferred tax assets to the amount likely to be realized. Based upon our qualification and election to be subject to taxation as a RIC, we are typically not subject to a material level of U.S. federal income taxes. We distributed 100% of our spillover earnings from ordinary income for our taxable year ended December 31, 2015 to our stockholders during 2016.
Net Increase in Net Assets Resulting from Operations and Earnings Per Share
For the years ended December 31, 2015 and 2014, the net increase in net assets resulting from operations totaled approximately $42.9 million and approximately $71.2 million, respectively. These changes are made up of the items previously described.
The basic and fully diluted net change in net assets per common share for the year ended December 31, 2015 were $0.60 and $0.59, respectively, whereas the basic and fully diluted net change in net assets per common share for the year ended December 31, 2014 was $1.12 and $1.10, respectively.
93
For the purpose of calculating diluted earnings per share for years ended December 31, 2015 and 2014, the dilutive effect of the 2016 Convertible Notes under the treasury stock method is included in this calculation as our share price was greater than the conversion price of $11.03 in effect as of December 31, 2015 and $11.36 as of December 31, 2014 for the 2016 Convertible Notes for such periods.
Financial Condition, Liquidity, and Capital Resources
Our liquidity and capital resources are derived from our Credit Facilities, SBA debentures, 2024 Notes, 2021 Asset-Backed Notes, 2022 Convertible Notes and cash flows from operations, including investment sales and repayments, and income earned. Our primary use of funds from operations includes investments in portfolio companies and payments of fees and other operating expenses we incur. We have used, and expect to continue to use, our borrowings and the proceeds from the turnover of our portfolio and from public and private offerings of securities to finance our investment objectives. We may also raise additional equity or debt capital through registered offerings off a shelf registration, ATM and private offerings of securities, by securitizing a portion of our investments, or by borrowing from the SBA through our SBIC subsidiaries.
On August 16, 2013, we entered into an ATM equity distribution agreement (the “Equity Distribution Agreement”) with JMP Securities LLC (“JMP”). On March 7, 2016, we renewed the Equity Distribution Agreement and on December 21, 2016, we further amended the agreement to increase the total shares available under the program. The Equity Distribution Agreement, as amended, provides that we may offer and sell up to 12.0 million shares of our common stock from time to time through JMP, as our sales agent. Sales of our common stock, if any, may be made in negotiated transactions or transactions that are deemed to be “at the market,” as defined in Rule 415 under the Securities Act including sales made directly on the NYSE or similar securities exchange or sales made to or through a market maker other than on an exchange, at prices related to the prevailing market prices or at negotiated prices.
During the six months ended June 30, 2017 we sold 3.3 million shares of common stock for total accumulated net proceeds of approximately $46.9 million, including $532,000 of offering expenses. We did not sell any shares under the program during the three months ended June 30, 2017. During the three and six months ended June 30, 2016 we sold 1.0 million and 2.1 million shares of common stock for total accumulated net proceeds of approximately $11.3 million and $23.7 million, respectively, including $420,000 and $822,000 of offering expenses, respectively. We generally use the net proceeds from these offerings to make investments, repurchase or pay down liabilities and for general corporate purposes. As of June 30, 2017, approximately 751,000 shares remained available for issuance and sale under the ATM program.
On August 27, 2015, our Board of Directors authorized a stock repurchase plan permitting us to repurchase up to $50.0 million of our common stock until August 23, 2016, after which the plan expired. In January 2016, we repurchased 449,588 shares of our common stock at an average price per share of $10.64 per share and a total cost of approximately $4.8 million.
Our 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016. Throughout the life of the 2016 Convertible Notes, holders of approximately $74.8 million of our 2016 Convertible Notes exercised their conversion rights. These 2016 Convertible Notes were settled with a combination of cash equal to the outstanding principal amount of the converted notes and approximately 1.6 million shares of our common stock, or $24.3 million.
On May 2, 2016, we closed an underwritten public offering of an additional $72.9 million in aggregate principal amount of our 6.25% unsecured notes due 2024 (the “2024 Notes”). The $72.9 million in aggregate principal amount includes $65.4 million from the initial offering on April 21, 2016 and $7.5 million as a result of underwriters exercising a portion of their option to cover overallotments on April 29, 2016. On June 27, 2016, we closed an underwritten public offering of an additional $60.0 million in aggregate principal amount of the 2024 Notes. On June 30, 2016, the underwriters exercised their option to purchase up to an additional $9.0 million in aggregate principal to cover overallotments, resulting in total aggregate principal of $69.0 million from the
94
offering. The 2024 Notes rank equally in right of payment and form a single series of notes. We intend to invest the net proceeds of these public offerings to fund investments in debt and equity securities in accordance with its investment objective and for other general corporate purposes.
On May 5, 2016, we, through a special purpose wholly-owned subsidiary, Hercules Funding III, as borrower, entered into the Union Bank Facility with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Union Bank Facility from time to time. The Union Bank Facility replaced our credit facility (the “Prior Union Bank Facility”) entered into on August 14, 2014 (as amended and restated from time to time) with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Prior Union Bank Facility from time to time. Any references to amounts related to the Union Bank Facility prior to May 5, 2016 were incurred and relate to the Prior Union Bank Facility.
On October 11, 2016, we entered into a debt distribution agreement with FBR Capital Markets & Co. (“FBR”), pursuant to which we may offer for sale, from time to time, up to $150.0 million in aggregate principal amount of 2024 Notes through FBR acting as our sales agent. Sales of the 2024 Notes, if any, may be made in negotiated transactions or transactions that are deemed to be “at the market offerings” as defined in Rule 415 under the Securities Act, including sales made directly on the NYSE, or similar securities exchange or sales made through a market maker other than on an exchange at prices related to prevailing market prices or at negotiated prices.
During the six months ended June 30, 2017, we sold 225,457 notes for approximately $5.6 million in aggregate principal amount. We did not sell any notes under the program during the three months ended June 30, 2017. During the year ended December 31, 2016, we sold 317,125 notes for approximately $7.9 million in aggregate principal amount. As of June 30, 2017, approximately $136.4 million in aggregate principal amount remains available for issuance and sale under the debt distribution agreement.
On January 25, 2017, we issued $230.0 million in aggregate principal amount of 2022 Convertible Notes, which amount includes the additional $30.0 million aggregate principal amount issued pursuant to the initial purchaser’s exercise in full of its overallotment option. The sale generated net proceeds of approximately $225.7 million, including $4.3 million of debt issuance costs. Aggregate issuances costs include the initial purchaser’s discount of approximately $5.2 million, offset by the reimbursement of $1.2 million by the initial purchaser. We intend to use the net proceeds from this offering (i) to repurchase or otherwise redeem all of our 2019 Notes, (ii) to fund investments in debt and equity securities in accordance with our investment objective and (iii) for working capital and other general corporate purposes.
On February 24, 2017, we redeemed the $110.4 million remaining outstanding balance of our 2019 Notes in full.
At June 30, 2017, we had $230.0 million of 2022 Convertible Notes, $258.5 million of 2024 Notes, $87.7 million of 2021 Asset-Backed Notes, and $190.2 million of SBA debentures payable. We had no borrowings outstanding under the Wells Facility or the Union Bank Facility.
At June 30, 2017, we had $355.4 million in available liquidity, including $160.4 million in cash and cash equivalents. We had available borrowing capacity of $120.0 million under the Wells Facility and $75.0 million under the Union Bank Facility, both subject to existing terms and advance rates and regulatory requirements. We primarily invest cash on hand in interest bearing deposit accounts.
At June 30, 2017, we had $118.5 million of capital outstanding in restricted accounts related to our SBIC that we may use to fund new investments in the SBIC. With our net investments of $44.0 million and $74.5 million in HT II and HT III, respectively, we have the combined capacity to issue a total of $190.2 million of SBA guaranteed debentures, subject to SBA approval. At June 30, 2017, we have issued $190.2 million in SBA guaranteed debentures in our SBIC subsidiaries.
95
At June 30, 2017, we had approximately $17.2 million of restricted cash, which consists of collections of interest and principal payments on assets that are securitized. In accordance with the terms of the related securitized 2021 Asset-Backed Notes, based on current characteristics of the securitized debt investment portfolios, the restricted funds may be used to pay monthly interest and principal on the securitized debt and are not distributed to us or available for our general operations.
During the six months ended June 30, 2017, we principally funded our operations from (i) cash receipts from interest, dividend and fee income from our investment portfolio and (ii) cash proceeds from the realization of portfolio investments through the repayments of debt investments and the sale of debt and equity investments.
During the six months ended June 30, 2017, our operating activities provided $67.6 million of cash and cash equivalents, compared to $81.9 million used during the six months ended June 30, 2016. This $149.5 million increase in cash provided by operating activities is primarily related to an increase in investment repayments of $128.2 million and an increase in proceeds from the sale of investments of $12.4 million, partially offset by an increase in investment purchases of $9.9 million.
During the six months ended June 30, 2017, our investing activities used approximately $9.0 million of cash, compared to $5.4 million provided during the six months ended June 30, 2016. This $14.4 million increase in cash used in investing activities was primarily due to an increase of approximately $14.5 million in cash, classified as restricted cash, on assets that are securitized.
During the six months ended June 30, 2017, our financing activities provided $88.8 million of cash, compared to $41.0 million provided during the six months ended June 30, 2016. The $47.8 million increase in cash provided by financing activities was primarily due to the net issuance of $225.7 million of the 2022 Convertible Notes, offset by the repayment of $110.4 million of 2019 Notes and the decrease in 2024 Notes issuance during the six months ended June 30, 2017.
As of June 30, 2017, net assets totaled $817.5 million, with a NAV per share of $9.87. We intend to continue to operate in order to generate cash flows from operations, including income earned from investments in our portfolio companies. Our primary use of funds will be investments in portfolio companies and cash distributions to holders of our common stock.
As required by the 1940 Act, our asset coverage must be at least 200% after each issuance of senior securities. As of June 30, 2017 our asset coverage ratio under our regulatory requirements as a business development company was 241.9% excluding our SBA debentures as a result of our exemptive order from the SEC that allows us to exclude all SBA leverage from our asset coverage ratio. As a result of the SEC exemptive order, our ratio of total assets on a consolidated basis to outstanding indebtedness may be less than 200%, which while providing increased investment flexibility, also may increase our exposure to risks associated with leverage. Total asset coverage ratio when including our SBA debentures was 206.7% at June 30, 2017.
96
Outstanding Borrowings
At June 30, 2017 and December 31, 2016, we had the following available borrowings and outstanding amounts:
| June 30, 2017 | December 31, 2016 | |||||||||||||||||||||||
| (in thousands) |
Total Available |
Principal | Carrying Value(1) |
Total Available |
Principal | Carrying Value(1) |
||||||||||||||||||
| SBA Debentures(2) |
$ | 190,200 | $ | 190,200 | $ | 187,824 | $ | 190,200 | $ | 190,200 | $ | 187,501 | ||||||||||||
| 2019 Notes(3) |
— | — | — | 110,364 | 110,364 | 108,818 | ||||||||||||||||||
| 2024 Notes |
258,510 | 258,510 | 251,478 | 252,873 | 252,873 | 245,490 | ||||||||||||||||||
| 2021 Asset-Backed Notes |
87,678 | 87,678 | 86,865 | 109,205 | 109,205 | 107,972 | ||||||||||||||||||
| 2022 Convertible Notes |
230,000 | 230,000 | 222,898 | — | — | — | ||||||||||||||||||
| Wells Facility(4) |
120,000 | — | — | 120,000 | 5,016 | 5,016 | ||||||||||||||||||
| Union Bank Facility(4) |
75,000 | — | — | 75,000 | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 961,388 | $ | 766,388 | $ | 749,065 | $ | 857,642 | $ | 667,658 | $ | 654,797 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | Except for the Wells Facility and Union Bank Facility, all carrying values represent the principal amount outstanding less the remaining unamortized debt issuance costs and unaccreted discount, if any, associated with the loan as of the balance sheet date. See below for the amount of debt issuance cost associated with each borrowing. |
| (2) | At both June 30, 2017 and December 31, 2016, the total available borrowings under the SBA debentures were $190.2 million, of which $41.2 million was available in HT II and $149.0 million was available in HT III. |
| (3) | The 2019 Notes were redeemed in full on February 24, 2017. |
| (4) | Availability subject to us meeting the borrowing base requirements. |
Debt issuance costs are fees and other direct incremental costs we incur in obtaining debt financing and are recognized as prepaid expenses and amortized over the life of the related debt instrument using the effective yield method or the straight line method, which closely approximates the effective yield method. In accordance with ASC Subtopic 835-30 (“Interest—Imputation of Interest”), debt issuance costs are presented as a reduction to the associated liability balance on the Consolidated Statement of Assets and Liabilities, except for debt issuance costs associated with line-of-credit arrangements. Debt issuance costs, net of accumulated amortization, as of June 30, 2017 and December 31, 2016 were as follows:
| (in thousands) |
June 30, 2017 | December 31, 2016 | ||||||
| SBA Debentures |
$ | 2,376 | $ | 2,699 | ||||
| 2019 Notes |
— | 1,546 | ||||||
| 2024 Notes |
7,141 | 7,482 | ||||||
| 2021 Asset-Backed Notes |
813 | 1,233 | ||||||
| 2022 Convertible Notes |
3,969 | — | ||||||
| Wells Facility(1) |
337 | 501 | ||||||
| Union Bank Facility(1) |
543 | 768 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 15,179 | $ | 14,229 | ||||
|
|
|
|
|
|||||
| (1) | As the Wells Facility and Union Bank Facility are line-of-credit arrangements, the debt issuance costs associated with these instruments are presented separately as an asset on the Consolidated Statement of Assets and Liabilities in accordance with ASC Subtopic 835-30. |
Refer to “Note 4—Borrowings” included in the notes to our consolidated financial statements appearing elsewhere in this prospectus for a discussion of the contract terms, interest expense, and fees associated with each outstanding borrowing as of and for the year ended December 31, 2016.
Commitments
In the normal course of business, we are party to financial instruments with off-balance sheet risk. These consist primarily of unfunded contractual commitments to extend credit, in the form of loans, to our portfolio
97
companies. Unfunded contractual commitments to provide funds to portfolio companies are not reflected on our balance sheet. Our unfunded contractual commitments may be significant from time to time. A portion of these unfunded contractual commitments are dependent upon the portfolio company reaching certain milestones before the debt commitment becomes available. Furthermore, our credit agreements contain customary lending provisions which allow us relief from funding obligations for previously made commitments in instances where the underlying company experiences materially adverse events that affect the financial condition or business outlook for the company. These commitments will be subject to the same underwriting and ongoing portfolio maintenance as are the on-balance sheet financial instruments that we hold. Since these commitments may expire without being drawn upon, the total commitment amount does not necessarily represent future cash requirements. As such, our disclosure of unfunded contractual commitments includes only those which are available at the request of the portfolio company and unencumbered by milestones.
At June 30, 2017, we had approximately $57.6 million of unfunded commitments, including undrawn revolving facilities, which were available at the request of the portfolio company and unencumbered by milestones. We intend to use cash flow from normal and early principal repayments, and proceeds from borrowings and notes to fund these commitments.
We also had approximately $70.0 million of non-binding term sheets outstanding to three new companies, which generally convert to contractual commitments within approximately 90 days of signing. Non-binding outstanding term sheets are subject to completion of our due diligence and final investment committee approval process, as well as the negotiation of definitive documentation with the prospective portfolio companies. Not all non-binding term sheets are expected to close and do not necessarily represent future cash requirements.
The fair value of our unfunded commitments is considered to be immaterial as the yield determined at the time of underwriting is expected to be materially consistent with the yield upon funding, given that interest rates are generally pegged to market indices and given the existence of milestones, conditions and/or obligations imbedded in the borrowing agreements.
As of June 30, 2017, our unfunded contractual commitments available at the request of the portfolio company, including undrawn revolving facilities, and unencumbered by milestones are as follows:
| (in thousands) Portfolio Company |
Unfunded Commitments(1) |
|||
| NewVoiceMedia Limited |
$ | 15,000 | ||
| Evernote Corporation |
10,000 | |||
| Aquantia Corp. |
6,500 | |||
| Audentes Therapeutics, Inc. |
5,000 | |||
| Wrike, Inc. |
5,000 | |||
| Vela Trading Technologies |
4,800 | |||
| MDX Medical Inc. |
4,500 | |||
| 908 DEVICES INC. |
2,500 | |||
| Verastem, Inc. |
2,500 | |||
| RedSeal Inc. |
1,795 | |||
|
|
|
|||
| Total |
$ | 57,595 | ||
|
|
|
|||
| (1) | Amount represents unfunded commitments, including undrawn revolving facilities, which are available at the request of the portfolio company. Amount excludes unfunded commitments which are unavailable due to the borrower having not met certain milestones. |
98
Contractual Obligations
The following table shows our contractual obligations as of June 30, 2017:
| Payments due by period (in thousands) | ||||||||||||||||||||
| Contractual Obligations(1) |
Total | Less than 1 year |
1 - 3 years | 3 - 5 years | After 5 years |
|||||||||||||||
| Borrowings(2)(3) |
$ | 766,388 | $ | 87,678 | $ | 21,800 | $ | 349,400 | $ | 307,510 | ||||||||||
| Operating Lease Obligations(4) |
2,616 | 1,744 | 872 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 769,004 | $ | 89,422 | $ | 22,672 | $ | 349,400 | $ | 307,510 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Excludes commitments to extend credit to our portfolio companies. |
| (2) | Includes $190.2 million in principal outstanding under the SBA debentures, $258.5 million of the 2024 Notes, $230.0 million of the Convertible Notes and $87.7 million of the 2021 Asset-Backed Notes as of June 30, 2017. |
| (3) | Amounts represent future principal repayments and not the carrying value of each liability. See Note 4 to our consolidated financial statements. |
| (4) | Facility leases. |
Certain premises are leased under agreements which expire at various dates through March 2020. Total rent expense amounted to approximately $449,000 and $893,000 during the three and six months ended June 30, 2017. Total rent expense amounted to approximately $436,000 and $872,000 during the same periods ended June 30, 2016.
Indemnification Agreements
We have entered into indemnification agreements with our directors and executive officers. The indemnification agreements are intended to provide our directors and executive officers the maximum indemnification permitted under Maryland law and the 1940 Act. Each indemnification agreement provides that we shall indemnify the director or executive officer who is a party to the agreement, or an “Indemnitee,” including the advancement of legal expenses, if, by reason of his or her corporate status, the Indemnitee is, or is threatened to be, made a party to or a witness in any threatened, pending, or completed proceeding, to the maximum extent permitted by Maryland law and the 1940 Act.
We and our executives and directors are covered by Directors and Officers Insurance, with the directors and officers being indemnified by us to the maximum extent permitted by Maryland law subject to the restrictions in the 1940 Act.
Borrowings
Long-Term SBA Debentures
On September 27, 2006, HT II received a license to operate as a SBIC under the SBIC program and is able to borrow funds from the SBA against eligible investments and additional contributions to regulatory capital. Under the Small Business Investment Company Act and current SBA policy applicable to SBICs, a SBIC can have outstanding at any time SBA guaranteed debentures up to twice the amount of its regulatory capital. With our net investment of $44.0 million in HT II as of June 30, 2017, HT II has the capacity to issue a total of $41.2 million of SBA guaranteed debentures, subject to SBA approval, of which $41.2 million was outstanding as of June 30, 2017. As of June 30, 2017, HT II has paid the SBA commitment fees and facility fees of approximately $1.5 million and $3.6 million, respectively. As of June 30, 2017, we held investments in HT II in 33 companies with a fair value of approximately $98.7 million, accounting for approximately 7.1% of our total investment portfolio at June 30, 2017. HT II held approximately $104.8 million in assets and accounted for approximately 5.8% of our total assets prior to consolidation at June 30, 2017.
On May 26, 2010, HT III received a license to operate as a SBIC under the SBIC program and is able to borrow funds from the SBA against eligible investments and additional contributions to regulatory capital. With
99
our net investment of $74.5 million in HT III as of June 30, 2017, HT III has the capacity to issue a total of $149.0 million of SBA guaranteed debentures, subject to SBA approval, of which $149.0 million was outstanding as of June 30, 2017. As of June 30, 2017, HT III has paid the SBA commitment fees and facility fees of approximately $1.5 million and $3.6 million, respectively. As of June 30, 2017, we held investments in HT III in 49 companies with a fair value of approximately $245.8 million, accounting for approximately 17.6% of our total investment portfolio at June 30, 2017. HT III held approximately $271.5 million in assets and accounted for approximately 14.9% of our total assets prior to consolidation at June 30, 2017.
SBICs are designed to stimulate the flow of private equity capital to eligible small businesses. Under present SBA regulations, eligible small businesses include businesses that have a tangible net worth not exceeding $19.5 million and have average annual fully taxed net income not exceeding $6.5 million for the two most recent fiscal years. In addition, SBICs must devote 25.0% of its investment activity to “smaller” enterprises as defined by the SBA. A smaller enterprise is one that has a tangible net worth not exceeding $6.0 million and has average annual fully taxed net income not exceeding $2.0 million for the two most recent fiscal years. SBA regulations also provide alternative size standard criteria to determine eligibility, which depend on the industry in which the business is engaged and are based on such factors as the number of employees and gross sales. According to SBA regulations, SBICs may make long-term loans to small businesses, invest in the equity securities of such businesses and provide them with consulting and advisory services. Through our wholly owned subsidiaries HT II and HT III, we plan to provide long-term loans to qualifying small businesses, and in connection therewith, make equity investments.
HT II and HT III are periodically examined and audited by the SBA’s staff to determine their compliance with SBA regulations. If HT II or HT III fails to comply with applicable SBA regulations, the SBA could, depending on the severity of the violation, limit or prohibit HT II’s or HT III’s use of debentures, declare outstanding debentures immediately due and payable, and/or limit HT II or HT III from making new investments. In addition, HT II or HT III may also be limited in their ability to make distributions to us if they do not have sufficient capital in accordance with SBA regulations. Such actions by the SBA would, in turn, negatively affect us because HT II and HT III are our wholly owned subsidiaries. HT II and HT III were in compliance with the terms of the SBIC’s leverage as of June 30, 2017 as a result of having sufficient capital as defined under the SBA regulations.
The rates of borrowings under various draws from the SBA beginning in March 2009 are set semiannually in March and September and range from 2.25% to 4.62% excluding annual fees. Interest payments on SBA debentures are payable semiannually. There are no principal payments required on these issues prior to maturity and no prepayment penalties. Debentures under the SBA generally mature ten years after being borrowed. Based on the initial draw down date of March 2009, the initial maturity of SBA debentures will occur in March 2019. In addition, the SBA charges a fee that is set annually, depending on the Federal fiscal year the leverage commitment was delegated by the SBA, regardless of the date that the leverage was drawn by the SBIC. The annual fees related to HT II debentures that pooled on September 22, 2010 were 0.406% and 0.285%, depending upon the year in which the underlying commitment was closed. The annual fees on other debentures have been set at 0.906%. The annual fees related to HT III debentures that pooled on March 27, 2013 were 0.804%. The annual fees on other debentures have been set at 0.515%. The rates of borrowings on our SBA debentures range from 3.05% to 5.53% when including these annual fees.
The average amount of debentures outstanding for the three and six months ended June 30, 2017 for HT II was approximately $41.2 million with an average interest rate of approximately 4.51% and 4.48%, respectively. The average amount of debentures outstanding for the three and six months ended June 30, 2017 for HT III was approximately $149.0 million with an average interest rate of approximately 3.42% and 3.40%, respectively.
100
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the SBA debentures are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 1,737 | $ | 1,737 | $ | 3,456 | $ | 3,475 | ||||||||
| Amortization of debt issuance cost (loan fees) |
156 | 168 | 324 | 336 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 1,893 | $ | 1,905 | $ | 3,780 | $ | 3,811 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | — | $ | 3,442 | $ | 3,461 | ||||||||
In aggregate, at June 30, 2017, with our net investment of $118.5 million, HT II and HT III have the capacity to issue a total of $190.2 million of SBA-guaranteed debentures, subject to SBA approval. At June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries.
We reported the following SBA debentures outstanding principal balances as of June 30, 2017 and December 31, 2016:
| (in thousands) Issuance/Pooling Date |
Maturity Date | Interest Rate(1) |
June 30, 2017 |
December 31, 2016 |
||||||||||||
| March 25, 2009 |
March 1, 2019 | 5.53 | % | $ | 18,400 | $ | 18,400 | |||||||||
| September 23, 2009 |
September 1, 2019 | 4.64 | % | 3,400 | 3,400 | |||||||||||
| September 22, 2010 |
September 1, 2020 | 3.62 | % | 6,500 | 6,500 | |||||||||||
| September 22, 2010 |
September 1, 2020 | 3.50 | % | 22,900 | 22,900 | |||||||||||
| March 29, 2011 |
March 1, 2021 | 4.37 | % | 28,750 | 28,750 | |||||||||||
| September 21, 2011 |
September 1, 2021 | 3.16 | % | 25,000 | 25,000 | |||||||||||
| March 21, 2012 |
March 1, 2022 | 3.28 | % | 25,000 | 25,000 | |||||||||||
| March 21, 2012 |
March 1, 2022 | 3.05 | % | 11,250 | 11,250 | |||||||||||
| September 19, 2012 |
September 1, 2022 | 3.05 | % | 24,250 | 24,250 | |||||||||||
| March 27, 2013 |
March 1, 2023 | 3.16 | % | 24,750 | 24,750 | |||||||||||
|
|
|
|
|
|||||||||||||
| Total SBA Debentures |
$ | 190,200 | $ | 190,200 | ||||||||||||
|
|
|
|
|
|||||||||||||
| (1) | Interest rate includes annual charge |
2019 Notes
In April and July 2012, we issued $84.5 million in aggregate principal amount of 7.00% notes due 2019 (the “April 2019 Notes”). In September and October 2012, we issued $85.9 million in aggregate principal amount of 7.00% notes due 2019 (the “September 2019 Notes”). The April 2019 Notes and September 2019 Notes are together referred to as the “2019 Notes”.
In April 2015, we redeemed $20.0 million of the $84.5 million issued and outstanding aggregate principal amount of April 2019 Notes, as previously approved by the Board of Directors. In December 2015, we redeemed $40.0 million of the $85.9 million issued and outstanding aggregate principal amount of September 2019 Notes, as previously approved by the Board of Directors. The remaining 2019 Notes were fully redeemed on February 24, 2017.
101
As of December 31, 2016, the 2019 Notes payable outstanding principal balance consisted of:
| (in thousands) |
December 31, 2016 | |||
| April 2019 Notes |
$ | 64,490 | ||
| September 2019 Notes |
45,874 | |||
|
|
|
|||
| Total 2019 Notes principal outstanding |
$ | 110,364 | ||
|
|
|
|||
The April 2019 Notes bore interest at a rate of 7.00% per year and traded on the NYSE under the trading symbol “HTGZ.” The September 2019 Notes bore interest at a rate of 7.00% per year and traded on the NYSE under the trading symbol “HTGY.” For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the 2019 Notes are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 1,931 | $ | 1,159 | $ | 3,863 | ||||||||
| Amortization of debt issuance cost (loan fees) |
— | 160 | 1,546 | 320 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | — | $ | 2,091 | $ | 2,705 | $ | 4,183 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | 1,931 | $ | 1,911 | $ | 3,863 | ||||||||
2024 Notes
On July 14, 2014, we and U.S. Bank, N.A. (the “2024 Trustee”), entered into the Third Supplemental Indenture (the “Third Supplemental Indenture”) to the Base Indenture between us and the 2024 Trustee, dated July 14, 2014, relating to our issuance, offer and sale of $100.0 million aggregate principal amount of the 2024 Notes. On August 6, 2014, the underwriters issued notification to exercise their over-allotment option for an additional $3.0 million in aggregate principal amount of the 2024 Notes.
On May 2, 2016, we closed an underwritten public offering of an additional $72.9 million in aggregate principal amount of the 2024 Notes. The $72.9 million in aggregate principal amount includes $65.4 million from the initial offering on April 21, 2016 and $7.5 million as a result of underwriters exercising a portion of their option to purchase up to an additional $9.8 million in aggregate principal to cover overallotments on April 29, 2016.
On June 27, 2016, we closed an underwritten public offering of an additional $60.0 million in aggregate principal amount of the 2024 Notes. On June 30, 2016, the underwriters exercised their option to purchase up to an additional $9.0 million in aggregate principal to cover overallotments, resulting in total aggregate principal of $69.0 million from the offering.
On October 11, 2016, we entered into a debt distribution agreement, pursuant to which it may offer for sale, from time to time, up to $150.0 million in aggregate principal amount of 2024 Notes through FBR Capital Markets & Co. acting as its sales agent (the “2024 Notes Agent”). Sales of the 2024 Notes may be made in negotiated transactions or transactions that are deemed to be “at the market offerings” as defined in Rule 415 under the Securities Act, including sales made directly on the NYSE, or similar securities exchange or sales made through a market maker other than on an exchange at prices related to prevailing market prices or at negotiated prices.
The 2024 Notes Agent receives a commission from us equal to up to 2.00% of the gross sales of any 2024 Notes sold through the 2024 Notes Agent under the debt distribution agreement. The 2024 Notes Agent is not required to sell any specific principal amount of 2024 Notes, but will use its commercially reasonable efforts consistent with its sales and trading practices to sell the 2024 Notes. The 2024 Notes are expected to trade “flat,”
102
which means that purchasers in the secondary market will not pay, and sellers will not receive, any accrued and unpaid interest on the 2024 Notes that is not reflected in the trading price.
During the six months ended June 30, 2017, we sold 225,457 notes for approximately $5.6 million in aggregate principal amount. We did not sell any notes under the debt distribution agreement during the three months ended June 30, 2017. During the year ended December 31, 2016, we sold 317,125 notes for approximately $7.9 million in aggregate principal amount. As of June 30, 2017 approximately $136.4 million in aggregate principal amount remains available for issuance and sale under the debt distribution agreement. See “Summary—Recent Developments”.
All issuances of 2024 Notes rank equally in right of payment and form a single series of notes.
The 2024 Notes will mature on July 30, 2024 and may be redeemed in whole or in part at our option at any time or from time to time on or after July 30, 2017, upon not less than 30 days nor more than 60 days written notice by mail prior to the date fixed for redemption thereof, at a redemption price of 100% of the outstanding principal amount thereof plus accrued and unpaid interest payments otherwise payable for the then-current quarterly interest period accrued to but not including the date fixed for redemption. The 2024 Notes bear interest at a rate of 6.25% per year payable quarterly on January 30, April 30, July 30 and October 30 of each year, commencing on July 30, 2014, and trade on the NYSE under the trading symbol “HTGX.”
The 2024 Notes are our direct unsecured obligations and rank: (i) pari passu with our other outstanding and future senior unsecured indebtedness; (ii) senior to any of our future indebtedness that expressly provides it is subordinated to the 2024 Notes; (iii) effectively subordinated to all of our existing and future secured indebtedness (including indebtedness that is initially unsecured to which we subsequently grant security), to the extent of the value of the assets securing such indebtedness; (iv) structurally subordinated to all existing and future indebtedness and other obligations of any of our subsidiaries.
The Base Indenture, as supplemented by the Third Supplemental Indenture, contains certain covenants including covenants requiring us to comply with (regardless of whether it is subject to) the asset coverage requirements set forth in Section 18(a)(1)(A) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act and to comply with the restrictions on dividends and other distributions as well as the purchase of capital stock set forth in Section 18(a)(1)(B) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act. These covenants are subject to important limitations and exceptions that are described in the Base Indenture, as supplemented by the Third Supplemental Indenture. The Base Indenture, as supplemented by the Third Supplemental Indenture, also contains certain reporting requirements, including a requirement that we provide financial information to the holders of the 2024 Notes and the 2024 Trustee if we should no longer be subject to the reporting requirements under the Exchange Act. The Base Indenture provides for customary events of default and further provides that the 2024 Trustee or the holders of 25% in aggregate principal amount of the outstanding 2024 Notes in a series may declare such 2024 Notes immediately due and payable upon the occurrence of any event of default after expiration of any applicable grace period. As of June 30, 2017, we were in compliance with the terms of the Base Indenture as supplemented by the Third Supplemental Indenture.
As of June 30, 2017 and December 31, 2016, the components of the carrying value of the 2024 Notes were as follows:
| (in thousands) |
June 30, 2017 |
December 31, 2016 |
||||||
| Principal amount of debt |
$ | 258,510 | $ | 252,873 | ||||
| Unamortized debt issuance cost |
(7,141 | ) | (7,482 | ) | ||||
| Original issue premium, net of amortization |
109 | 99 | ||||||
|
|
|
|
|
|||||
| Carrying value of 2024 Notes |
$ | 251,478 | $ | 245,490 | ||||
|
|
|
|
|
|||||
103
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the 2024 Notes are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 4,039 | $ | 2,375 | $ | 8,026 | $ | 3,984 | ||||||||
| Amortization of debt issuance cost (loan fees) |
252 | 135 | 501 | 218 | ||||||||||||
| Amortization of original issue premium |
(13 | ) | — | (29 | ) | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 4,278 | $ | 2,510 | $ | 8,498 | $ | 4,202 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 4,039 | $ | 1,609 | $ | 8,016 | $ | 3,219 | ||||||||
2021 Asset-Backed Notes
On November 13, 2014, we completed a $237.4 million term debt securitization in connection with which an affiliate of ours made an offer of $129.3 million in aggregate principal amount of fixed rate asset-backed notes (the “2021 Asset-Backed Notes”), which were rated A(sf) by Kroll Bond Rating Agency, Inc. The 2021 Asset-Backed Notes were sold by Hercules Capital Funding Trust 2014-1 pursuant to a note purchase agreement, dated as of November 13, 2014, by and among us, the 2014 Trust Depositor, the 2014 Securitization Issuer, and Guggenheim Securities, LLC, as initial purchaser, and are backed by a pool of senior loans made to certain of our portfolio companies and secured by certain assets of those portfolio companies and are to be serviced by us. The securitization has an 18-month reinvestment period during which time principal collections may be reinvested into additional eligible loans. Interest on the 2021 Asset-Backed Notes is paid, to the extent of funds available, at a fixed rate of 3.524% per annum. The 2021 Asset-Backed Notes have a stated maturity of April 16, 2021.
As part of this transaction, we entered into a sale and contribution agreement with the 2014 Trust Depositor under which we have agreed to sell or have contributed to the 2014 Trust Depositor the 2014 Loans. We have made customary representations, warranties and covenants in the sale and contribution agreement with respect to the 2014 Loans as of the date of their transfer to the 2014 Trust Depositor.
In connection with the issuance and sale of the 2021 Asset-Backed Notes, we have made customary representations, warranties and covenants in the note purchase agreement. The 2021 Asset-Backed Notes are secured obligations of the 2014 Securitization Issuer and are non-recourse to us. The 2014 Securitization Issuer also entered into an indenture governing the 2021 Asset-Backed Notes, which includes customary representations, warranties and covenants. The 2021 Asset-Backed Notes were sold without being registered under the Securities Act (A) in the United States to “qualified institutional buyers” as defined in Rule 144A under the Securities Act and to institutional “accredited investors” (as defined in Rules 501(a)(1), (2), (3) or (7) under the Securities Act) who in each case, are “qualified purchasers” as defined in Sec. 2 (a)(51)(A) of the 1940 Act and pursuant to an exemption under the Securities Act and (B) to non-U.S. purchasers acquiring interest in the 2021 Asset-Backed Notes outside the United States in accordance with Regulation S under the Securities Act. The 2014 Securitization Issuer is not registered under the 1940 Act in reliance on an exemption provided by Section 3(c)(7) thereof and Rule 3a-7 thereunder. In addition, the 2014 Trust Depositor entered into an amended and restated trust agreement in respect of the 2014 Securitization Issuer, which includes customary representation, warranties and covenants.
The 2014 Loans are serviced by us pursuant to a sale and servicing agreement, which contains customary representations, warranties and covenants. We perform certain servicing and administrative functions with respect to the 2014 Loans. We are entitled to receive a monthly fee from the 2014 Securitization Issuer for servicing the 2014 Loans. This servicing fee is equal to the product of one-twelfth (or in the case of the first payment date, a fraction equal to the number of days from and including October 5, 2014 through and including December 5, 2014 over 360) of 2.00% and the aggregate outstanding principal balance of the 2014 Loans plus
104
collections on deposit in the 2014 Securitization Issuer’s collections account, as of the first day of the related collection period (the period from the 5th day of the immediately preceding calendar month through the 4th day of the calendar month in which a payment date occurs, and for the first payment date, the period from and including October 5, 2014, to the close of business on December 5, 2014). We also serve as administrator to the 2014 Securitization Issuer under an administration agreement, which includes customary representations, warranties and covenants.
At June 30, 2017 and December 31, 2016, the 2021 Asset-Backed Notes had an outstanding principal balance of $87.7 million and $109.2 million, respectively.
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the 2021 Asset-Backed Notes are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 807 | $ | 1,139 | $ | 1,695 | $ | 2,278 | ||||||||
| Amortization of debt issuance cost (loan fees) |
211 | 234 | 421 | 466 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 1,018 | $ | 1,373 | $ | 2,116 | $ | 2,744 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 848 | $ | 1,139 | $ | 1,788 | $ | 2,278 | ||||||||
Under the terms of the 2021 Asset-Backed Notes, we are required to maintain a reserve cash balance, funded through interest and principal collections from the underlying securitized debt portfolio, which may be used to pay monthly interest and principal payments on the 2021 Asset-Backed Notes. We have segregated these funds and classified them as restricted cash. There was approximately $17.2 million and $8.3 million of restricted cash as of June 30, 2017 and December 31, 2016, respectively, funded through interest collections.
Convertible Notes
2016 Convertible Notes
In April 2011, we issued $75.0 million in aggregate principal amount of 2016 Convertible Notes. The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016.
Prior to the close of business on October 14, 2015, holders were able to convert their 2016 Convertible Notes only under certain circumstances set forth in the indenture governing the 2016 Convertible Notes. On or after October 15, 2015 until the close of business on the scheduled trading day immediately preceding the maturity date, holders were able to convert their 2016 Convertible Notes at any time. Throughout the life of the 2016 Convertible Notes, holders of approximately $74.8 million of the 2016 Convertible Notes exercised their conversion rights. These 2016 Convertible Notes were settled with a combination of cash equal to the outstanding principal amount of the 2016 Convertible Notes and approximately 1.6 million shares of our common stock, or $24.3 million.
The 2016 Convertible Notes were accounted for in accordance with ASC Subtopic 470-20 (“Debt Instruments with Conversion and Other Options”). In accounting for the 2016 Convertible Notes, we estimated at the time of issuance that the values of the debt and the embedded conversion feature of the 2016 Convertible Notes were approximately 92.8% and 7.2%, respectively. The original issue discount of 7.2% attributable to the conversion feature of the 2016 Convertible Notes was recorded in “capital in excess of par value” in the Consolidated Statement of Assets and Liabilities. As a result, we recorded interest expense comprised of both stated interest expense as well as accretion of the original issue discount resulting in an estimated effective interest rate of approximately 8.1%.
105
For the three and six months ended June 30, 2016, the components of interest expense, fees and cash paid for interest expense for the 2016 Convertible Notes were as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 88 | $ | — | $ | 352 | ||||||||
| Amortization of debt issuance cost (loan fees) |
— | 11 | — | 43 | ||||||||||||
| Accretion of original issue discount |
— | 21 | — | 82 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | — | $ | 120 | $ | — | $ | 477 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | 440 | $ | — | $ | 440 | ||||||||
The estimated effective interest rate of the debt component of the 2016 Convertible Notes, equal to the stated interest of 6.0% plus the accretion of the original issue discount, was approximately 8.1% for the three and six months ended June 30, 2016.
2022 Convertible Notes
On January 25, 2017, we issued $230.0 million in aggregate principal amount of 2022 Convertible Notes, which amount includes the additional $30.0 million aggregate principal amount of 2022 Convertible Notes issued pursuant to the initial purchaser’s exercise in full of its overallotment option. The 2022 Convertible Notes were issued pursuant to the 2022 Convertible Notes Indenture. The sale of the 2022 Convertible Notes generated net proceeds of approximately $225.7 million, including $4.3 million of debt issuance costs.
The 2022 Convertible Notes mature on February 1, 2022, unless previously converted or repurchased in accordance with their terms. The 2022 Convertible Notes bear interest at a rate of 4.375% per year payable semiannually in arrears on February 1 and August 1 of each year, commencing on August 1, 2017.
The 2022 Convertible Notes are our unsecured obligations and rank senior in right of payment to our future indebtedness that is expressly subordinated in right of payment to the 2022 Convertible Notes; equal in right of payment to our existing and future indebtedness that is not so subordinated; effectively junior in right of payment to any of our secured indebtedness (including unsecured indebtedness that we later secure) to the extent of the value of the assets securing such indebtedness; and structurally junior to all existing and future indebtedness (including trade payables) incurred by our subsidiaries, financing vehicles or similar facilities.
Prior to the close of business on the business day immediately preceding August 1, 2021, holders may convert their 2022 Convertible Notes only under certain circumstances set forth in the 2022 Convertible Notes Indenture. On or after August 1, 2021 until the close of business on the scheduled trading day immediately preceding the maturity date, holders may convert their 2022 Convertible Notes at any time. Upon conversion, we will pay or deliver, as the case may be, at our election, cash, shares of our common stock or a combination of cash and shares of our common stock. The conversion rate is initially 60.9366 shares of common stock per $1,000 principal amount of 2022 Convertible Notes (equivalent to an initial conversion price of approximately $16.41 per share of common stock). The conversion rate will be subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, if certain corporate events occur prior to the maturity date, we will increase the conversion rate for a holder who elects to convert our 2022 Convertible Notes in connection with such a corporate event in certain circumstances. As of June 30, 2017, the conversion rate was 60.9366 shares of common stock per $1,000 principal amount of Convertible Senior Notes (equivalent to an adjusted conversion price of approximately $16.41 per share of common stock).
We may not redeem the 2022 Convertible Notes at our option prior to maturity. No sinking fund is provided for the 2022 Convertible Notes. In addition, if certain corporate events occur, holders of the 2022 Convertible
106
Notes may require us to repurchase for cash all or part of their 2022 Convertible Notes at a repurchase price equal to 100% of the principal amount of the 2022 Convertible Notes to be repurchased, plus accrued and unpaid interest through, but excluding, the required repurchase date.
The 2022 Convertible Notes Indenture contains certain covenants, including covenants requiring us to comply with Section 18(a)(1)(A) as modified by Section 61(a)(1) of the 1940 Act and to provide financial information to the holders of the 2022 Convertible Notes and the 2022 Trustee if we cease to be subject to the reporting requirements of the Exchange Act. These covenants are subject to important limitations and exceptions that are described in the 2022 Convertible Notes Indenture. The Company offered and sold the 2022 Convertible Notes to the initial purchaser in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act, for resale by the initial purchaser to qualified institutional buyers (as defined in the Securities Act) pursuant to the exemption from registration provided by Rule 144A under the Securities Act. We relied on these exemptions from registration based in part on representations made by the initial purchaser in connection with the sale of the 2022 Convertible Notes.
The 2022 Convertible Notes are accounted for in accordance with ASC Subtopic 470-20 (“Debt Instruments with Conversion and Other Options”). In accounting for the 2022 Convertible Notes, we estimated at the time of issuance that the values of the debt and the embedded conversion feature of the 2022 Convertible Notes were approximately 98.5% and 1.5%, respectively. The original issue discount of 1.5%, or $3.4 million, attributable to the conversion feature of the 2022 Convertible Notes was recorded in “capital in excess of par value” in the Consolidated Statement of Assets and Liabilities. As a result, we record interest expense comprised of both stated interest expense as well as accretion of the OID resulting in an estimated effective interest rate of approximately 4.76%.
As of June 30, 2017, the components of the carrying value of the 2022 Convertible Notes were as follows:
| (in thousands) |
June 30, 2017 |
|||
| Principal amount of debt |
$ | 230,000 | ||
| Unamortized debt issuance cost |
(3,969 | ) | ||
| Original issue discount, net of accretion |
(3,133 | ) | ||
|
|
|
|||
| Carrying value of 2022 Convertible Notes |
$ | 222,898 | ||
|
|
|
|||
For the three and six months ended June 30, 2017, the components of interest expense, fees and cash paid for interest expense for the 2022 Convertible notes were as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 2,516 | $ | — | $ | 4,274 | $ | — | ||||||||
| Amortization of debt issuance cost (loan fees) |
212 | — | 345 | — | ||||||||||||
| Accretion of original issue discount |
168 | — | 280 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 2,896 | $ | — | $ | 4,899 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | — | $ | — | $ | — | ||||||||
The estimated effective interest of the debt component of the 2022 Convertible Notes, equal to the stated interest rate of 4.375% plus the accretion of the original issue discount, was approximately 4.76% for the three and six months ended June 30, 2017. As of June 30, 2017, we are in compliance with the terms of the indentures governing the 2022 Convertible Notes.
107
Credit Facilities
As of June 30, 2017 and December 31, 2016, we have two available credit facilities, the Wells Facility and the Union Bank Facility.
Wells Facility
On June 29, 2015, we, through a special purpose wholly owned subsidiary, Hercules Funding II LLC (“Hercules Funding II”), entered into the Wells Facility with Wells Fargo Capital Finance, LLC, as a lender and as the arranger and the administrative agent, and the lenders party thereto from time to time.
The Wells Facility matures on August 2, 2019, unless terminated sooner in accordance with its terms.
Under the Wells Facility, Wells Fargo Capital Finance, LLC made commitments of $75.0 million, Alostar Bank of Commerce made commitments of $20.0 million, and Everbank Commercial Finance Inc. made commitments of $25.0 million. The Wells Facility contains an accordion feature, in which we can increase the credit line up to an aggregate of $300.0 million, funded by additional lenders and with the agreement of Wells Fargo and subject to other customary conditions. We expect to continue discussions with various other potential lenders to join the facility; however, there can be no assurances that additional lenders will join the Wells Facility. Borrowings under the Wells Facility generally bear interest at a rate per annum equal to LIBOR plus 3.25%, and the Wells Facility has an advance rate of 50% against eligible debt investments. The Wells Facility is secured by all of the assets of Hercules Funding II. The Wells Facility requires payment of a non-use fee on a scale of 0.0% to 0.50% depending on the average monthly outstanding balance under the facility relative to the maximum amount of commitments at such time. For the three and six months ended June 30, 2017, this non-use fee was $152,000 and $297,000, respectively. For the three and six months ended June 30, 2016, this non-use fee was $115,000 and $181,000, respectively.
The Wells Facility also includes various financial and other covenants applicable to us and our subsidiaries, in addition to those applicable to Hercules Funding II, including covenants relating to certain changes of control of us and Hercules Funding II. Among other things, these covenants also require us to maintain certain financial ratios, including a maximum debt to worth ratio, minimum interest coverage ratio, minimum portfolio funding liquidity, and a minimum tangible net worth in an amount, when added to outstanding subordinated indebtedness, that is in excess of $500.0 million plus 90% of the cumulative amount of equity raised after June 30, 2014. As of June 30, 2017, the minimum tangible net worth covenant increased to $718.6 million as a result of the March 2015 follow-on public offering of 7.6 million shares of common stock for total gross proceeds of approximately $100.4 million, the issuance of 7.3 million shares of common stock issued under the Equity Distribution Agreement for gross proceeds of $95.0 million during the year ended December 31, 2016, and the issuance of 3.3 million shares of common stock issued under the Equity Distribution Agreement for gross proceeds of $47.4 million during the six months ended June 30, 2017. The Wells Facility provides for customary events of default, including, without limitation, with respect to payment defaults, breach of representations and covenants, certain key person provisions, cross acceleration provisions to certain other debt, lien and judgment limitations, and bankruptcy.
On June 20, 2011, we paid $1.1 million in structuring fees in connection with the original Wells Facility. In connection with an amendment to the original Wells Facility in August 2014, we paid an additional $750,000 in structuring fees and in connection with the amendment in December 2015, we paid an additional $188,000 in structuring fees. These fees are being amortized through the end of the term of the Wells Facility.
We had aggregate draws of $8.5 million on the available facility during the six months ended June 30, 2017 offset by repayments of $13.5 million. At December 31, 2016 there was $5.0 million, respectively, of borrowings outstanding on this facility. There were no borrowings outstanding on the facility as of June 30, 2017.
108
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the Wells Facility are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 226 | $ | 2 | $ | 500 | ||||||||
| Amortization of debt issuance cost (loan fees) |
106 | 122 | 213 | 227 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 106 | $ | 348 | $ | 215 | $ | 727 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 214 | $ | 333 | $ | 470 | $ | 577 | ||||||||
Union Bank Facility
On May 5, 2016, we, through a special purpose wholly owned subsidiary, Hercules Funding III LLC (“Hercules Funding III”), as borrower, entered into the Union Bank Facility with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Union Bank Facility from time to time. The Union Bank Facility replaced the Prior Union Bank. Any references to amounts related to the Union Bank Facility prior to May 5, 2016 were incurred and relate to the Prior Union Bank Facility.
On July 18, 2016, we entered into the First Amendment to the Loan and Security Agreement, dated as of May 5, 2016 with MUFG Union Bank, N.A. The Amendment amends certain definitions relating to borrowings which accrue interest based on the London Interbank Offered Rate (“LIBOR Loans”) and (ii) the method(s) for calculating interest on and the paying of certain fees related to such LIBOR Loans.
Under the Union Bank Facility, MUFG Union Bank made commitments of $75.0 million. The Union Bank Facility contains an accordion feature, in which we can increase the credit line up to an aggregate of $200.0 million, funded by additional lenders and with the agreement of MUFG Union Bank and subject to other customary conditions. There can be no assurances that additional lenders will join the Union Bank Facility to increase available borrowings. Borrowings under the Union Bank Facility generally bear interest at either (i) if such borrowing is a base rate loan, a base rate per annum equal to the federal funds rate plus 1.00%, LIBOR plus 1.00% or MUFG Union Bank’s prime rate, in each case, plus a margin of 1.25% or (ii) if such borrowing is a LIBOR loan, a rate per annum equal to LIBOR plus 3.25%, and the Union Bank Facility generally has an advance rate of 50% against eligible debt investments. The Union Bank Facility is secured by all of the assets of Hercules Funding III.
We paid a one-time $562,500 structuring fee in connection with the Union Bank Facility. The Union Bank Facility requires payment of a non-use fee during the revolving credit availability period on a scale of 0.25% to 0.50% depending on the average monthly outstanding balance under the facility relative to the maximum amount of commitments at such time. For the three and six months ended June 30, 2017, we incurred non-use of $95,000 and $189,000, respectively. For the three and six months ended June 30, 2016, we incurred non-use fees under the Prior Union Bank Facility of $87,000 and $182,000, respectively.
The Union Bank Facility also includes various financial and other covenants applicable to us and our subsidiaries, in addition to those applicable to Hercules Funding III, including covenants relating to certain changes of control of the Company and Hercules Funding III. Among other things, these covenants also require us to maintain certain financial ratios, including a maximum debt to worth ratio, minimum interest coverage ratio, minimum portfolio funding liquidity, and a minimum tangible net worth in an amount that is in excess of $500.0 million plus 90% of the cumulative amount of equity raised after June 30, 2014. As of June 30, 2017, the minimum tangible net worth covenant increased to $765.9 million as a result of the March 2015 follow-on public offering of 7.6 million shares of common stock for total net proceeds of approximately $100.1 million, the issuance of 7.3 million shares of common stock issued under the Equity Distribution Agreement for net proceeds
109
of $92.8 million during the year ended December 31, 2016, and the issuance of 3.3 million shares of common stock issued under the Equity Distribution Agreement for net proceeds of $46.9 million during the six months ended June 30, 2017. The Union Bank Facility provides for customary events of default, including with respect to payment defaults, breach of representations and covenants, servicer defaults, certain key person provisions, cross default provisions to certain other debt, lien and judgment limitations, and bankruptcy.
The Union Bank Facility matures on May 5, 2020, unless sooner terminated in accordance with its terms.
In connection with the Union Bank Facility, we and Hercules Funding III also entered into the Sale and Servicing Agreement, dated as of May 5, 2016 (the “Sale Agreement”), by and among Hercules Funding III, as borrower, us, as originator and servicer, and MUFG Union Bank, as agent. Under the Sale Agreement, we agree to (i) sell or transfer certain loans to Hercules Funding III under the Union Bank Facility and (ii) act as servicer for the loans sold or transferred.
We did not make any draws or repayments on the available facility during the six months ended June 30, 2017. We had aggregate draws of $25.0 million on the available facility during the six months ended June 30, 2016 offset by repayments of $25.0 million. At June 30, 2017 and December 31, 2016, there were no borrowings outstanding on the Union Bank Facility.
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the previous and current Union Bank Facility are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 55 | $ | — | $ | 55 | ||||||||
| Amortization of debt issuance cost (loan fees) |
112 | 95 | 224 | 133 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 112 | $ | 150 | $ | 224 | $ | 188 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 96 | $ | 333 | $ | 238 | $ | 577 | ||||||||
Distributions
The following table summarizes our distributions declared and paid, to be paid or reinvested on all shares, including restricted stock, for the fiscal years ended December 31, 2015, 2016 and 2017:
| Date Declared |
Record Date | Payment Date | Amount Per Share | |||||||||
| February 24, 2015 |
March 12, 2015 | March 19, 2015 | $ | 0.31 | ||||||||
| May 4, 2015 |
May 18, 2015 | May 25, 2015 | 0.31 | |||||||||
| July 29, 2015 |
August 17, 2015 | August 24, 2015 | 0.31 | |||||||||
| October 28, 2015 |
November 16, 2015 | November 23, 2015 | 0.31 | |||||||||
| February 17, 2016 |
March 7, 2016 | March 14, 2016 | 0.31 | |||||||||
| April 27, 2016 |
May 16, 2016 | May 23, 2016 | 0.31 | |||||||||
| July 27, 2016 |
August 15, 2016 | August 22, 2016 | 0.31 | |||||||||
| October 24, 2016 |
November 14, 2016 | November 21, 2016 | 0.31 | |||||||||
| February 16, 2017 |
March 6, 2017 | March 13, 2017 | 0.31 | |||||||||
| April 26, 2017 |
May 15, 2017 | May 22, 2017 | 0.31 | |||||||||
| July 26, 2017 |
August 14, 2017 | August 21, 2017 | 0.31 | |||||||||
|
|
|
|||||||||||
| $ | 3.41 | |||||||||||
|
|
|
|||||||||||
| * | Distribution paid in cash and stock. |
On July 26, 2017, the Board of Directors declared a cash distribution of $0.31 per share to be paid on August 21, 2017 to stockholders of record as of August 14, 2017. This distribution represented our forty-eighth consecutive distribution since our IPO, bringing the total cumulative distribution to date to $13.40 per share.
110
Our Board of Directors maintains a variable distribution policy with the objective of distributing four quarterly distributions in an amount that approximates 90—100% of our taxable quarterly income or potential annual income for a particular taxable year. In addition, at the end of our taxable year, our Board of Directors may choose to pay an additional special distribution, or fifth distribution, so that we may distribute approximately all of our annual taxable income in the taxable year in which it was earned, or may elect to maintain the option to spill over our excess taxable income into the following taxable year as part of any future distribution payments.
Distributions in excess of our current and accumulated earnings and profits would generally be treated first as a return of capital to the extent of a stockholder’s tax basis in our shares, and any remaining distributions would be treated as a capital gain. The determination of the tax attributes of our distributions is made annually as of the end of our taxable year based upon our taxable income for the full taxable year and distributions paid for the full taxable year. Of the distributions declared during the fiscal years ended December 31, 2016, 2015, and 2014, 100% were distributions derived from our current and accumulated earnings and profits. There can be no certainty to stockholders that this determination is representative of the tax attributes of our 2017 distributions to stockholders.
We maintain an “opt out” dividend reinvestment plan that provides for reinvestment of our distribution on behalf of our stockholders, unless a stockholder elects to receive cash. As a result, if our Board of Directors authorizes, and we declare a cash distribution, then our stockholders who have not “opted out” of our dividend reinvestment plan will have their cash distribution automatically reinvested in additional shares of our common stock, rather than receiving the cash distributions.
Shortly after the close of each calendar year information identifying the source of the distribution (i.e., paid from ordinary income, paid from net capital gains on the sale of securities, and/or a return of paid-in-capital surplus which is a nontaxable distribution, if any) will be provided to our stockholders subject to information reporting. To the extent our taxable earnings fall below the total amount of our distributions for any taxable year, a portion of those distributions may be deemed a tax return of capital to our stockholders.
We expect to qualify to be subject to tax as a RIC under Subchapter M of the Code. In order to be subject to tax as a RIC, we are required to satisfy certain annual gross income and quarterly asset composition tests, as well as make distributions to our stockholders each taxable year treated as dividends for U.S. federal income tax purposes of an amount at least equal to 90% of the sum of our investment company taxable income, determined without regard to any deduction for dividends paid, plus our net tax-exempt income, if any. Upon being eligible to be subject to tax as a RIC, we would be entitled to deduct such distributions we pay to our stockholders in determining the overall components of our “taxable income.” Components of our taxable income include our taxable interest, dividend and fee income, reduced by certain deductions, as well as taxable net realized securities gains. Taxable income generally differs from net income for financial reporting purposes due to temporary and permanent differences in the recognition of income and expenses and generally excludes net unrealized appreciation or depreciation as such gains or losses are not included in taxable income until they are realized. In connection with maintaining our ability to be subject to tax as a RIC, among other things, we have made and intend to continue to make the requisite distributions to our stockholders each taxable year, which generally should relieve us from corporate-level U.S. federal income taxes.
As a RIC, we will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income and gains unless we make distributions treated as dividends for U.S. federal income tax purposes in a timely manner to our stockholders in respect of each calendar year of an amount generally at least equal to the Excise Tax Avoidance Requirement. We will not be subject to this excise tax on any amount on which we incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains).
Depending on the level of taxable income earned in a taxable year, we may choose to carry over taxable income in excess of current taxable year distributions treated as dividends for U.S. federal income tax purposes
111
from such taxable income into the next taxable year and incur a 4% excise tax on such taxable income, as required. The maximum amount of excess taxable income that may be carried over for distribution in the next taxable year under the Code is the total amount of distributions treated as dividends for U.S. federal income tax purposes paid in the following taxable year, subject to certain declaration and payment guidelines. To the extent we choose to carry over taxable income into the next taxable year, distributions declared and paid by us in a taxable year may differ from our taxable income for that taxable year as such distributions may include the distribution of current taxable year taxable income, the distribution of prior taxable year taxable income carried over into and distributed in the current taxable year, or returns of capital.
We can offer no assurance that we will achieve results that will permit the payment of any cash distributions and, if we issue senior securities, we will be prohibited from making distributions if doing so causes us to fail to maintain the asset coverage ratios stipulated by the 1940 Act or if distributions are limited by the terms of any of our borrowings. Our ability to make distributions will be limited by the asset coverage requirements under the 1940 Act.
We intend to distribute 100% of our spillover earnings, which consists of ordinary income and long term capital gains, from the year ended December 31, 2016 to our stockholders during 2017.
Critical Accounting Policies
The preparation of consolidated financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and revenues and expenses during the period reported. On an ongoing basis, our management evaluates its estimates and assumptions, which are based on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results could differ from those estimates. Changes in our estimates and assumptions could materially impact our results of operations and financial condition.
Reclassification
Certain balances from prior years have been reclassified in order to conform to the current year presentation.
Valuation of Investments
The most significant estimate inherent in the preparation of our consolidated financial statements is the valuation of investments and the related amounts of unrealized appreciation and depreciation of investments recorded.
At June 30, 2017, approximately 87.8% of our total assets represented investments in portfolio companies whose fair value is determined in good faith by the Board of Directors. Value, as defined in Section 2(a)(41) of the 1940 Act, is (i) the market price for those securities for which a market quotation is readily available and (ii) for all other securities and assets, fair value is as determined in good faith by the Board of Directors. Our investments are carried at fair value in accordance with the 1940 Act and ASC Topic 946 and measured in accordance with ASC Topic 820. Our debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of our investments in these portfolio companies are considered Level 3 assets under ASC Topic 820 because there is no known or accessible market or market indexes for these investment securities to be traded or exchanged. As such, we value substantially all of our investments at fair value as determined in good faith pursuant to a consistent valuation policy by our Board of Directors in accordance with the provisions of ASC Topic 820 and the 1940 Act. Due to the inherent uncertainty
112
in determining the fair value of investments that do not have a readily available market value, the fair value of our investments determined in good faith by our Board of Directors may differ significantly from the value that would have been used had a readily available market existed for such investments, and the differences could be material.
See “Determination of Net Asset Value” for a discussion of our investment valuation process.
Investments measured at fair value on a recurring basis are categorized in the tables below based upon the lowest level of significant input to the valuations as of June 30, 2017 and as of December 31, 2016. We transfer investments in and out of Level 1, 2 and 3 as of the beginning balance sheet date, based on changes in the use of observable and unobservable inputs utilized to perform the valuation for the period. During the six months ended June 30, 2017, there were no transfers between Levels 1 or 2.
| (in thousands) Description |
Balance June 30, 2017 |
Quoted Prices In Active Markets For Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Senior Secured Debt |
$ | 1,287,623 | $ | — | $ | — | $ | 1,287,623 | ||||||||
| Preferred Stock |
43,385 | — | — | 43,385 | ||||||||||||
| Common Stock |
31,931 | 8,361 | — | 23,570 | ||||||||||||
| Warrants |
32,530 | — | 8,426 | 24,104 | ||||||||||||
| Escrow Receivable |
2,171 | — | — | 2,171 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,397,640 | $ | 8,361 | $ | 8,426 | $ | 1,380,853 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (in thousands) Description |
Balance December 31, 2016 |
Quoted Prices In Active Markets For Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Senior Secured Debt |
$ | 1,328,803 | $ | — | $ | 4,825 | $ | 1,323,978 | ||||||||
| Preferred Stock |
39,418 | — | — | 39,418 | ||||||||||||
| Common Stock |
28,236 | 17,271 | — | 10,965 | ||||||||||||
| Warrants |
27,485 | — | 3,239 | 24,246 | ||||||||||||
| Escrow Receivable |
1,382 | — | — | 1,382 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,425,324 | $ | 17,271 | $ | 8,064 | $ | 1,399,989 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The table below presents a reconciliation for all financial assets and liabilities measured at fair value on a recurring basis, excluding accrued interest components, using significant unobservable inputs (Level 3) for the six months ended June 30, 2017 and the year ended December 31, 2016.
| (in thousands) |
Balance January 1, 2017 |
Net Realized Gains (Losses)(1) |
Net Change in Unrealized Appreciation (Depreciation)(2) |
Purchases(5) | Sales | Repayments(6) | Gross Transfers into Level 3(3) |
Gross Transfers out of Level 3(3) |
Balance June 30, 2017 |
|||||||||||||||||||||||||||
| Senior Debt |
$ | 1,323,978 | $ | — | $ | 17,265 | $ | 347,275 | $ | — | $ | (338,224 | ) | $ | — | $ | (62,671 | ) | $ | 1,287,623 | ||||||||||||||||
| Preferred Stock |
39,418 | (7,263 | ) | 11,293 | 173 | (236 | ) | — | — | — | 43,385 | |||||||||||||||||||||||||
| Common Stock |
10,965 | — | (53,814 | ) | 3,748 | — | — | 62,671 | — | 23,570 | ||||||||||||||||||||||||||
| Warrants |
24,246 | 1,173 | 4,389 | 1,286 | (6,990 | ) | — | — | — | 24,104 | ||||||||||||||||||||||||||
| Escrow Receivable |
1,382 | 20 | — | 2,737 | (1,968 | ) | — | — | — | 2,171 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 1,399,989 | $ | (6,070 | ) | $ | (20,867 | ) | $ | 355,219 | $ | (9,194 | ) | $ | (338,224 | ) | $ | 62,671 | $ | (62,671 | ) | $ | 1,380,853 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
113
| (in thousands) |
Balance January 1, 2016 |
Net Realized Gains (Losses)(1) |
Net Change in Unrealized Appreciation (Depreciation)(2) |
Purchases(5) | Sales | Repayments(6) | Gross Transfers into Level 3(4) |
Gross Transfers out of Level 3(4) |
Balance December 31, 2016 |
|||||||||||||||||||||||||||
| Senior Debt |
$ | 1,102,396 | $ | (6,968 | ) | $ | (12,675 | ) | $ | 687,353 | $ | — | $ | (441,567 | ) | $ | — | $ | (4,561 | ) | $ | 1,323,978 | ||||||||||||||
| Preferred Stock |
35,245 | (334 | ) | (7,864 | ) | 13,873 | (1,367 | ) | — | 626 | (761 | ) | 39,418 | |||||||||||||||||||||||
| Common Stock |
1,527 | — | (1,404 | ) | 6,081 | — | — | 4,761 | — | 10,965 | ||||||||||||||||||||||||||
| Warrants |
18,565 | (116 | ) | 3,465 | 4,082 | (1,186 | ) | — | — | (564 | ) | 24,246 | ||||||||||||||||||||||||
| Escrow Receivable |
2,967 | (6 | ) | — | 2,009 | (3,588 | ) | — | — | — | 1,382 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 1,160,700 | $ | (7,424 | ) | $ | (18,478 | ) | $ | 713,398 | $ | (6,141 | ) | $ | (441,567 | ) | $ | 5,387 | $ | (5,886 | ) | $ | 1,399,989 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| (1) | Included in net realized gains or losses in the accompanying Consolidated Statement of Operations. |
| (2) | Included in net change in unrealized appreciation (depreciation) in the accompanying Consolidated Statement of Operations. |
| (3) | Transfers into Level 3 during the six months ended June 30, 2017 relate to the conversion of our debt investment in Sungevity, Inc. and a portion of our debt investment in Gamma Medica, Inc. to common stock through bankruptcy transactions. Transfers out of Level 3 during the six months ended June 30, 2017 relate to the conversion of our debt investment in Sungevity, Inc. and a portion of our debt investment in Gamma Medica, Inc. to common stock through bankruptcy transactions. |
| (4) | Transfers into Level 3 during the year ended December 31, 2016 relate to the acquisition of preferred stock as a result of the exercise of warrants in Ping Identity Corporation, the conversion of debt to equity in Optiscan Biomedical Corp and Achilles Technology Management Co II, Inc. and the conversion of our preferred shares to common shares in SCIEnergy, Inc. Transfers out of Level 3 during the year ended December 31, 2016 relate to the exercise of warrants in TPI Composites, Inc. and Touchcommerce, Inc. to common stock in an initial public offering, or IPO, and acquisition, respectively; the exercise of warrants in Ping Identity Corporation to preferred stock; the conversion of debt to equity in Optiscan Biomedical Corp and Achilles Technology Management Co II, Inc. and the conversion of our preferred shares to common shares in SCIEnergy, Inc. |
| (5) | Amounts listed above are inclusive of loan origination fees received at the inception of the loan which are deferred and amortized into fee income as well as the accretion of existing loan discounts and fees during the period. Escrow receivable purchases may include additions due to proceeds held in escrow from the liquidation of level 3 investments. |
| (6) | Amounts listed above include the acceleration and payment of loan discounts and loan fees due to early payoffs or restructures. |
For six months ended June 30, 2017, approximately $3.8 million in net unrealized appreciation and $53.8 million in net unrealized depreciation was recorded for preferred stock and common stock Level 3 investments, respectively, relating to assets still held at the reporting date. The depreciation on common stock during the period reflects the conversion of our debt investment in Sungevity, Inc. to common stock at cost through a bankruptcy transaction and subsequent depreciation to fair value. For the same period, approximately $2.6 million in net unrealized depreciation and $5.3 million in net unrealized appreciation was recorded for debt and warrant Level 3 investments, respectively, relating to assets still held at the reporting date.
For the year ended December 31, 2016, approximately $9.1 million and $1.4 million in net unrealized depreciation was recorded for preferred stock and common stock Level 3 investments, respectively, relating to assets still held at the reporting date. For the same period, approximately $25.7 million in net unrealized depreciation and $2.8 million in net unrealized appreciation was recorded for debt and warrant Level 3 investments, respectively, relating to assets still held at the reporting date.
114
The following tables provide quantitative information about our Level 3 fair value measurements as of June 30, 2017. In addition to the techniques and inputs noted in the table below, according to our valuation policy we may also use other valuation techniques and methodologies when determining our fair value measurements. The tables below are not intended to be all-inclusive, but rather provide information on the significant Level 3 inputs as they relate to our fair value measurements.
The significant unobservable input used in the fair value measurement of our escrow receivables is the amount recoverable at the contractual maturity date of the escrow receivable.
| Investment Type - Level Three Debt Investments |
Fair Value at June 30, 2017 (in thousands) |
Valuation Techniques/ Methodologies |
Unobservable Input(a) |
Range |
Weighted Average(b) |
|||||||||
| Pharmaceuticals |
$ | 107,497 | Originated Within 6 Months | Origination Yield | 11.25% - 13.41% | 12.60 | % | |||||||
| 484,125 | Market Comparable Companies | Hypothetical Market Yield | 8.93% - 15.48% | 12.88 | % | |||||||||
| Premium/(Discount) | (0.25%) - 0.75% | |||||||||||||
| — | Liquidation(c) | Probability weighting of
|
100.00% | |||||||||||
| Technology |
130,894 | Originated Within 6 Months | Origination Yield | 10.98% - 14.88% | 12.17 | % | ||||||||
| 183,058 | Market Comparable Companies | Hypothetical Market Yield | 9.13% - 18.50% | 13.48 | % | |||||||||
| Premium/(Discount) | (0.25%) - 1.00% | |||||||||||||
| 4,576 | Liquidation(c) | Probability weighting of
|
100.00% | |||||||||||
| Sustainable and Renewable |
82,118 | Market Comparable Companies | Hypothetical Market Yield | 11.91% - 14.63% | 13.37 | % | ||||||||
| Premium/(Discount)
|
0.00% - 0.25% | |||||||||||||
| Medical Devices |
45,082 | Originated Within 6 Months | Origination Yield | 10.38% - 13.18% | 11.06 | % | ||||||||
| 47,142 | Market Comparable Companies | Hypothetical Market Yield | 9.49% - 18.53% | 13.57 | % | |||||||||
| Premium/(Discount) | 0.00% - 0.75% | |||||||||||||
| — | Liquidation(c) | Probability weighting of
|
50.00% | |||||||||||
| Lower Middle Market |
19,991 | Market Comparable Companies | Hypothetical Market Yield | 9.03% | 9.03 | % | ||||||||
| Premium/(Discount) | 0.50% | |||||||||||||
| — | Liquidation(c) | Probability weighting of alternative outcomes |
100.00% | |||||||||||
| Debt Investments Where Fair Value Approximates Cost |
||||||||||||||
| 40,466 | Imminent Payoffs(d) | |||||||||||||
| 142,674 | Debt Investments Maturing in Less than One Year | |||||||||||||
|
|
|
|||||||||||||
| $ | 1,287,623 | Total Level Three Debt Investments | ||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of our debt securities are hypothetical market yields and premiums/(discounts). The hypothetical market yield is defined as the exit price of an investment in a hypothetical market to hypothetical market participants where buyers and sellers are willing participants. The premiums (discounts) relate to company specific characteristics such as underlying investment performance, security liens, and other characteristics of the investment. Significant increases (decreases) in the inputs in isolation may result in a significantly lower (higher) fair value measurement, depending on the materiality of the investment. Debt investments in the industries noted in our Consolidated Schedule of Investments are included in the industries noted above as follows: |
| • | Pharmaceuticals, above, is comprised of debt investments in the Specialty Pharmaceuticals, Drug Discovery and Development, Drug Delivery and Biotechnology Tools industries in the Consolidated Schedule of Investments. |
| • | Technology, above, is comprised of debt investments in the Software, Semiconductors, Internet Consumer and Business Services, Consumer and Business Products, Information Services, and Communications and Networking industries in the Consolidated Schedule of Investments. |
| • | Sustainable and Renewable Technology, above, aligns with the Sustainable and Renewable Technology Industry in the Consolidated Schedule of Investments. |
| • | Medical Devices, above, is comprised of debt investments in the Surgical Devices and Medical Devices and Equipment industries in the Consolidated Schedule of Investments. |
| • | Lower Middle Market, above, is comprised of debt investments in the Communications and Networking, Electronics and Computer Hardware, Healthcare Services - Other, Information Services, Internet Consumer and Business Services, Media/Content/Info, and Specialty Pharmaceuticals industries in the Consolidated Schedule of Investments. |
| (b) | The weighted averages are calculated based on the fair market value of each investment. |
| (c) | The significant unobservable input used in the fair value measurement of impaired debt securities is the probability weighting of alternative outcomes. |
| (d) | Imminent payoffs represent debt investments that we expect to be fully repaid within the next three months, prior to their scheduled maturity date. |
115
| Investment Type - Level Three Debt |
Fair Value at December 31, 2016 (in thousands) |
Valuation Techniques/Methodologies | Unobservable Input(a) |
Range | Weighted Average(b) |
|||||||||
| Pharmaceuticals |
$ | 102,412 | Originated Within 6 Months | Origination Yield | 12.24% - 14.59% | 13.64 | % | |||||||
| 434,718 | Market Comparable Companies | Hypothetical Market Yield | 9.07% - 15.62% | 12.44 | % | |||||||||
| Premium/(Discount) |
(0.25%) - 0.75% | |||||||||||||
| 2,693 | Liquidation(c) | Probability weighting of alternative outcomes |
25.00% - 100.00% | |||||||||||
| Technology |
93,674 | Originated Within 6 Months | Origination Yield | 7.29% - 16.53% | 13.69 | % | ||||||||
| 325,553 | Market Comparable Companies | Hypothetical Market Yield | 10.14% - 21.66% | 12.69 | % | |||||||||
| Premium/(Discount) | (0.50%) - 0.50% | |||||||||||||
| 24,706 | Liquidation(c) | Probability weighting of alternative outcomes |
20.00% - 100.00% | |||||||||||
| Sustainable and Renewable |
99,286 | Market Comparable Companies | Hypothetical Market Yield | 11.77% - 16.84% | 13.45 | % | ||||||||
| Technology |
Premium/(Discount) | 0.00% - 0.25% | ||||||||||||
| 44,626 | Liquidation(c) | Probability weighting of alternative outcomes
|
10.00% - 40.00% | |||||||||||
| Medical Devices |
88,983 | Market Comparable Companies | Hypothetical Market Yield | 10.25% - 18.60% | 14.01 | % | ||||||||
| Premium/(Discount) | (0.25%) - 0.75% | |||||||||||||
| Lower Middle Market |
25,017 | Market Comparable Companies | Hypothetical Market Yield | 8.85% - 15.79% | 10.10 | % | ||||||||
| Premium/(Discount) | 0.00% - 0.25% | |||||||||||||
| 13,148 | Liquidation(c) | Probability weighting of alternative outcomes |
100.00% | |||||||||||
| Debt Investments Where Fair Value Approximates Cost | ||||||||||||||
| 25,000 | Imminent Payoffs(d) | |||||||||||||
| 44,162 | Debt Investments Maturing in Less than One Year | |||||||||||||
|
|
|
|||||||||||||
| $ | 1,323,978 | Total Level Three Debt Investments | ||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of our debt securities are hypothetical market yields and premiums/(discounts). The hypothetical market yield is defined as the exit price of an investment in a hypothetical market to hypothetical market participants where buyers and sellers are willing participants. The premiums (discounts) relate to company specific characteristics such as underlying investment performance, security liens, and other characteristics of the investment. Significant increases (decreases) in the inputs in isolation may result in a significantly lower (higher) fair value measurement, depending on the materiality of the investment. Debt investments in the industries noted in our Consolidated Schedule of Investments are included in the industries noted above as follows: |
| • | Pharmaceuticals, above, is comprised of debt investments in the Specialty Pharmaceuticals, Drug Discovery and Development, and Drug Delivery industries in the Consolidated Schedule of Investments. |
| • | Technology, above, is comprised of debt investments in the Software, Semiconductors, Internet Consumer and Business Services, Consumer and Business Products, Information Services, and Communications and Networking industries in the Consolidated Schedule of Investments. |
| • | Sustainable and Renewable Technology, above, aligns with the Sustainable and Renewable Technology Industry in the Consolidated Schedule of Investments. |
| • | Medical Devices, above, is comprised of debt investments in the Surgical Devices and Medical Devices and Equipment industries in the Consolidated Schedule of Investments. |
| • | Lower Middle Market, above, is comprised of debt investments in the Communications and Networking, Electronics and Computer Hardware, Healthcare Services—Other, Information Services, Internet Consumer and Business Services, Media/Content/Info, and Specialty Pharmaceuticals industries in the Consolidated Schedule of Investments. |
| (b) | The weighted averages are calculated based on the fair market value of each investment. |
| (c) | The significant unobservable input used in the fair value measurement of impaired debt securities is the probability weighting of alternative outcomes. |
| (d) | Imminent payoffs represent debt investments that we expect to be fully repaid within the next three months, prior to their scheduled maturity date. |
116
| Investment Type - Level Three Equity and Warrant Investments |
Fair Value at June 30, 2017 (in thousands) |
Valuation Techniques/ Methodologies |
Unobservable Input(a) |
Range |
Weighted Average(f) | |||||||
| Equity Investments |
$ | 9,102 | Market Comparable Companies |
EBITDA Multiple(b) |
5.2x - 57.5x | 16.8x | ||||||
| Revenue Multiple(b) |
0.8x - 11.3x | 3.9x | ||||||||||
|
Discount for Lack of Marketability(c) |
11.18% - 22.41% | 13.91% | ||||||||||
| Average Industry Volatility(d) |
39.43% - 65.04% | 50.92% | ||||||||||
| Risk-Free Interest Rate |
1.20% - 1.49% | 1.25% | ||||||||||
| Estimated Time to Exit (in months)
|
9 - 32 | 14 | ||||||||||
| 23,388 | Market Adjusted OPM Backsolve | Market Equity Adjustment(e) |
(20.33%) - 42.06% | 16.12% | ||||||||
| Average Industry Volatility(d) |
28.99%- 109.39% | 78.43% | ||||||||||
| Risk-Free Interest Rate |
0.70% - 1.44% | 1.10% | ||||||||||
| Estimated Time to Exit (in months) | 5 - 29 | 11 | ||||||||||
| 34,465 | Other(g) |
|||||||||||
| Warrant Investments | 16,570 | Market Comparable Companies | EBITDA Multiple(b) |
5.0x - 57.5x | 15.6x | |||||||
| Revenue Multiple(b) |
0.3x - 7.7x | 2.9x | ||||||||||
| Discount for Lack of Marketability(c) |
10.15% - 33.15% | 16.25% | ||||||||||
| Average Industry Volatility(d) |
33.86% - 105.93% | 52.46% | ||||||||||
| Risk-Free Interest Rate |
1.14% - 1.70% | 1.31% | ||||||||||
| Estimated Time to Exit (in months)
|
6 - 47 | 18 | ||||||||||
| 7,534 | Market Adjusted OPM Backsolve | Market Equity Adjustment(e) |
(83.25%) - 149.47% | 12.71% | ||||||||
| Average Industry Volatility(d) |
28.99% - 109.88% | 76.99% | ||||||||||
| Risk-Free Interest Rate |
0.73% - 1.82% | 1.19% | ||||||||||
| Estimated Time to Exit (in months) | 10 - 48 | 17 | ||||||||||
|
|
|
|||||||||||
| Total Level Three Warrant and Equity Investments | $ | 91,059 | ||||||||||
|
|
|
|||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s warrant and equity-related securities are revenue and/or EBITDA multiples, market equity adjustment factors, and discounts for lack of marketability. Additional inputs used in the Black Scholes option pricing model include industry volatility, risk free interest rate and estimated time to exit. Significant increases (decreases) in the inputs in isolation would result in a significantly higher (lower) fair value measurement, depending on the materiality of the investment. For some investments, additional consideration may be given to data from the last round of financing or merger/acquisition events near the measurement date. |
| (b) | Represents amounts used when we have determined that market participants would use such multiples when pricing the investments. |
| (c) | Represents amounts used when we have determined market participants would take into account these discounts when pricing the investments. |
| (d) | Represents the range of industry volatility used by market participants when pricing the investment. |
| (e) | Represents the range of changes in industry valuations since the portfolio company’s last external valuation event. |
| (f) | Weighted averages are calculated based on the fair market value of each investment. |
| (g) | The fair market value of these investments is derived based on recent private market and merger and acquisition transaction prices. |
117
| Investment Type - Level Three |
Fair Value at December 31, 2016 (in thousands) |
Valuation
Techniques/ |
Unobservable Input(a) |
Range | Weighted Average(e) | |||||||
| Equity Investments |
$ | 9,258 | Market Comparable Companies |
EBITDA Multiple(b) |
0.0x - 38.7x | 12.3x | ||||||
| Revenue Multiple(b) |
0.9x - 8.7x | 3.1x | ||||||||||
|
Discount for Lack of Marketability(c) |
13.75% - 25.97% | 16.73% | ||||||||||
| Average Industry Volatility(d) |
45.54% - 113.16% | 61.06% | ||||||||||
| Risk-Free Interest Rate |
0.79% - 1.50% | 0.91% | ||||||||||
| Estimated Time to Exit (in months)
|
10 - 38 | 15 | ||||||||||
| 19,836 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) | 29.93% - 109.95% | 73.49% | ||||||||
| Risk-Free Interest Rate |
0.65% - 1.44% | 0.92% | ||||||||||
| Estimated Time to Exit (in months)
|
10 - 34 | 15 | ||||||||||
| 21,289 | Other(f) | |||||||||||
| Warrant Investments |
8,959 | Market Comparable Companies |
EBITDA Multiple(b) |
2.6x - 51.4x | 13.8x | |||||||
| Revenue Multiple(b) |
0.4x - 6.1x | 2.5x | ||||||||||
|
Discount for Lack of Marketability(c) |
11.74% - 27.25% | 19.02% | ||||||||||
| Average Industry Volatility(d) |
38.58% - 111.15% | 62.03% | ||||||||||
| Risk-Free Interest Rate |
0.68% - 1.68% | 1.04% | ||||||||||
| Estimated Time to Exit (in months)
|
7 - 47 | 20 | ||||||||||
| 9,713 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) | 29.93% - 116.29% | 67.20% | ||||||||
| Risk-Free Interest Rate |
0.45% - 1.84% | 0.99% | ||||||||||
| Estimated Time to Exit (in months) |
3 - 47 | 20 | ||||||||||
| 5,574 | Other(f) |
|||||||||||
|
|
|
|||||||||||
| Total Level Three Warrant and Equity Investments | $ | 74,629 | ||||||||||
|
|
|
|||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of our warrant and equity-related securities are revenue and/or EBITDA multiples and discounts for lack of marketability. Additional inputs used in the Black Scholes OPM include industry volatility, risk free interest rate and estimated time to exit. Significant increases (decreases) in the inputs in isolation may result in a significantly higher (lower) fair value measurement, depending on the materiality of the investment. For some investments, additional consideration may be given to data from the last round of financing or merger/acquisition events near the measurement date. |
| (b) | Represents amounts used when we have determined that market participants would use such multiples when pricing the investments. |
| (c) | Represents amounts used when we have determined market participants would take into account these discounts when pricing the investments. |
| (d) | Represents the range of industry volatility used by market participants when pricing the investment. |
| (e) | Weighted averages are calculated based on the fair market value of each investment. |
| (f) | The fair market value of these investments is derived based on recent private market and merger and acquisition transaction prices. |
Income Recognition
We record interest income on an accrual basis and recognize it as earned in accordance with the contractual terms of the loan agreement, to the extent that such amounts are expected to be collected. OID initially represents the value of detachable equity warrants obtained in conjunction with the acquisition of debt securities and is accreted into interest income over the term of the loan as a yield enhancement. When a loan becomes 90 days or more past due, or if management otherwise does not expect that principal, interest and other obligations due will be collected in full, we will generally place the loan on non-accrual status and cease recognizing interest income on that loan until all principal and interest due has been paid or we believe the portfolio company has demonstrated the ability to repay our current and future contractual obligations. Any uncollected interest related to prior periods is reversed from income in the period that collection of the interest receivable is determined to be doubtful. However, we may make exceptions to this policy if the investment has sufficient collateral value and is in the process of collection.
At June 30, 2017, we had seven debt investments on non-accrual with a cumulative investment cost and approximate fair value of $43.6 million and $3.6 million, respectively. At December 31, 2016, we had five debt investments on non-accrual with cumulative investment cost and fair value of approximately $43.9 million and $6.2 million, respectively.
Fee income, generally collected in advance, includes loan commitment and facility fees for due diligence and structuring, as well as fees for transaction services and management services rendered by us to portfolio companies and
118
other third parties. Loan and commitment fees are amortized into income over the contractual life of the loan. Management fees are generally recognized as income when the services are rendered. Loan origination fees are capitalized and then amortized into interest income using the effective interest rate method. In certain loan arrangements, warrants or other equity interests are received from the borrower as additional origination fees. We had approximately $35.3 million of unamortized fees at June 30, 2017, of which approximately $31.8 million was included as an offset to the cost basis of our current debt investments and approximately $3.5 million was deferred contingent upon the occurrence of a funding or milestone. At December 31, 2016, we had approximately $38.2 million of unamortized fees, of which approximately $35.8 million was included as an offset to the cost basis of our current debt investments and approximately $2.4 million was deferred contingent upon the occurrence of a funding or milestone.
We recognize nonrecurring fees amortized over the remaining term of the loan commencing in the quarter relating to specific loan modifications. Certain fees may still be recognized as one-time fees, including prepayment penalties, fees related to select covenant default, waiver fees and acceleration of previously deferred loan fees and OID related to early loan pay-off or material modification of the specific debt outstanding. We recorded approximately $5.5 million and $791,000 in one-time fee income during the three months ended June 30, 2017 and 2016, respectively. We recorded approximately $6.1 million and $956,000 in one-time fee income during the six months ended June 30, 2017 and 2016, respectively.
In addition, we may also be entitled to an exit fee that is amortized into income over the life of the loan. Loan exit fees to be paid at the termination of the loan are accreted into interest income over the contractual life of the loan. At June 30, 2017, we had approximately $25.5 million in exit fees receivable, of which approximately $22.9 million was included as a component of the cost basis of our current debt investments and approximately $2.6 million was a deferred receivable related to expired commitments. At December 31, 2016, we had approximately $32.8 million in exit fees receivable, of which approximately $30.3 million was included as an offset to the cost basis of our current debt investments and approximately $2.5 million was deferred related to expired commitments.
We have debt investments in our portfolio that contain a PIK provision. Contractual PIK interest, which represents contractually deferred interest added to the loan balance that is generally due at the end of the loan term, is generally recorded on the accrual basis to the extent such amounts are expected to be collected. We will generally cease accruing PIK interest if there is insufficient value to support the accrual or we do not expect the portfolio company to be able to pay all principal and interest due. We recorded approximately $2.5 million and $1.8 million in PIK income during the three months ended June 30, 2017 and 2016, respectively. We recorded approximately $4.7 million and $3.5 million in PIK income during the six months ended June 30, 2017 and 2016, respectively.
To maintain our status as a RIC, PIK and exit fee income must be paid out to stockholders in the form of distributions even though the cash has not yet been collected. Amounts necessary to pay these distributions may come from available cash or the liquidation of certain investments.
In certain investment transactions, we may provide advisory services. For services that are separately identifiable and external evidence exists to substantiate fair value, income is recognized as earned, which is generally when the investment transaction closes. We had no income from advisory services in the three and six months ended June 30, 2017 and 2016.
Stock Based Compensation
We have issued and may, from time to time, issue stock options and restricted stock to employees under our 2004 Equity Incentive Plan and members of the Board of Directors under our 2006 Equity Incentive Plan prior to its expiration on June 21, 2017. We follow the guidelines set forth under ASC Topic 718, (“Compensation—Stock Compensation”) to account for stock options granted. Under ASC Topic 718, compensation expense
119
associated with stock based compensation is measured at the grant date based on the fair value of the award and is recognized over the vesting period. Determining the appropriate fair value model and calculating the fair value of stock-based awards at the grant date requires judgment, including estimating stock price volatility, forfeiture rate and expected option life.
Income Taxes
We intend to operate so as to qualify to be subject to tax as a RIC under Subchapter M of the Code and, as such, will not be subject to U.S. federal income tax on the portion of taxable income and gains distributed to stockholders. Taxable income includes our taxable interest, dividend and fee income, reduced by certain deductions, as well as taxable net realized securities gains. Taxable income generally differs from net income for financial reporting purposes due to temporary and permanent differences in the recognition of income and expenses, and generally excludes net unrealized appreciation or depreciation, as such gains or losses are not included in taxable income until they are realized.
To qualify and be subject to tax as a RIC, we are required to meet certain income and asset diversification tests in addition to distributing dividends of an amount generally at least equal to 90% of its investment company taxable income, as defined by the Code and determined without regard to any deduction for distributions paid, to its stockholders. The amount to be paid out as a distribution is determined by the Board of Directors each quarter and is based upon the annual earnings estimated by us. To the extent that our earnings fall below the amount of dividend distributions declared, however, a portion of the total amount of our distributions for the fiscal year may be deemed a return of capital for tax purposes to our stockholders.
During the three months ended June 30, 2017, we declared a distribution of $0.31 per share. The determination of the tax attributes of our distributions is made annually as of the end of our taxable year generally based upon its taxable income for the full taxable year and distributions paid for the full taxable year. As a result, a determination made on a quarterly basis may not be representative of the actual tax attributes of our distributions for a full taxable year. If we had determined the tax attributes of our distributions taxable year-to-date as of June 30, 2017, 100% would be from our current and accumulated earnings and profits. However, there can be no certainty to stockholders that this determination is representative of what the actual tax attributes of our 2017 distributions to stockholders will be.
As a RIC, we will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income unless we make distributions treated as dividends for U.S. federal income tax purposes in a timely manner to our stockholders in respect of each calendar year of an amount at least equal to the Excise Tax Avoidance Requirement. We will not be subject to this excise tax on any amount on which we incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains). Depending on the level of taxable income earned in a taxable year, we may choose to carry over taxable income in excess of current taxable year distributions from such taxable income into the next taxable year and incur a 4% excise tax on such taxable income, as required. The maximum amount of excess taxable income that may be carried over for distribution in the next taxable year under the Code is the total amount of distributions paid in the following taxable year, subject to certain declaration and payment guidelines. To the extent we choose to carry over taxable income into the next taxable year, distributions declared and paid by us in a taxable year may differ from taxable income for that taxable year as such distributions may include the distribution of current taxable year taxable income, the distribution of prior taxable year taxable income carried over into and distributed in the current taxable year, or returns of capital.
We have taxable subsidiaries which are designed to hold certain portfolio investments in an effort to limit potential legal liability and/or comply with source-income type requirements contained in the RIC tax provisions of the Code. These taxable subsidiaries are consolidated for GAAP financial reporting purposes and the portfolio investments held by the taxable subsidiaries are included in our consolidated financial statements, and recorded at fair value. These taxable subsidiaries are not consolidated with us for income tax purposes and may generate
120
income tax expense, or benefit, and tax assets and liabilities as a result of their ownership of certain portfolio investments. Any income generated by these taxable subsidiaries would be taxed at normal corporate tax rates based on its taxable income.
Taxable income for the six months ended June 30, 2017 was approximately $42.3 million or $0.52 per share. Taxable net realized losses for the same period was $1.2 million or approximately $0.01 per share. Taxable income for the six months ended June 30, 2016 was approximately $43.8 million or $0.61 per share. Taxable net realized losses for the same period were $2.4 million or approximately $0.03 per share.
For the six months ended June 30, 2017, we paid approximately $1.0 million of tax expense and had no accrued but unpaid tax expense as of the balance sheet date. For the six months ended June 30, 2016, we paid approximately $18,000 of tax expense and had approximately $498,000 of accrued but unpaid tax expense as of the balance sheet date.
We intend to distribute 100% of our spillover earnings, which consists of ordinary income and long term capital gains, from the taxable year ended December 31, 2016 to the our stockholders during 2017.
Recent Accounting Pronouncements
In January 2016, the FASB issued ASU 2016-01, “Financial Instruments—Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities,” which, among other things, requires that (i) all equity investments, other than equity-method investments, in unconsolidated entities generally be measured at fair value through earnings and (ii) an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments. Additionally, the ASU changes the disclosure requirements for financial instruments. ASU 2016-01 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2017. Early adoption is permitted for certain provisions. We do not believe that ASU 2016-01 will have a material impact on our consolidated financial statements and disclosures.
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842),” which, among other things, requires recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous GAAP. Additionally, the ASU requires the classification of all cash payments on leases within operating activities in the Consolidated Statement of Cash Flows. ASU 2016-02 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2018. Early adoption is permitted. We do not believe that ASU 2016-02 will have a material impact on our consolidated financial statements and disclosures.
In March 2016, the FASB issued ASU 2016-09, “Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting,” which, among other things, simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. ASU 2016-09 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2016. There is not a material impact from adopting this standard on our financial statements. We have adopted this standard for the six months ended June 30, 2017.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments,” which addresses eight specific cash flow issues including, among other things, the classification of debt prepayment or debt extinguishment costs. ASU 2016-15 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2017. Early adoption is permitted. We do not believe that ASU 2016-15 will have a material impact on our consolidated financial statements and disclosures.
121
In November 2016, the FASB issued ASU 2016-18, “Statement of Cash Flows (Topic 230),” which requires that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The new guidance is effective for interim and annual periods beginning after December 15, 2017 and early adoption is permitted. The amendment should be adopted retrospectively. We do not believe that ASU 2016-18 will have a material impact on our consolidated financial statements and disclosures.
Quantitative and Qualitative Disclosure About Market Risk
We are subject to financial market risks, including changes in interest rates. Interest rate risk is defined as the sensitivity of our current and future earnings to interest rate volatility, variability of spread relationships, the difference in re-pricing intervals between our assets and liabilities and the effect that interest rates may have on our cash flows. Changes in interest rates may affect both our cost of funding and our interest income from portfolio investments, cash and cash equivalents and idle fund investments. Our investment income will be affected by changes in various interest rates, including LIBOR and Prime rates, to the extent our debt investments include variable interest rates. As of June 30, 2017, approximately 94.5% of the loans in our portfolio had variable rates based on floating Prime or LIBOR rates with a floor. Changes in interest rates can also affect, among other things, our ability to acquire and originate loans and securities and the value of our investment portfolio.
Based on our Consolidated Statement of Assets and Liabilities as of June 30, 2017, the following table shows the approximate annualized increase (decrease) in components of net assets resulting from operations of hypothetical base rate changes in interest rates, assuming no changes in our investments and borrowings.
| (in thousands) Basis Point Change |
Interest Income |
Interest Expense |
Net Income |
EPS(1) | ||||||||||||
| 25 |
$ | 2,544 | $ | — | $ | 2,544 | $ | 0.03 | ||||||||
| 50 |
$ | 5,401 | $ | — | $ | 5,401 | $ | 0.07 | ||||||||
| 75 |
$ | 8,258 | $ | — | $ | 8,258 | $ | 0.10 | ||||||||
| 100 |
$ | 11,220 | $ | — | $ | 11,220 | $ | 0.14 | ||||||||
| 200 |
$ | 23,766 | $ | — | $ | 23,766 | $ | 0.29 | ||||||||
| 300 |
$ | 36,589 | $ | — | $ | 36,589 | $ | 0.44 | ||||||||
| (1) | Earnings per share impact calculated based on basic weighted average shares outstanding of 82,292. |
We do not currently engage in any hedging activities. However, we may, in the future, hedge against interest rate fluctuations (and foreign currency) by using standard hedging instruments such as futures, options, and forward contracts. While hedging activities may insulate us against changes in interest rates (and foreign currency), they may also limit our ability to participate in the benefits of lower interest rates with respect to our borrowed funds and higher interest rates with respect to our portfolio of investments. During the six months ended June 30, 2017 we did not engage in interest rate (or foreign currency) hedging activities.
Although we believe that the foregoing analysis is indicative of our sensitivity to interest rate changes, it does not adjust for potential changes in the credit market, credit quality, size and composition of the assets in our portfolio. It also does not adjust for other business developments, including borrowings under our Credit Facilities, SBA debentures, 2024 Notes, 2022 Convertible Notes and 2021 Asset-Backed Notes that could affect the net increase in net assets resulting from operations, or net income. It also does not assume any repayments from borrowers. Accordingly, no assurances can be given that actual results would not differ materially from the statement above.
122
Because we currently borrow, and plan to borrow in the future, money to make investments, our net investment income is dependent upon the difference between the rate at which we borrow funds and the rate at which we invest the funds borrowed. Accordingly, there can be no assurance that a significant change in market interest rates will not have a material adverse effect on our net investment income. In periods of rising interest rates, our cost of funds would increase, which could reduce our net investment income if there is not a corresponding increase in interest income generated by variable rate assets in our investment portfolio.
For additional information regarding the interest rate associated with each of our Credit Facilities, SBA debentures, 2024 Notes, 2022 Convertible Notes, and 2021 Asset-Backed Notes, please refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity and Capital Resources—Borrowings” in this prospectus.
Disclosure Controls and Procedures
The Company’s chief executive and chief financial officers, under the supervision and with the participation of the Company’s management, conducted an evaluation of the Company’s disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. As of the end of the period covered by this prospectus, the Company’s chief executive and chief financial officers have concluded that the Company’s disclosure controls and procedures were effective to ensure that information required to be disclosed by the Company in reports that the Company files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed by the Company in the reports that the Company files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including the Company’s chief executive and chief financial officers, as appropriate to allow timely decisions regarding required disclosure.
Internal Control Over Financial Reporting
a. Management’s Report on Internal Control over Financial Reporting
The Company is responsible for establishing and maintaining adequate internal control over financial reporting and for the assessment of the effectiveness of internal control over financial reporting. As defined by the SEC, internal control over financial reporting is a process designed under the supervision of the Company’s principal executive and principal financial and accounting officer, approved and monitored by the Company’s Board of Directors, and implemented by management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP.
The Company’s internal control over financial reporting is supported by written policies and procedures, that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the Company’s assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorizations of the Company’s management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
123
Management of the Company conducted an assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2016 based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“the COSO Framework”). Based on this assessment, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2016.
Report of the Independent Registered Public Accounting Firm
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2016 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm who also audited the Company’s consolidated financial statements, as stated in their report, which is included in this prospectus.
Changes in Internal Control over Financial Reporting
There have been no changes in the Company’s internal control over financing reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act, which occurred during the Company’s most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
124
We are a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences and sustainable and renewable technology industries. We source our investments through our principal office located in Palo Alto, CA, as well as through our additional offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT and San Diego, CA.
Our goal is to be the leading structured debt financing provider for venture capital-backed companies in technology-related industries requiring sophisticated and customized financing solutions. Our strategy is to evaluate and invest in a broad range of technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology and to offer a full suite of growth capital products. We focus our investments in companies active in the technology industry sub-sectors characterized by products or services that require advanced technologies, including, but not limited to, computer software and hardware, networking systems, semiconductors, semiconductor capital equipment, information technology infrastructure or services, internet consumer and business services, telecommunications, telecommunications equipment, renewable or alternative energy, media and life sciences. Within the life sciences sub-sector, we generally focus on medical devices, bio-pharmaceutical, drug discovery, drug delivery, health care services and information systems companies. Within the sustainable and renewable technology sub-sector, we focus on sustainable and renewable energy technologies and energy efficiency and monitoring technologies. We refer to all of these companies as “technology-related” companies and intend, under normal circumstances, to invest at least 80% of the value of our total assets in such businesses.
We invest primarily in structured debt with warrants and, to a lesser extent, in senior debt and equity investments. We invest primarily in private companies but also have investments in public companies. We use the term “structured debt with warrants” to refer to any debt investment, such as a senior or subordinated secured loan, that is coupled with an equity component, including warrants, options or other rights to purchase common or preferred stock. Our structured debt with warrants investments typically are secured by some or all of the assets of the portfolio company.
Our investment objective is to maximize our portfolio’s total return by generating current income from our debt investments and capital appreciation from our warrant and equity-related investments. Our primary business objectives are to increase our net income, net operating income and NAV by investing in structured debt with warrants and equity of venture capital-backed companies in technology-related industries with attractive current yields and the potential for equity appreciation and realized gains. Our equity ownership in our portfolio companies may exceed 25% of the voting securities of such companies, which represents a controlling interest under the 1940 Act. In some cases, we receive the right to make additional equity investments in our portfolio companies in connection with future equity financing rounds. Capital that we provide directly to venture capital-backed companies in technology-related industries is generally used for growth and general working capital purposes as well as in select cases for acquisitions or recapitalizations.
We also make investments in qualifying small businesses through our two wholly-owned small business investment companies, or SBICs. Our SBIC subsidiaries, Hercules Technology II, L.P., or HT II, and Hercules Technology III, L.P., or HT III, hold approximately $104.8 million and $271.5 million in assets, respectively, and accounted for approximately 5.8% and 14.9% of our total assets, respectively, prior to consolidation at June 30, 2017. At June 30, 2017, we have issued $190.2 million in SBA-guaranteed debentures in our SBIC subsidiaries. See “Regulation—Small Business Administration Regulations” for additional information regarding our SBIC subsidiaries.
We regularly engage in discussions with third parties with respect to various potential transactions. We may acquire an investment or a portfolio of investments or an entire company or sell a portion of our portfolio on an opportunistic basis. We, our subsidiaries or our affiliates may also agree to manage certain other funds that invest
125
in debt, equity or provide other financing or services to companies in a variety of industries for which we may earn management or other fees for our services. We may also invest in the equity of these funds, along with other third parties, from which we would seek to earn a return and/or future incentive allocations. Some of these transactions could be material to our business. Consummation of any such transaction will be subject to completion of due diligence, finalization of key business and financial terms (including price) and negotiation of final definitive documentation as well as a number of other factors and conditions including, without limitation, the approval of our Board of Directors and required regulatory or third party consents and, in certain cases, the approval of our stockholders. Accordingly, there can be no assurance that any such transaction would be consummated. Any of these transactions or funds may require significant management resources either during the transaction phase or on an ongoing basis depending on the terms of the transaction.
CORPORATE HISTORY AND OFFICES
We are a Maryland corporation formed in December 2003 that began investment operations in September 2004. On February 25, 2016, we changed our name from “Hercules Technology Growth Capital, Inc.” to “Hercules Capital, Inc.” We are an internally managed, non-diversified closed-end investment company that has elected to be regulated as a business development company under the 1940 Act. As a business development company, we are required to comply with certain regulatory requirements. For instance, we generally have to invest at least 70% of our total assets in “qualifying assets,” including securities of private U.S. companies, cash, cash equivalents, U.S. government securities and high-quality debt investments that mature in one year or less. A business development company also must meet a coverage ratio of total net assets to total senior securities, which include all of our borrowings (including accrued interest payable) except for debentures issued by the SBA and any preferred stock we may issue in the future, of at least 200% subsequent to each borrowing or issuance of senior securities. See “Regulation.”
Our portfolio is comprised of, and we anticipate that our portfolio will continue to be comprised of, investments primarily in technology-related companies at various stages of their development. Consistent with regulatory requirements, we invest primarily in United States based companies and, to a lesser extent, in foreign companies.
Effective January 1, 2006, we elected to be treated for tax purposes as a RIC under the Code. Pursuant to this election, we generally will not have to pay corporate-level taxes on any income that we distribute to our stockholders. However, our qualification and election to be treated as a RIC requires that we comply with provisions contained in the Code. For example, as a RIC we must receive 90% or more of our income from qualified earnings, typically referred to as “good income,” as well as satisfy asset diversification and income distribution requirements. As an investment company, we follow accounting and reporting guidance as set forth in Topic 946 of FASB’s ASC.
Our principal executive offices are located at 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301, and our telephone number is (650) 289-3060. We also have offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT and San Diego, CA. We maintain a website on the Internet at www.htgc.com. We make available, free of charge, on our website our proxy statement, annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider that information to be part of this prospectus.
We file annual, quarterly and current periodic reports, proxy statements and other information with the SEC under the Exchange Act. This information is available at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information about the operation of the SEC’s public reference room by calling the SEC at (202) 551-8090. In addition, the SEC maintains an Internet website, at www.sec.gov, that
126
contains reports, proxy and information statements, and other information regarding issuers, including us, who file documents electronically with the SEC.
OUR MARKET OPPORTUNITY
We believe that technology-related companies compete in one of the largest and most rapidly growing sectors of the U.S. economy and that continued growth is supported by ongoing innovation and performance improvements in technology products as well as the adoption of technology across virtually all industries in response to competitive pressures. We believe that an attractive market opportunity exists for a specialty finance company focused primarily on investments in structured debt with warrants in technology- related companies for the following reasons:
| • | technology-related companies have generally been underserved by traditional lending sources; |
| • | unfulfilled demand exists for structured debt financing to technology-related companies due to the complexity of evaluating risk in these investments; and |
| • | structured debt with warrants products are less dilutive and complement equity financing from venture capital and private equity funds. |
Technology-Related Companies are Underserved by Traditional Lenders. We believe many viable technology-related companies backed by financial sponsors have been unable to obtain sufficient growth financing from traditional lenders, including financial services companies such as commercial banks and finance companies because traditional lenders have continued to consolidate and have adopted a more risk-averse approach to lending. More importantly, we believe traditional lenders are typically unable to underwrite the risk associated with these companies effectively.
The unique cash flow characteristics of many technology-related companies typically include significant research and development expenditures and high projected revenue growth thus often making such companies difficult to evaluate from a credit perspective. In addition, the balance sheets of these companies often include a disproportionately large amount of intellectual property assets, which can be difficult to value. Finally, the speed of innovation in technology and rapid shifts in consumer demand and market share add to the difficulty in evaluating technology-related companies.
Due to the difficulties described above, we believe traditional lenders generally refrain from entering the structured debt financing marketplace, instead preferring the risk-reward profile of asset based lending. Traditional lenders generally do not have flexible product offerings that meet the needs of technology-related companies. The financing products offered by traditional lenders typically impose on borrowers many restrictive covenants and conditions, including limiting cash outflows and requiring a significant depository relationship to facilitate rapid liquidation.
Unfulfilled Demand for Structured Debt Financing to Technology-Related Companies. Private debt capital in the form of structured debt financing from specialty finance companies continues to be an important source of funding for technology-related companies. We believe that the level of demand for structured debt financing is a function of the level of annual venture equity investment activity.
We believe that demand for structured debt financing is currently underserved. The venture capital market for the technology-related companies in which we invest has been active. Therefore, to the extent we have capital available, we believe this is an opportune time to be active in the structured lending market for technology-related companies.
Structured Debt with Warrants Products Complement Equity Financing From Venture Capital and Private Equity Funds. We believe that technology-related companies and their financial sponsors will continue
127
to view structured debt securities as an attractive source of capital because it augments the capital provided by venture capital and private equity funds. We believe that our structured debt with warrants products provide access to growth capital that otherwise may only be available through incremental investments by existing equity investors. As such, we provide portfolio companies and their financial sponsors with an opportunity to diversify their capital sources. Generally, we believe many technology-related companies at all stages of development target a portion of their capital to be debt in an attempt to achieve a higher valuation through internal growth. In addition, because financial sponsor-backed companies have reached a more mature stage prior to reaching a liquidity event, we believe our investments could provide the debt capital needed to grow or recapitalize during the extended period sometimes required prior to liquidity events.
OUR BUSINESS STRATEGY
Our strategy to achieve our investment objective includes the following key elements:
Leverage the Experience and Industry Relationships of Our Management Team and Investment Professionals. We have assembled a team of experienced investment professionals with extensive experience as venture capitalists, commercial lenders, and originators of structured debt and equity investments in technology-related companies. Our investment professionals have, on average, more than 15 years of experience as equity investors in, and/or lenders to, technology-related companies. In addition, our team members have originated structured debt, debt with warrants and equity investments in over 380 technology-related companies, representing approximately $6.9 billion in commitments from inception to June 30, 2017, and have developed a network of industry contacts with investors and other participants within the venture capital and private equity communities. In addition, members of our management team also have operational, research and development and finance experience with technology-related companies. We have established contacts with leading venture capital and private equity fund sponsors, public and private companies, research institutions and other industry participants, which we believe will enable us to identify and attract well-positioned prospective portfolio companies.
We focus our investing activities generally in industries in which our investment professionals have investment experience. We believe that our focus on financing technology-related companies will enable us to leverage our expertise in structuring prospective investments, to assess the value of both tangible and intangible assets, to evaluate the business prospects and operating characteristics of technology-related companies and to identify and originate potentially attractive investments with these types of companies.
Mitigate Risk of Principal Loss and Build a Portfolio of Equity-Related Securities. We expect that our investments have the potential to produce attractive risk-adjusted returns through current income, in the form of interest and fee income, as well as capital appreciation from warrant and equity-related securities. We believe that we can mitigate the risk of loss on our debt investments through the combination of loan principal amortization, cash interest payments, relatively short maturities (typically between 24-48 months), security interests in the assets of our portfolio companies, and on select investment covenants requiring prospective portfolio companies to have certain amounts of available cash at the time of our investment and the continued support from a venture capital or private equity firm at the time we make our investment. Although we do not currently engage in hedging transactions, we may engage in hedging transactions in the future utilizing instruments such as forward contracts, currency options and interest rate swaps, caps, collars, and floors.
Historically our structured debt investments to technology-related companies typically include warrants or other equity interests, giving us the potential to realize equity-like returns on a portion of our investment. In addition, in some cases, we receive the right to make additional equity investments in our portfolio companies, including the right to convert some portion of our debt into equity, in connection with future equity financing rounds. We believe these equity interests will create the potential for meaningful long-term capital gains in connection with the future liquidity events of these technology-related companies.
128
Provide Customized Financing Complementary to Financial Sponsors’ Capital. We offer a broad range of investment structures and possess expertise and experience to effectively structure and price investments in technology-related companies. Unlike many of our competitors that only invest in companies that fit a specific set of investment parameters, we have the flexibility to structure our investments to suit the particular needs of our portfolio companies. We offer customized financing solutions ranging from senior debt, including below-investment grade debt instruments (also known as “junk bonds”), to equity capital, with a focus on structured debt with warrants.
We use our relationships in the financial sponsor community to originate investment opportunities. Because venture capital and private equity funds typically invest solely in the equity securities of their portfolio companies, we believe that our debt investments will be viewed as an attractive and complimentary source of capital, both by the portfolio company and by the portfolio company’s financial sponsor. In addition, we believe that many venture capital and private equity fund sponsors encourage their portfolio companies to use debt financing for a portion of their capital needs as a means of potentially enhancing equity returns, minimizing equity dilution and increasing valuations prior to a subsequent equity financing round or a liquidity event.
Invest at Various Stages of Development. We provide growth capital to technology-related companies at all stages of development, including select publicly listed companies and select special opportunity lower middle market companies that require additional capital to fund acquisitions, recapitalizations and refinancings and established-stage companies. We believe that this provides us with a broader range of potential investment opportunities than those available to many of our competitors, who generally focus their investments on a particular stage in a company’s development. Because of the flexible structure of our investments and the extensive experience of our investment professionals, we believe we are well positioned to take advantage of these investment opportunities at all stages of prospective portfolio companies’ development.
Benefit from Our Efficient Organizational Structure. We believe that the perpetual nature of our corporate structure enables us to be a long-term partner for our portfolio companies in contrast to traditional investment funds, which typically have a limited life. In addition, because of our access to the equity markets, we believe that we may benefit from a lower cost of capital than that available to private investment funds. We are not subject to requirements to return invested capital to investors nor do we have a finite investment horizon. Capital providers that are subject to such limitations are often required to seek a liquidity event more quickly than they otherwise might, which can result in a lower overall return on an investment.
Deal Sourcing Through Our Proprietary Database. We have developed a proprietary and comprehensive SQL database system to track various aspects of our investment process including sourcing, originations, transaction monitoring and post-investment performance. As of June 30, 2017, our proprietary SQL-based database system included approximately 48,000 technology-related companies and approximately 9,600 venture capital firms, private equity sponsors/investors, as well as various other industry contacts. This proprietary SQL system allows us to maintain, cultivate and grow our industry relationships while providing us with comprehensive details on companies in the technology-related industries and their financial sponsors.
OUR INVESTMENTS AND OPERATIONS
We principally invest in debt securities and, to a lesser extent, equity securities, with a particular emphasis on structured debt with warrants.
We generally seek to invest in companies that have been operating for at least six to 12 months prior to the date of our investment. We anticipate that such entities may, at the time of investment, be generating revenues or will have a business plan that anticipates generation of revenues within 24 to 48 months. Further, we anticipate that on the date of our investment we will generally obtain a lien on available assets, which may or may not include intellectual property, and these companies will have sufficient cash on their balance sheet to operate as
129
well as potentially amortize their debt for at least three to nine months following our investment. We generally require that a prospective portfolio company, in addition to having sufficient capital to support leverage, demonstrate an operating plan capable of generating cash flows or raising the additional capital necessary to cover its operating expenses and service its debt, for an additional six to twelve months subject to market conditions.
We expect that our investments will generally range from $12.0 million to $40.0 million, although we may make investments in amounts above or below this range. We typically structure our debt securities to provide for amortization of principal over the life of the loan, but may include a period of interest-only payments. Our loans will typically be collateralized by a security interest in the borrower’s assets, although we may not have the first claim on these assets and the assets may not include intellectual property. Our debt investments carry fixed or variable contractual interest rates which generally ranged from approximately 5.8% to 12.0% as of June 30, 2017. As of June 30, 2017, approximately 94.5% of our loans were at floating rates or floating rates with a floor and 5.5% of the loans were at fixed rates.
In addition to the cash yields received on our loans, in some instances, our loans generally include one or more of the following: exit fees, balloon payment fees, commitment fees, success fees or prepayment fees. In some cases our loans also include contractual PIK interest arrangements. The increases in loan balances as a result of contractual PIK arrangements are included in income for the period in which such PIK interest was accrued, which is often in advance of receiving cash payment, and are separately identified on our statements of cash flows. We also may be required to include in income for tax purposes certain other amounts prior to receiving the related cash.
In addition, the majority of our investments in the structured debt of venture capital-backed companies generally have equity enhancement features, typically in the form of warrants or other equity-related securities that are considered OID to our loans and are designed to provide us with an opportunity for potential capital appreciation. The warrants typically will be immediately exercisable upon issuance and generally will remain exercisable for the lesser of five to ten years or three to five years after completion of an IPO. The exercise prices for the warrants varies from nominal exercise prices to exercise prices that are at or above the current fair market value of the equity for which we receive warrants. We may structure warrants to provide minority rights provisions or on a very select basis put rights upon the occurrence of certain events. We generally target a total annualized return (including interest, fees and value of warrants) of 12% to 25% for our debt investments.
Typically, our structured debt and equity investments take one of the following forms:
| • | Structured Debt with Warrants. We seek to invest a majority of our assets in structured debt with warrants of prospective portfolio companies. Our investments in structured debt with warrants may be the only debt capital on the balance sheet of our portfolio companies, and in many cases we have a first priority security interest in all of our portfolio company’s assets, or in certain investments we may have a negative pledge on intellectual property. Our structured debt with warrants typically has a maturity of between two and seven years, and they may provide for full amortization after an interest only period. Our structured debt with warrants generally carries a contractual interest rate up to 12.0% and may include an additional exit fee payment or contractual PIK interest arrangements. We may structure our structured debt with warrants with restrictive affirmative and negative covenants, default penalties, prepayment penalties, lien protection, equity calls, change-in-control provisions or board observation rights. |
| • | Senior Debt. We seek to invest a limited portion of our assets in senior debt. Senior debt may be collateralized by accounts receivable and/or inventory financing of prospective portfolio companies. Senior debt has a senior position with respect to a borrower’s scheduled interest and principal payments and holds a first priority security interest in the assets pledged as collateral. Senior debt also may impose covenants on a borrower with regard to cash flows and changes in capital structure, among other items. We generally collateralize our investments by obtaining security interests in our portfolio |
130
| companies’ assets, which may include their intellectual property. In other cases we may obtain a negative pledge covering a company’s intellectual property. Our senior loans, in certain instances, may be tied to the financing of specific assets. In connection with a senior debt investment, we may also provide the borrower with a working capital line-of-credit that will carry an interest rate ranging from Prime or LIBOR plus a spread with a floor, generally maturing in one to three years, and typically secured by accounts receivable and/or inventory. |
| • | Equipment Loans. We intend to invest a limited portion of our assets in equipment-based loans to early-stage prospective portfolio companies. Equipment-based loans are secured by a first priority security interest in only the specific assets financed. These loans are generally for amounts of $1.0 million to $3.0 million but may be up to $15.0 million, carry a contractual interest rate between Prime and Prime plus 9.0%, and have an average term between three and four years. Equipment loans may also include exit fee payments. |
| • | Equity-Related Securities. The equity-related securities we hold consist primarily of warrants or other equity interests generally obtained in connection with our structured debt investments. In addition to the warrants received as a part of a structured debt financing, we typically receive the right to make equity investments in a portfolio company in connection with that company’s next round of equity financing. We may also on certain debt investments have the right to convert a portion of the debt investment into equity. These rights will provide us with the opportunity to further enhance our returns over time through opportunistic equity investments in our portfolio companies. These equity-related investments are typically in the form of preferred or common equity and may be structured with a dividend yield, providing us with a current return, and with customary anti-dilution protection and preemptive rights. We may achieve liquidity through a merger or acquisition of a portfolio company, a public offering of a portfolio company’s stock or by exercising our right, if any, to require a portfolio company to buy back the equity-related securities we hold. We may also make stand-alone direct equity investments into portfolio companies in which we may not have any debt investment in the company. As of June 30, 2017, we held warrant and equity-related securities in 164 portfolio companies. |
131
A comparison of the typical features of our various investment alternatives is set forth in the chart below.
| Structured Debt with Warrants |
Senior Debt | Equipment Loans | Equity-Related Securities | |||||
| Typical Structure | Term debt with warrants | Term or revolving debt | Term debt with warrants | Preferred stock or common stock | ||||
| Investment Horizon | Long-term, ranging from 2 to 7 years, with an average of 3 years | Usually under 3 years | Ranging from 3 to 4 years | Ranging from 3 to 7 years | ||||
| Ranking/Security | Senior secured, either first out or last out, or second lien | Senior / First lien | Secured only by underlying equipment | None/unsecured | ||||
| Covenants | Less restrictive; mostly financial |
Generally borrowing base and financial | None | None | ||||
| Risk Tolerance | Medium / High | Low | High | High | ||||
| Coupon/Dividend | Cash pay—fixed and floating rate; PIK in limited cases | Cash pay—fixed or floating rate | Cash pay—fixed or floating rate and may include PIK | Generally none | ||||
| Customization or Flexibility | More flexible | Little to none | Little to none | Flexible | ||||
| Equity Dilution | Low to medium | None to low | Low | High |
Investment Criteria
We have identified several criteria, among others, that we believe are important in achieving our investment objective with respect to prospective portfolio companies. These criteria, while not inclusive, provide general guidelines for our investment decisions.
Portfolio Composition. While we generally focus our investments in venture capital-backed companies in technology-related industries, we seek to invest across various financial sponsors as well as across various stages of companies’ development and various technology industry sub-sectors and geographies. As of June 30, 2017, approximately 76.1% of the fair value of our portfolio was composed of investments in five industries: 31.5% investments in the drug discovery & development industry, 18.2% investments in the software industry, 10.4% investments in the media/content/info industry, 8.8% investments in the drug delivery industry, and 7.2% investments in the internet consumer & business services industry.
Continuing Support from One or More Financial Sponsors. We generally invest in companies in which one or more established financial sponsors have previously invested and continue to make a contribution to the management of the business. We believe that having established financial sponsors with meaningful commitments to the business is a key characteristic of a prospective portfolio company. In addition, we look for representatives of one or more financial sponsors to maintain seats on the Board of Directors of a prospective portfolio company as an indication of such commitment.
Company Stage of Development. While we invest in companies at various stages of development, we generally require that prospective portfolio companies be beyond the seed stage of development and generally
132
have received or anticipate having commitments for their first institutional round of equity financing for early stage companies. We expect a prospective portfolio company to demonstrate progress in its product development or demonstrate a path towards revenue generation or increase its revenues and operating cash flow over time. The anticipated growth rate of a prospective portfolio company is a key factor in determining the value that we ascribe to any warrants or other equity securities that we may acquire in connection with an investment in debt securities.
Operating Plan. We generally require that a prospective portfolio company, in addition to having potential access to capital to support leverage, demonstrate an operating plan capable of generating cash flows or the ability to potentially raise the additional capital necessary to cover its operating expenses and service its debt for a specific period. Specifically, we require that a prospective portfolio company demonstrate at the time of our proposed investment that in addition to having sufficient capital to support leverage, it has an operating plan capable of generating cash flows or raising the additional capital necessary to cover its operating expenses and service its debt for an additional six to twelve months subject to market conditions.
Security Interest. In many instances we seek a first priority security interest in all of the portfolio companies’ tangible and intangible assets as collateral for our debt investment, subject in some cases to permitted exceptions. In other cases we may obtain a negative pledge prohibiting a company from pledging or otherwise encumbering their intellectual property. Although we do not intend to operate as an asset-based lender, the estimated liquidation value of the assets, if any, collateralizing the debt securities that we hold is an important factor in our credit analysis and subject to assumptions that may change over the life of the investment especially when attempting to estimate the value of intellectual property. We generally evaluate both tangible assets, such as accounts receivable, inventory and equipment, and intangible assets, such as intellectual property, customer lists, networks and databases.
Covenants. Our investments may include one or more of the following covenants: cross-default; material adverse change provisions; requirements that the portfolio company provide periodic financial reports and operating metrics; and limitations on the portfolio company’s ability to incur additional debt, sell assets, dividend recapture, engage in transactions with affiliates and consummate an extraordinary transaction, such as a merger or recapitalization without our consent. In addition, we may require other performance or financial based covenants, as we deem appropriate.
Exit Strategy. Prior to making a debt investment that is accompanied by an equity-related security in a prospective portfolio company, we analyze the potential for that company to increase the liquidity of its equity through a future event that would enable us to realize appreciation in the value of our equity interest. Liquidity events may include an IPO, a private sale of our equity interest to a third party, a merger or an acquisition of the company or a purchase of our equity position by the company or one of its stockholders.
Investment Process
We have organized our management team around the four key elements of our investment process:
| • | Origination; |
| • | Underwriting; |
| • | Documentation; and |
| • | Loan and Compliance Administration. |
133
Our investment process is summarized in the following chart:
Origination
The origination process for our investments includes sourcing, screening, preliminary due diligence and deal structuring and negotiation, all leading to an executed non-binding term sheet. As of June 30, 2017, our investment origination team, which consists of approximately 34 investment professionals, is headed by our Chief Investment Officer and our Chief Executive Officer. The origination team is responsible for sourcing potential investment opportunities and members of the investment origination team use their extensive relationships with various leading financial sponsors, management contacts within technology-related companies, trade sources, technology conferences and various publications to source prospective portfolio companies. Our investment origination team is divided into life sciences, technology, sustainable and renewable technology, and special situation sub-teams to better source potential portfolio companies.
In addition, we have developed a proprietary and comprehensive SQL-based database system to track various aspects of our investment process including sourcing, originations, transaction monitoring and post-investment performance. This proprietary SQL system allows our origination team to maintain, cultivate and grow our industry relationships while providing our origination team with comprehensive details on companies in the technology-related industries and their financial sponsors.
If a prospective portfolio company generally meets certain underwriting criteria, we perform preliminary due diligence, which may include high level company and technology assessments, evaluation of its financial sponsors’ support, market analysis, competitive analysis, identifying key management, risk analysis and transaction size, pricing, return analysis and structure analysis. If the preliminary due diligence is satisfactory, and the origination team recommends moving forward, we then structure, negotiate and execute a non-binding term sheet with the potential portfolio company. Upon execution of a term sheet, the investment opportunity moves to the underwriting process to complete formal due diligence review and approval.
134
Underwriting
The underwriting review includes formal due diligence and approval of the proposed investment in the portfolio company.
Due Diligence. Our due diligence on a prospective investment is typically completed by two or more investment professionals whom we define as the underwriting team. The underwriting team for a proposed investment consists of the deal sponsor who typically possesses general industry knowledge and is responsible for originating and managing the transaction, other investment professional(s) who perform due diligence, credit and corporate financial analyses and, as needed, our legal professionals. To ensure consistent underwriting, we generally use our standardized due diligence methodologies, which include due diligence on financial performance and credit risk as well as an analysis of the operations and the legal and applicable regulatory framework of a prospective portfolio company. The members of the underwriting team work together to conduct due diligence and understand the relationships among the prospective portfolio company’s business plan, operations and financial performance.
As part of our evaluation of a proposed investment, the underwriting team prepares an investment memorandum for presentation to the investment committee. In preparing the investment memorandum, the underwriting team typically interviews select key management of the company and select financial sponsors and assembles information necessary to the investment decision. If and when appropriate, the investment professionals may also contact industry experts and customers, vendors or, in some cases, competitors of the company.
Approval Process. The sponsoring managing director or principal presents the investment memorandum to our investment committee for consideration. The approval of a majority of our investment committee and an affirmative vote by our Chief Executive Officer is required before we proceed with any investment. The members of our investment committee are our Chief Executive Officer, our Chief Financial Officer, and our Chief Investment Officer. The investment committee generally meets weekly and more frequently on an as-needed basis.
Documentation
Our legal department administers the documentation process for our investments. This department is responsible for documenting the transactions approved by our investment committee with a prospective portfolio company. This department negotiates loan documentation and, subject to appropriate approvals, final documents are prepared for execution by all parties. The legal department generally uses the services of external law firms to complete the necessary documentation.
Loan and Compliance Administration
Our investment committee, supported by our investment team, credit team, and finance department, administers loans and track covenant compliance, if applicable, of our investments and oversees periodic reviews of our critical functions to ensure adherence with our internal policies and procedures. After funding of a loan in accordance with the investment committee’s approval, the loan is recorded in our loan administration software and our SQL-based database system. The investment team, credit team, and finance department are responsible for ensuring timely interest and principal payments and collateral management as well as advising the investment committee on the financial performance and trends of each portfolio company, including any covenant violations that occur, to aid us in assessing the appropriate course of action for each portfolio company and evaluating overall portfolio quality. In addition, the investment team and credit team advise the investment committee and the Audit Committee of our Board of Directors, accordingly, regarding the credit and investment grading for each portfolio company as well as changes in the value of collateral that may occur.
The investment team and credit team monitor our portfolio companies in order to determine whether the companies are meeting our financing criteria and their respective business plans and also monitors the financial
135
trends of each portfolio company from its monthly or quarterly financial statements to assess the appropriate course of action for each company and to evaluate overall portfolio quality. In addition, our management team closely monitors the status and performance of each individual company through our SQL-based database system and periodic contact with our portfolio companies’ management teams and their respective financial sponsors.
Credit and Investment Grading System. Our investment team and credit team use an investment grading system to characterize and monitor our outstanding loans. Our investment team and credit team monitors and, when appropriate, recommends changes to investment grading. Our investment committee reviews the recommendations and/or changes to the investment grading, which are submitted on a quarterly basis to the Audit Committee and our Board of Directors for approval.
From time to time, we will identify investments that require closer monitoring or become workout assets. We develop a workout strategy for workout assets and our investment committee monitors the progress against the strategy. We may incur losses from our investing activities, however, we work with our troubled portfolio companies in order to recover as much of our investments as is practicable, including possibly taking control of the portfolio company. There can be no assurance that principal will be recovered.
We use the following investment grading system approved by our Board of Directors:
| Grade 1. | Loans involve the least amount of risk in our portfolio. The borrower is performing above expectations, and the trends and risk profile is generally favorable. |
| Grade 2. | The borrower is performing as expected and the risk profile is neutral to favorable. All new loans are initially graded 2. |
| Grade 3. | The borrower may be performing below expectations, and the loan’s risk has increased materially since origination. We increase procedures to monitor a borrower that may have limited amounts of cash remaining on the balance sheet, is approaching its next equity capital raise within the next three to six months, or if the estimated fair value of the enterprise may be lower than when the loan was originated. We will generally lower the loan grade to a level 3 even if the company is performing in accordance to plan as it approaches the need to raise additional cash to fund its operations. Once the borrower closes its new equity capital raise, we may increase the loan grade back to grade 2 or maintain it at a grade 3 as the company continues to pursue its business plan. |
| Grade 4. | The borrower is performing materially below expectations, and the loan risk has substantially increased since origination. Loans graded 4 may experience some partial loss or full return of principal but are expected to realize some loss of interest which is not anticipated to be repaid in full, which, to the extent not already reflected, may require the fair value of the loan to be reduced to the amount we anticipate will be recovered. Grade 4 investments are closely monitored. |
| Grade 5. | The borrower is in workout, materially performing below expectations and a significant risk of principal loss is probable. Loans graded 5 will experience some partial principal loss or full loss of remaining principal outstanding is expected. Grade 5 loans will require the fair value of the loans be reduced to the amount, if any, we anticipate will be recovered. |
At June 30, 2017, our investments had a weighted average investment grading of 2.27.
Managerial Assistance
As a business development company, we are required to offer, and provide upon request, managerial assistance to our portfolio companies. This assistance could involve, among other things, monitoring the operations of our portfolio companies, participating in board and management meetings, consulting with and advising officers of portfolio companies and providing other organizational and financial guidance. We may, from time to time, receive fees for these services. In the event that such fees are received, they are incorporated
136
into our operating income and are passed through to our stockholders, given the nature of our structure as an internally managed business development company. See “Regulation—Significant Managerial Assistance” for additional information.
COMPETITION
Our primary competitors provide financing to prospective portfolio companies and include non-bank financial institutions, federally or state chartered banks, venture debt funds, financial institutions, venture capital funds, private equity funds, investment funds and investment banks. Many of these entities have greater financial and managerial resources than we have, and the 1940 Act imposes certain regulatory restrictions on us as a business development company to which many of our competitors are not subject. Additionally, competition is especially intense from commercial venture banks. However, we believe that few of our competitors possess the expertise to properly structure and price debt investments to venture capital-backed companies in technology-related industries. We believe that our specialization in financing technology-related companies will enable us to determine a range of potential values of intellectual property assets, evaluate the business prospects and operating characteristics of prospective portfolio companies and, as a result, identify investment opportunities that produce attractive risk-adjusted returns. For additional information concerning the competitive risks we face, see “Risk Factors—Risks Related to our Business and Structure—We operate in a highly competitive market for investment opportunities, and we may not be able to compete effectively.”
BROKERAGE ALLOCATIONS AND OTHER PRACTICES
Because we generally acquire and dispose of our investments in privately negotiated transactions, we typically do not use brokers in the normal course of business. However, from time to time, we may work with brokers to sell positions we have acquired in the securities of publicly listed companies or to acquire positions (principally equity) in companies where we see a market opportunity to acquire such securities at attractive valuations. In cases where we do use a broker, we do not execute transactions through any particular broker or dealer, but will seek to obtain the best net results for the Company, taking into account such factors as price (including the applicable brokerage commission or dealer spread), size of order, difficulty of execution, and operational facilities of the firm and the firm’s risk and skill in positioning blocks of securities. While we generally seek reasonably competitive execution costs, we may not necessarily pay the lowest spread or commission available. Subject to applicable legal requirements, we may select a broker based partly upon brokerage or research services provided to us. In return for such services, we may pay a higher commission than other brokers would charge if we determine in good faith that such commission is reasonable in relation to the services provided.
EMPLOYEES
As of June 30, 2017, we had 68 employees, including approximately 34 investment and portfolio management professionals, all of whom have extensive experience working on financing transactions for technology-related companies.
LEGAL PROCEEDINGS
We may, from time to time, be involved in litigation arising out of our operations in the normal course of business or otherwise. Furthermore, third parties may try to seek to impose liability on us in connection with the activities of our portfolio companies. While the outcome of any current legal proceedings cannot at this time be predicted with certainty, we do not expect any current matters will materially affect our financial condition or results of operations; however, there can be no assurance whether any pending legal proceedings will have a material adverse effect on our financial condition or results of operations in any future reporting period.
137
(dollars in thousands)
The following tables set forth certain information as of June 30, 2017 regarding each portfolio company in which we had a debt or equity investment. The general terms of our loans and other investments are described in “Business—Our Investments and Operations.” Other than these investments, our only formal relationship with our portfolio companies is the offer to make available significant managerial assistance. In addition, we may receive rights to observe the Board of Directors’ meetings of our portfolio companies. Amounts are presented in thousands.
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Debt Investments |
||||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Exicure, Inc.(11)(14A) 8045 Lamon Avenue, Suite 410 Skokie, IL 60077 |
Biotechnology Tools | Senior Secured | September 2019 | Interest rate PRIME + 6.45% or Floor rate of 9.95% | $ | 6,000 | $ | 6,046 | $ | 6,124 | ||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
6,046 | 6,124 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Biotechnology Tools (0.75%)* |
|
6,046 | 6,124 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Communications & Networking |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(13)(14B) 1441 Knightsbridge Drive Blue Bell, PA 19422 |
Communications & Networking |
Senior Secured |
August 2017 |
PIK Interest 10.50% |
$ |
819 |
|
|
928 |
|
|
928 |
| |||||||
| OpenPeak, Inc.(7) One Riverfront Plaza 1037 Raymond Boulevard Sixteenth Floor Newark, NJ 07102 |
Communications & Networking | Senior Secured | April 2018 | Interest rate PRIME + 8.75% or Floor rate of 12.00% | $ | 11,464 | 8,228 | — | ||||||||||||
| SkyCross, Inc.(6)(7)(13)(14B)(15) 1441 Knightsbridge Drive Blue Bell, PA 19422 |
Communications & Networking | Senior Secured | January 2018 | Interest rate FIXED 10.95%, PIK Interest 5.00% | $ | 14,916 | 15,058 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
24,214 | 928 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Spring Mobile Solutions, Inc.(12)(14B) 11710 Plaza America Drive, Suite 2000 Reston, VA 20190 |
Communications & Networking |
Senior Secured |
January 2019 |
Interest rate PRIME + 6.70% or Floor rate of 9.95% |
$ | 2,739 | 2,826 | 2,827 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
2,826 | 2,827 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Communications & Networking (0.46%)* |
|
27,040 | 3,755 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(14A)(15) 709N 400 W #3 North Salt Lake, UT 84054 |
Consumer & Business Products |
Senior Secured |
December 2019 |
Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ |
20,000 |
|
|
19,988 |
|
|
20,146 |
| |||||||
| Consumer & Business Products | Senior Secured | December 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.50% | $ | 1,000 | 1,000 | 1,000 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Antenna79 (p.k.a. Pong Research Corporation) |
$ | 21,000 | 20,988 | 21,146 | ||||||||||||||||
138
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Second Time Around (Simplify Holdings, LLC)(7)(14A)(15) 560 Harrison Ave., Suite 501 Boston, MA 02118 |
Consumer & Business Products |
Senior Secured |
February 2019 |
Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ |
1,886 |
|
$ |
1,920 |
|
$ |
— |
| |||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
22,908 | 21,146 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Consumer & Business Products (2.59%)* |
|
22,908 | 21,146 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Delivery |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| BioQ Pharma Incorporated(10)(14A)(14B) 185 Berry St., Ste 160 San Francisco, CA 94107 |
Drug Delivery |
Senior Secured |
May 2018 |
Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 6,356 | 6,850 | 6,850 | ||||||||||||
| Drug Delivery | Senior Secured | May 2018 | Interest rate PRIME + 7.00% or Floor rate of 10.25% | $ | 1,898 | 1,979 | 1,979 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total BioQ Pharma Incorporated |
$ | 8,254 | 8,829 | 8,829 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
8,829 | 8,829 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(9)(10)(14C)(15) 351 Galveston Drive Redwood City, CA 94063 |
Drug Delivery |
Senior Secured |
March 2020 |
Interest rate PRIME + 6.05% or Floor rate of 9.55% |
$ | 20,466 | 21,340 | 21,425 | ||||||||||||
| Agile Therapeutics, Inc.(10)(14A) 101 Poor Farm Road Princeton, NJ 08540 |
Drug Delivery | Senior Secured | December 2018 | Interest rate PRIME + 4.75% or Floor rate of 9.00% | $ | 14,004 | 14,234 | 14,150 | ||||||||||||
| Antares Pharma Inc.(9)(14A)(15) 100 Princeton South, Suite 300 Ewing, NJ 08628 |
Drug Delivery | Senior Secured | July 2022 | Interest rate PRIME + 4.50% or Floor rate of 9.50% | $ | 25,000 | 24,862 | 24,862 | ||||||||||||
| Aprecia Pharmaceuticals Company(11)(14A) 2010 Cabot Blvd., West Suite F Langhorne, PA 19047 |
Drug Delivery |
Senior Secured |
January 2020 |
Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 15,000 | 15,221 | 15,215 | ||||||||||||
| Edge Therapeutics, Inc.(11)(14A) 300 Connell Dr., Suite 4000 Berkeley Heights, NJ 07922 |
Drug Delivery | Senior Secured | February 2020 | Interest rate PRIME + 4.65% or Floor rate of 9.15% | $ | 20,000 | 20,131 | 20,226 | ||||||||||||
| Pulmatrix Inc.(8)(10)(14A) 99 Hayden Avenue, Suite 390 Lexington, MA 02421 |
Drug Delivery | Senior Secured | July 2018 | Interest rate PRIME + 6.25% or Floor rate of 9.50% | $ | 4,639 | 4,772 | 4,807 | ||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(10)(14A) 34790 Ardentech Court Fremont, CA 94555 |
Drug Delivery |
Senior Secured |
December 2018 |
Interest rate PRIME + 2.70% or Floor rate of 7.95% |
$ |
9,277 |
|
|
9,495 |
|
|
9,465 |
| |||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
110,055 | 110,150 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Delivery (14.55%)* |
|
118,884 | 118,979 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Cerecor, Inc.(11)(14A) 400 East Pratt Street, Suite 606 Baltimore, MD 21202 |
Drug Discovery & Development | Senior Secured | August 2017 | Interest rate PRIME + 4.70% or Floor rate of 7.95% | $ | 607 | 792 | 792 | ||||||||||||
139
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Epirus Biopharmaceuticals, 99 High Street Boston, MA 02110-2320 |
Drug Discovery & Development |
Senior Secured |
April 2018 |
Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ |
3,066 |
|
$ |
3,349 |
|
$ |
— |
| |||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
4,141 | 792 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Auris Medical Holding, AG(4)(9)(14B) Dornacherstrasse 210 CH-4053, Basel Switzerland |
Drug Discovery & Development | Senior Secured | January 2020 | Interest rate PRIME + 6.05% or Floor rate of 9.55% | $ | 12,500 | 12,547 | 12,586 | ||||||||||||
| Aveo Pharmaceuticals, Inc.(9)(12)(14A)(14B) One Broadway, 9th Floor Cambridge, MA 02142 |
Drug Discovery & Development | Senior Secured | December 2019 | Interest rate PRIME + 6.90% or Floor rate of 11.90% | $ | 10,000 | 10,339 | 10,377 | ||||||||||||
| Drug Discovery & Development | Senior Secured | December 2019 | Interest rate PRIME + 6.90% or Floor rate of 11.90% | $ | 10,000 | 9,842 | 9,858 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Aveo Pharmaceuticals, Inc. |
$ | 20,000 | 20,181 | 20,235 | ||||||||||||||||
| Axovant Sciences Ltd.(4)(9) 2 Church Street Hamilton, Bermuda HM11 |
Drug Discovery & Development | Senior Secured | March 2021 | Interest rate PRIME + 6.80% or Floor rate of 10.55% | $ | 55,000 | 53,333 | 53,333 | ||||||||||||
| Bellicum Pharmaceuticals, Inc.(14A)(14B)(15) 2130 West Holcombe Boulevard, Suite 800 Houston, TX 77030 |
Drug Discovery & Development | Senior Secured | March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% | $ | 15,000 | 15,421 | 15,640 | ||||||||||||
| Drug Discovery & Development | Senior Secured | March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% | $ | 5,000 | 5,022 | 5,114 | |||||||||||||
| Drug Discovery & Development | Senior Secured | March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% | $ | 10,000 | 10,030 | 10,163 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Bellicum Pharmaceuticals, Inc. |
$ | 30,000 | 30,473 | 30,917 | ||||||||||||||||
| Brickell Biotech, Inc.(11)(14B) 5777 Central Ave, Suite 102 Boulder, CO 80301 |
Drug Discovery & Development | Senior Secured | September 2019 | Interest rate PRIME + 5.70% or Floor rate of 9.20% | $ | 7,262 | 7,426 | 7,458 | ||||||||||||
| Concert Pharmaceuticals, Inc.(14A)(15) 99 Hayden Avenue, Suite 500 Lexington, MA 02421-7966 |
Drug Discovery & Development | Senior Secured | June 2021 | Interest rate PRIME + 4.05% or Floor rate of 8.55% | $ | 30,000 | 29,540 | 29,540 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(10)(14A) 3101 Western Avenue, Suite 600 Seattle, WA 98121 |
Drug Discovery & Development |
Senior Secured |
December 2018 |
Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ |
15,639 |
|
|
15,469 |
|
|
15,589 |
| |||||||
| CytRx Corporation(10)(14B)(15) 11726 San Vicente Blvd., Suite 650 Los Angeles, CA 90049 |
Drug Discovery & Development | Senior Secured | February 2020 | Interest rate PRIME + 6.00% or Floor rate of 9.50% | $ | 22,573 | 23,068 | 23,265 | ||||||||||||
| Genocea Biosciences, Inc.(10)(14A) 100 Acorn Park Drive, 5th Floor Cambridge, MA 02140 |
Drug Discovery & Development | Senior Secured | January 2019 | Interest rate PRIME + 2.25% or Floor rate of 7.25% | $ | 17,000 | 17,475 | 17,532 | ||||||||||||
| Immune Pharmaceuticals(10)(14B) 430 East 29th St., Suite 940 New York, NY 10016 |
Drug Discovery & Development | Senior Secured | September 2018 | Interest rate PRIME + 4.75% or Floor rate of 10.00% | $ | 2,398 | 2,551 | 2,551 | ||||||||||||
| Insmed, Incorporated (10)(14A) 10 Finderne Avenue, Building 10 Bridgewater, NJ 08807 |
Drug Discovery & Development | Senior Secured | October 2020 | Interest rate PRIME + 4.75% or Floor rate of 9.25% | $ | 55,000 | 55,065 | 55,082 | ||||||||||||
140
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Metuchen Pharmaceuticals LLC(13)(14A) 11 Commerce Drive, First Floor Cranford, NJ 07016 |
Drug Discovery & Development | Senior Secured | October 2020 | Interest rate PRIME + 7.25% or Floor rate of 10.75%, PIK Interest 1.35% | $ | 35,322 | $ | 35,030 | $ | 35,221 | ||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(14A)(15) 75 Park Plaza, 4th Floor Boston, MA 02116 |
Drug Discovery & Development |
Senior Secured |
September 2020 |
Interest rate PRIME + 2.75% or Floor rate of 8.50% |
$ |
40,000 |
|
|
39,721 |
|
|
39,744 |
| |||||||
| Drug Discovery & Development | Senior Secured | September 2020 | Interest rate PRIME + 2.75% or Floor rate of 8.50% | $ | 10,000 | 9,934 | 9,937 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.) |
$ | 50,000 | 49,655 | 49,681 | ||||||||||||||||
|
PhaseRx,Inc.(14B)(15) 410 West Harrison Street, Suite 300 Seattle, WA 98119 |
Drug Discovery & Development | Senior Secured | December 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% | $ | 6,000 | 6,034 | 6,047 | ||||||||||||
| Sorrento Therapeutics, Inc. (9)(14A) 9380 Judicial Dr San Diego, CA 92121 |
Drug Discovery & Development |
Senior Secured |
December 2020 |
Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 30,000 | 28,879 | 28,736 | ||||||||||||
| Stealth Bio Therapeutics Corp.(4)(9)(14A) 275 Grove Street, Suite 3-107 Newton, MA 02466 |
Drug Discovery & Development |
Senior Secured |
January 2021 |
Interest rate PRIME + 5.50% or Floor rate of 9.50% |
$ | 12,500 | 12,260 | 12,260 | ||||||||||||
| uniQure B.V.(4)(9)(10)(14B) Tafelbergweg 51 Amsterdam, The Netherlands 1105 BD |
Drug Discovery & Development | Senior Secured | May 2020 | Interest rate PRIME + 3.00% or Floor rate of 8.25% | $ | 20,000 | 20,359 | 20,342 | ||||||||||||
| Verastem, Inc.(14A)(17) 117 Kendrick Street, Suite 500 Needham, MA 02494 |
Drug Discovery & Development | Senior Secured | December 2020 | Interest rate PRIME + 6.00% or Floor rate of 10.50% | $ | 2,500 | 2,465 | 2,465 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
421,810 | 422,840 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Discovery & Development (51.82%)* |
|
425,951 | 423,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| 908 DEVICES INC.(14A)(15)(17) 27 Drydock Avenue, 7th Floor Boston, MA 02210 |
Electronics & Computer Hardware |
Senior Secured |
September 2020 |
Interest rate PRIME + 4.00% or Floor rate of 8.25% |
$ | 7,500 | 7,470 | 7,470 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
7,470 | 7,470 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.91%)* |
|
7,470 | 7,470 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| PH Group Holdings 950 N Glebe Rd., Suite 4000 Arlington, VA 22203 |
Healthcare Services, Other | Senior Secured | September 2020 | Interest rate PRIME + 7.45% or Floor rate of 10.95% | $ | 20,000 | 19,841 | 19,955 | ||||||||||||
| Healthcare Services, Other | Senior Secured | September 2020 | Interest rate PRIME + 7.45% or Floor rate of 10.95% | $ | 10,000 | 9,899 | 9,899 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total PH Group Holdings |
$ | 30,000 | 29,740 | 29,854 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
29,740 | 29,854 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Healthcare Services, Other (3.65%)* |
|
29,740 | 29,854 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
141
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Information Services |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| MDX Medical, Inc.(13)(15)(17) 160 Chubb Avenue, Suite 301 Lyndhurst, NJ 07071 |
Information Services | Senior Secured | December 2020 | Interest rate PRIME + 4.00% or Floor rate of 8.25%, PIK Interest 1.70% | $ | 7,502 | $ | 7,264 | $ | 7,264 | ||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
7,264 | 7,264 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Information Services (0.89%)* |
|
7,264 | 7,264 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Aria Systems, Inc.(10)(13) 575 Market Street, 32nd Floor San Francisco, CA 94105 |
Internet Consumer & Business Services | Senior Secured | June 2019 | Interest rate PRIME + 3.20% or Floor rate of 6.95%, PIK Interest 1.95% | $ | 2,082 | 2,071 | 2,068 | ||||||||||||
| Internet Consumer & Business Services | Senior Secured | June 2019 | Interest rate PRIME + 5.20% or Floor rate of 8.95%, PIK Interest 1.95% | $ | 18,646 | 18,539 | 18,533 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Aria Systems, Inc. |
$ | 20,728 | 20,610 | 20,601 | ||||||||||||||||
| Intent Media, Inc.(13)(14A)(15) 315 Hudson St., 9th Floor New York, NY 10013 |
Internet Consumer & Business Services | Senior Secured | May 2019 | Interest rate PRIME + 5.25% or Floor rate of 8.75%, PIK Interest 1.00% | $ | 5,025 | 4,929 | 4,957 | ||||||||||||
| Internet Consumer & Business Services | Senior Secured | May 2019 | Interest rate PRIME + 5.50% or Floor rate of 9.00%, PIK Interest 2.35% | $ | 2,000 | 1,938 | 1,940 | |||||||||||||
| Internet Consumer & Business Services | Senior Secured | May 2019 | Interest rate PRIME + 5.50% or Floor rate of 9.00%, PIK Interest 2.50% | $ | 2,000 | 1,938 | 1,940 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Intent Media, Inc. |
$ | 9,025 | 8,805 | 8,837 | ||||||||||||||||
|
LogicSource(14B)(15) 20 Marshall Street Sourth Norwalk, CT 06854 |
Internet Consumer & Business Services | Senior Secured | October 2019 | Interest rate PRIME + 6.25% or Floor rate of 9.75% | $ | 8,001 | 8,147 | 8,241 | ||||||||||||
| Snagajob.com, Inc.(12)(13)(14A) 1919 N Lynn Street, 7th Floor Arlington, VA 22209 |
Internet Consumer & Business Services | Senior Secured | July 2020 | Interest rate PRIME + 5.15% or Floor rate of 9.15%, PIK Interest 1.95% | $ | 35,642 | 35,125 | 35,788 | ||||||||||||
| Tectura Corporation(6)(7)(8)(13) 951 Old County Road, Suite 2-317 Belmont, CA 94002 |
Internet Consumer & Business Services | Senior Secured | June 2021 | Interest rate FIXED 6.00%, PIK Interest 3.00% | $ | 19,991 | 19,991 | 19,991 | ||||||||||||
| Internet Consumer & Business Services | Senior Secured | June 2021 | PIK Interest 8.00% | $ | 11,015 | 240 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Tectura Corporation |
$ | 31,006 | 20,231 | 19,991 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
92,918 | 93,458 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (11.43%)* |
|
92,918 | 93,458 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
142
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Media/Content/Info |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| Machine Zone, Inc.(13)(16) 1050 Page Mill Road Palo Alto, CA 94304 |
Media/Content/Info | Senior Secured | May 2018 | Interest rate PRIME + 2.50% or Floor rate of 6.75%, PIK Interest 3.00% | $ | 105,369 | $ | 104,512 | $ | 104,512 | ||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
104,512 | 104,512 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| FanDuel, Inc.(14B) 300 Park Avenue South, 14th Floor New York, NY 10005 |
Media/Content/Info | Senior Secured | November 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.75% | $ | 20,000 | 19,871 | 19,851 | ||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9)(11)(14B) 4950 Yonge Street, Suite 208 Toronto, ON M2M 3V5 |
Media/Content/Info |
Senior Secured |
April 2020 |
Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ |
5,000 |
|
|
5,080 |
|
|
5,177 |
| |||||||
| Media/Content/Info | Senior Secured | April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% | $ | 5,000 | 4,997 | 5,077 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total WP Technology, Inc. (Wattpad, Inc.) |
$ | 10,000 | 10,077 | 10,254 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
29,948 | 30,105 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (16.47%)* |
|
134,460 | 134,617 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| Amedica Corporation(8)(14B)(15) 1885 West 2100 South Salt Lake City, UT 84119 |
Medical Devices & Equipment | Senior Secured | January 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95% | $ | 4,098 | 5,678 | 5,678 | ||||||||||||
| Gamma Medica, Inc.(7)(10)(14B) 12 Manor Parkway, Unit 3 Salem, NH 03079 |
Medical Devices & Equipment | Senior Secured | January 2018 | Interest rate PRIME + 6.50% or Floor rate of 9.75% | $ | 161 | 366 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
6,044 | 5,678 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Aspire Bariatrics, Inc.(14B)(15) 3200 Horizon Drive, Suite 100 King of Prussia, PA 19406 |
Medical Devices & Equipment | Senior Secured | October 2018 | Interest rate PRIME + 4.00% or Floor rate of 9.25% | $ | 3,943 | 4,173 | 4,126 | ||||||||||||
| Medical Devices & Equipment |
Senior Secured |
June 2019 |
Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 15,000 | 15,370 | 15,362 | |||||||||||||
| IntegenX, Inc.(14B)(15) 5720 Stoneridge Drive, Suite 300 Pleasanton, CA 94588 |
Medical Devices & Equipment | Senior Secured | June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% | $ | 2,500 | 2,528 | 2,528 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total IntegenX, Inc. |
$ | 17,500 | 17,898 | 17,890 | ||||||||||||||||
| Micell Technologies,
Inc.(11)(14B) 801 Capitola Drive,
Suite 1 |
Medical Devices & Equipment | Senior Secured | August 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.50% | $ | 6,909 | 7,006 | 7,070 | ||||||||||||
| Quanta Fluid Solutions(4)(9)(10)(14B) Tything Road Alcester, UK B49 6EU |
Medical Devices & Equipment | Senior Secured | April 2020 | Interest rate PRIME + 8.05% or Floor rate of 11.55% | $ | 11,625 | 11,811 | 11,847 | ||||||||||||
|
Quanterix
Corporation(10)(14A) 113 Hartwell
Avenue |
Medical Devices & Equipment | Senior Secured | March 2019 | Interest rate PRIME + 2.75% or Floor rate of 8.00% | $ | 9,043 | 9,427 | 9,424 | ||||||||||||
143
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
|
Sebacia(14B)(15) 2905 Premiere Parkway, Suite 150 Duluth, GA 30097 |
Medical Devices & Equipment |
Senior Secured |
July 2020 |
Interest rate PRIME + 4.35% or Floor rate of 8.85% |
$ | 8,000 | $ | 7,805 | $ | 7,805 | ||||||||||
| Tela Bio, Inc.(14A)(15) One Great Valley Pkwy, Suite 24 Malvern, PA 19355 |
Medical Devices & Equipment | Senior Secured | September 2020 | Interest rate PRIME + 4.95% or Floor rate of 9.45% | $ | 5,000 | 4,945 | 4,945 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
63,065 | 63,107 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Medical Devices & Equipment (8.41%)* |
|
69,109 | 68,785 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Semiconductors |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(15) 2953 Bunker Hill Lane, Suite 101 Santa Clara, CA 95054 |
Semiconductors | Senior Secured | November 2017 | Interest rate PRIME + 7.00% or Floor rate of 10.50% | $ | 4,025 | 4,025 | 4,025 | ||||||||||||
| Aquantia Corp.(17) 105 E. Tasman Drive San Jose, CA 95134 |
Semiconductors | Senior Secured | February 2018 | Interest rate PRIME + 3.95% or Floor rate of 7.20% |
$ | 5,000 | 5,000 | 5,000 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
9,025 | 9,025 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(14B)(15) 2953 Bunker Hill Lane, Suite 101 Santa Clara, CA 95054 |
Semiconductors |
Senior Secured |
July 2018 |
Interest rate PRIME + 8.25% or Floor rate of 11.50% |
$ |
2,356 |
|
|
2,623 |
|
|
2,607 |
| |||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
2,623 | 2,607 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Semiconductors (1.42%)* |
|
11,648 | 11,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Software |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Clickfox, Inc.(12)(14C) 3445 Peachtree Road, Suite 450 Atlanta, GA 30326 |
Software | Senior Secured | May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.50% | $ | 9,672 | 10,437 | 10,437 | ||||||||||||
| Cloud Technology Partners, Inc. 321 Summer Street, 5th Floor Boston, MA 02210 |
Software | Senior Secured | June 2018 | Interest rate PRIME + 3.05% or Floor rate of 7.05% | $ | 3,400 | 3,400 | 3,400 | ||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(7)(13)(14A)(14C)(15)(18) 21250 Hawthorne Boulevard, Suite 380 Torrance, CA 90503 |
Software |
Senior Secured |
March 2018 |
Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ |
13,000 |
|
|
12,747 |
|
|
3,220 |
| |||||||
| Software | Senior Secured | February 2017 | Interest rate FIXED 5.75%, PIK Interest 10.75% | $ | 1,566 | 1,698 | 429 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.) |
$ | 14,566 | 14,445 | 3,649 | ||||||||||||||||
| RedSeal Inc.(14A)(15)(17) 940 Stewart Drive, Suite 101 Sunnyvale, CA 94085 |
Software | Senior Secured | August 2017 | Interest rate PRIME + 3.25% or Floor rate of 6.50% | $ | 1,205 | 1,205 | 1,205 | ||||||||||||
| Software | Senior Secured | June 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% | $ | 3,431 | 3,581 | 3,581 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total RedSeal Inc. |
$ | 4,636 | 4,786 | 4,786 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
33,068 | 22,272 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
144
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Clarabridge, Inc.(13) 11400 Commerce Park Drive., Suite 500 Reston, VA 20191 |
Software | Senior Secured | April 2021 | Interest rate PRIME + 4.80% or Floor rate of 8.55%, PIK Interest 3.25% | $ | 40,224 | $ | 40,196 | $ | 40,196 | ||||||||||
| Cloud Technology Partners, Inc.(14A) 321 Summer Street, 5th Floor Boston, MA 02210 |
Software | Senior Secured | December 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.75% | $ | 10,000 | 9,982 | 9,914 | ||||||||||||
| Evernote Corporation(13)(15)(17) 305 Walnut Street Redwood City, CA 94063 |
Software | Senior Secured | October 2020 | Interest rate PRIME + 5.45% or Floor rate of 8.95% | $ | 6,000 | 5,967 | 6,134 | ||||||||||||
| Software | Senior Secured | July 2021 | Interest rate PRIME + 6.00% or Floor rate of 9.50%, PIK Interest 1.25% | $ | 4,000 | 3,972 | 3,972 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Evernote Corporation |
$ | 10,000 | 9,939 | 10,106 | ||||||||||||||||
| Fuze, Inc.(13)(14A)(15) 2 Copley Place, Floor 7 Boston, MA 02116 |
Software | Senior Secured | July 2021 | Interest rate PRIME + 3.70% or Floor rate of 7.95%, PIK Interest 1.55% | $ | 50,000 | 49,901 | 49,901 | ||||||||||||
| Impact Radius Holdings, Inc.(13)(14A) 223 East De La Guerra Street Santa Barbara, CA 93101 |
Software | Senior Secured | December 2020 | Interest rate PRIME + 4.25% or Floor rate of 8.75%, PIK Interest 1.55% | $ | 5,000 | 4,990 | 4,990 | ||||||||||||
| Lithium Technologies, Inc.(13)(14A)(15)(19) 225 Bush St. San Francisco, CA 94104 |
Software | Senior Secured | June 2020 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 1.80% | $ | 25,247 | 25,351 | 25,351 | ||||||||||||
| OneLogin, Inc.(13)(15) 150 Spear Street, Suite 1400 San Francisco, CA 94105 |
Software | Senior Secured | August 2019 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 3.25% | $ | 15,623 | 15,526 | 15,838 | ||||||||||||
| Quid, Inc.(13)(14A)(15) 600 Harrison Street, Suite 400 San Francisco, CA 94107 |
Software | Senior Secured | October 2019 | Interest rate PRIME + 4.75% or Floor rate of 8.25%, PIK Interest 2.25% | $ | 8,208 | 8,278 | 8,399 | ||||||||||||
| RedSeal Inc.(14A)(15)(17) 940 Stewart Drive, Suite 101 Sunnyvale, CA 94085 |
Software | Senior Secured | January 2020 | Interest rate PRIME + 7.75% or Floor rate of 11.25% | $ | 5,000 | 4,952 | 4,952 | ||||||||||||
| Signpost, Inc.(13)(14A)(15) 127 W 26th St., Floor 2 New York, NY 10001 |
Software | Senior Secured | February 2020 | Interest rate PRIME + 4.15% or Floor rate of 8.15%, PIK Interest 1.75% | $ | 15,373 | 15,306 | 15,447 | ||||||||||||
| Vela Trading Technologies(17) 211 East 43rd Street, 5th Floor New York, NY 10017 |
Software | Senior Secured | July 2022 | Interest rate LIBOR + 9.50% or Floor rate of 10.50% | $ | 15,200 | 14,782 | 14,782 | ||||||||||||
| Wrike, Inc.(13)(14A)(17) 10 Almaden Blvd, Suite 1000 San Jose, CA 95113 |
Software | Senior Secured | February 2021 | Interest rate PRIME + 6.00% or Floor rate of 9.50%, PIK Interest 2.00% | $ | 10,062 | 9,790 | 9,790 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
208,993 | 209,666 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (28.37%)* |
|
242,061 | 231,938 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
145
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Alimera Sciences, Inc.(10)(13)(14A) 6120 Windward Parkway, Suite 290 Alpharetta, GA 30005 |
Specialty Pharmaceuticals | Senior Secured | November 2020 | Interest rate PRIME + 7.50% or Floor rate of 11.00%, PIK Interest 1.00% | $ | 35,218 | $ | 35,049 | $ | 35,398 | ||||||||||
| Jaguar Animal Health, Inc.(10)(14B) 201 Mission Street, Suite 2375 San Francisco, CA 94105 |
Specialty Pharmaceuticals | Senior Secured | August 2018 | Interest rate PRIME + 5.65% or Floor rate of 9.90% | $ | 2,520 | 2,876 | 2,821 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
37,925 | 38,219 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (4.68%)* |
|
37,925 | 38,219 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Surgical Devices |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Transmedics, Inc.(12)(14B) 200 Minuteman Road, Suite 302 Andover, MA 01810 |
Surgical Devices | Senior Secured | February 2020 | Interest rate PRIME + 5.30% or Floor rate of 9.55% | $ | 8,500 | 8,621 | 8,632 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
8,621 | 8,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (1.06%)* |
|
8,621 | 8,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Sustainable and Renewable Technology |
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| FuelCell Energy, Inc.(11)(14B) 3 Great Pasture Road Danbury, CT 06810 |
Sustainable and Renewable Technology | Senior Secured | October 2018 | Interest rate PRIME + 5.50% or Floor rate of 9.50% | $ | 20,000 | 20,925 | 21,034 | ||||||||||||
| Proterra, Inc.(10)(14A)(14B) 1 Whitlee Ct. Greenville, SC 29607 |
Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate PRIME + 6.95% or Floor rate of 10.20% | $ | 5,000 | 5,109 | 5,137 | ||||||||||||
| Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate PRIME + 6.95% or Floor rate of 10.20% | $ | 25,000 | 25,872 | 25,814 | |||||||||||||
| Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% | $ | 10,000 | 10,089 | 10,115 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Proterra, Inc. |
$ | 40,000 | 41,070 | 41,066 | ||||||||||||||||
| Rive Technology, Inc.(14A)(15) 1 Deer Park Drive, Suite A Monmouth Junction, NJ 08852 |
Sustainable and Renewable Technology | Senior Secured | January 2019 | Interest rate PRIME + 6.20% or Floor rate of 9.45% | $ | 6,061 | 6,234 | 6,283 | ||||||||||||
| Tendril Networks(11)(14B) 2580 55th Street, Suite 100 Boulder, CO 80301 |
Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate FIXED 9.25% | $ | 13,156 | 13,765 | 13,735 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
81,994 | 82,118 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (10.05%)* |
|
81,994 | 82,118 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total: Debt Investments (157.52%)* |
|
1,324,039 | 1,287,623 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
146
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Equity Investments |
||||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||||
| NuGEN Technologies, Inc.(15) 201 Industrial Road, Suite 310 San Carlos, CA 94070 |
Biotechnology Tools | Equity | 0.69% | Common Stock | 55,780 | $ | 500 | $ | — | |||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Biotechnology Tools (0.00%)* |
|
500 | — | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Communications & Networking |
||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(15) 1441 Knightsbridge Drive Blue Bell, PA 19422 |
Communications & Networking |
Equity |
100.00% |
Common Stock |
100 | 4,000 | 1,188 | |||||||||||||
| GlowPoint, Inc.(3) 1776 Lincoln Street, 13th Floor Denver, CO 80203 |
Communications & Networking | Equity | 0.31% | Common Stock | 114,192 | 101 | 32 | |||||||||||||
| Peerless Network Holdings, Inc. 222 South Riverside Plaza, Suite 2730 Chicago, IL 60606 |
Communications & Networking | Equity | 0.21% | Preferred Series A | 1,000,000 | 1,000 | 4,585 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Communications & Networking (0.71%)* |
|
5,101 | 5,805 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||
| Market Force Information, Inc. PO Box 270355 Louisville, CO 80027 |
Consumer & Business Products | Equity | 0.67% | Common Stock | 480,261 | — | 433 | |||||||||||||
| Consumer & Business Products | Equity | 0.26% | Preferred Series B-1 | 187,970 | 500 | 280 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Market Force Information, Inc. |
668,231 | 500 | 713 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Consumer & Business Products (0.09%)* |
|
500 | 713 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Diagnostic |
||||||||||||||||||||
| Singulex, Inc. 1701 Harbor Way Parkway, Suite 200 Alameda, CA 94502 |
Diagnostic | Equity | 0.37% | Common Stock | 937,998 | 750 | 655 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Diagnostic (0.08%)* |
|
750 | 655 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Delivery |
||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9) 351 Galveston Drive Redwood City, CA 94063 |
Drug Delivery | Equity | 0.12% | Common Stock | 54,240 | 108 | 117 | |||||||||||||
| BioQ Pharma Incorporated(15) 185 Berry St., Ste 160 San Francisco, CA 94107 |
Drug Delivery | Equity | 0.56% | Preferred Series D | 165,000 | 500 | 599 | |||||||||||||
| Edge Therapeutics, Inc.(3) 300 Connell Dr., Suite 4000 Berkeley Heights, NJ 07922 |
Drug Delivery | Equity | 0.17% | Common Stock | 53,165 | 329 | 545 | |||||||||||||
| Merrion Pharmaceuticals, Plc(4)(9) 3200 Lake Drive, Citywest Business Campus Dublin, Ireland 24 |
Drug Delivery | Equity | 0.11% | Common Stock | 20,000 | 9 | — | |||||||||||||
| Neos Therapeutics, Inc.(3)(15) 2940 N. Highway 360, Suite 400 Grand Prarie, TX 75050 |
Drug Delivery | Equity | 0.46% | Common Stock | 125,000 | 1,500 | 913 | |||||||||||||
| Revance Therapeutics, Inc.(3) 7555 Gateway Blvd. Newark, CA 94560 |
Drug Delivery | Equity | 0.07% | Common Stock | 22,765 | 557 | 601 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Delivery (0.34%)* |
|
3,003 | 2,775 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
147
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9)(15) One Broadway, 9th Floor Cambridge, MA 02142 |
Drug Discovery & Development | Equity | 0.37% | Common Stock | 426,931 | $ | 1,060 | $ | 950 | |||||||||||
| Cerecor, Inc.(3) 400 East Pratt Street, Suite 606 Baltimore, MD 21202 |
Drug Discovery & Development | Equity | 0.85% | Common Stock | 119,087 | 1,000 | 68 | |||||||||||||
| Cerulean Pharma, Inc.(3) 35 Gatehouse Drive Waltham, MA 02451 |
Drug Discovery & Development |
Equity | 0.47% | Common Stock | 135,501 | 1,000 | 60 | |||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) 87 Cambridge Park Dr Cambridge, MA 02140 |
Drug Discovery & Development | Equity | 0.69% | Common Stock | 142,858 | 1,000 | 453 | |||||||||||||
| Dynavax Technologies(3)(9) 2929 Seventh Street, Suite 100 Berkley, CA 94710 |
Drug Discovery & Development | Equity | 0.04% | Common Stock | 20,000 | 550 | 193 | |||||||||||||
| Epirus Biopharmaceuticals, Inc. 99 High Street Boston, MA 02110-2320 |
Drug Discovery & Development | Equity | 0.76% | Common Stock | 200,000 | 1,000 | — | |||||||||||||
| Genocea Biosciences, Inc.(3) 100 Acorn Park Drive, 5th Floor Cambridge, MA 02140 |
Drug Discovery & Development | Equity | 0.78% | Common Stock | 223,463 | 2,000 | 1,166 | |||||||||||||
| Inotek Pharmaceuticals Corporation(3) 131 Hartwell Ave., Suite 105 Lexington, MA 02421 |
Drug Discovery & Development | Equity | 0.01% | Common Stock | 3,778 | 1,500 | 7 | |||||||||||||
| Insmed, Incorporated(3) 10 Finderne Avenue, Building 10 Bridgewater, NJ 08807 |
Drug Discovery & Development | Equity | 0.11% | Common Stock | 70,771 | 1,000 | 1,214 | |||||||||||||
| Melinta Therapeutics 300 TriState International, Suite 272 Lincolnshire, IL 60069 |
Drug Discovery & Development | Equity | 0.67% | Preferred Series 4 | 1,914,448 | 2,000 | 2,598 | |||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3) 75 Park Plaza, 4th Floor Boston, MA 02116 |
Drug Discovery & Development |
Equity |
0.28% |
Common Stock |
|
76,362 |
|
|
2,743 |
|
|
1,840 |
| |||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Discovery & Development (1.05%)* |
|
14,853 | 8,549 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||||
| Identiv, Inc.(3) 1900-B Carnegie Avenue, Building B Santa Ana, CA 92705 |
Electronics & Computer Hardware | Equity | 0.05% | Common Stock | 6,700 | 34 | 35 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.00%)* |
|
34 | 35 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Information Services |
||||||||||||||||||||
| DocuSign, Inc. 221 Main St., Suite 1000 San Francisco, CA 94105 |
Information Services | Equity | 0.24% | Common Stock | 385,000 | 6,081 | 7,201 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Information Services (0.88%)* |
|
6,081 | 7,201 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||||
| Blurb, Inc.(15) 580 California St., Suite 300 San Francisco, CA 94104 |
Internet Consumer & Business Services | Equity | 0.38% | Preferred Series B | 220,653 | 175 | 170 | |||||||||||||
148
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Brigade Group, Inc. (p.k.a. Philotic, Inc.) 548 4th street San Francisco, CA 94107 |
Internet Consumer & Business Services |
Equity |
0.05% |
Common Stock |
9,023 | $ | 93 | $ | — | |||||||||||
| Lightspeed POS, Inc.(4)(9) 700 St-Antoine Est, Suite 300 Montreal, Canada H2Y1A6 |
Internet Consumer & Business Services | Equity | 0.09% | Preferred Series C | 230,030 | 250 | 245 | |||||||||||||
| Internet Consumer & Business Services | Equity | 0.08% | Preferred Series D | 198,677 | 250 | 241 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Lightspeed POS, Inc. |
428,707 | 500 | 486 | |||||||||||||||||
| OfferUp, Inc. 701 5th Avenue, Suite 5100 Seattle, WA 98104 |
Internet Consumer & Business Services | Equity | 0.15% | Preferred Series A | 286,080 | 1,663 | 1,917 | |||||||||||||
| Internet Consumer & Business Services | Equity | 0.06% | Preferred Series A-1 | 108,710 | 632 | 728 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total OfferUp, Inc. |
394,790 | 2,295 | 2,645 | |||||||||||||||||
| Oportun (p.k.a. Progress Financial) 1600 Seaport Blvd., Suite 250 Redwood City, CA 94063 |
Internet Consumer & Business Services | Equity | 0.09% | Preferred Series G | 218,351 | 250 | 430 | |||||||||||||
| Internet Consumer & Business Services | Equity | 0.03% | Preferred Series H | 87,802 | 250 | 254 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Oportun (p.k.a. Progress Financial) |
306,153 | 500 | 684 | |||||||||||||||||
| RazorGator Interactive Group, Inc. 4216 3/4 Glencoe Ave Marina Del Rey, CA 90292 |
Internet Consumer & Business Services | Equity | 0.11% | Preferred Series AA | 34,783 | 15 | 46 | |||||||||||||
| Tectura Corporation(6) 951 Old County Road, Suite 2-317 Belmont, CA 94002 |
Internet Consumer & Business Services | Equity | 0.12% | Preferred Series BB | 1,000,000 | — | — | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.49%)* |
|
3,578 | 4,031 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Media/Content/Info |
||||||||||||||||||||
| Pinterest, Inc. 777 South Figueroa Street, Suite 3200 Los Angeles, CA 90017-5855 |
Media/Content/Info | Equity | 0.04% | Preferred Series Seed | 620,000 | 4,085 | 4,452 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (0.54%)* |
|
4,085 | 4,452 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||
| AtriCure, Inc.(3)(15) 7555 Innovation Way Mason, Ohio 45040 |
Medical Devices & Equipment | Equity | 0.02% | Common Stock | 7,536 | 266 | 168 | |||||||||||||
| Flowonix Medical Incorporated 500 International Drive, Suite 200 Mount Olive, NJ 07828 |
Medical Devices & Equipment | Equity | 1.08% | Preferred Series AA | 221,893 | 1,500 | — | |||||||||||||
| Gelesis, Inc.(15) 500 Boylston Street, Suite 1600 Boston, MA 02116 |
Medical Devices & Equipment | Equity | 1.25% | Common Stock | 198,202 | — | 888 | |||||||||||||
| Medical Devices & Equipment | Equity | 1.20% | Preferred Series A-1 | 191,210 | 425 | 954 | ||||||||||||||
| Medical Devices & Equipment | Equity | 1.20% | Preferred Series A-2 | 191,626 | 500 | 905 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Gelesis, Inc. |
581,038 | 925 | 2,747 | |||||||||||||||||
| HercGamma, Inc.(6) 400 Hamilton Ave., Suite 310 Palo Alto, CA 94301 |
Medical Devices & Equipment | Equity | 100.00% | Common Stock | 100 | 1,169 | 1,169 | |||||||||||||
149
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Medrobotics Corporation(15) 475 Paramount Drive Raynham, MA 02767 |
Medical Devices & Equipment | Equity | 0.12% | Preferred Series E | 136,798 | $ | 250 | $ | 236 | |||||||||||
| Medical Devices & Equipment | Equity | 0.07% | Preferred Series F | 73,971 | 155 | 185 | ||||||||||||||
| Medical Devices & Equipment | Equity | 0.15% | Preferred Series G | 163,934 | 500 | 486 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Medrobotics Corporation |
374,703 | 905 | 907 | |||||||||||||||||
| Optiscan Biomedical, Corp.(5)(15) 24590 Clawiter Road Hayward, CA 94545 |
Medical Devices & Equipment | Equity | 0.44% | Preferred Series B | 6,185,567 | 3,000 | 383 | |||||||||||||
| Medical Devices & Equipment | Equity | 0.14% | Preferred Series C | 1,927,309 | 655 | 110 | ||||||||||||||
| Medical Devices & Equipment | Equity | 3.92% | Preferred Series D | 55,103,923 | 5,257 | 3,826 | ||||||||||||||
| Medical Devices & Equipment | Equity | 1.11% | Preferred Series E | 15,638,888 | 1,308 | 1,492 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Optiscan Biomedical, Corp. |
78,855,687 | 10,220 | 5,811 | |||||||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) 1830 Bering Drive San Jose, CA 95112 |
Medical Devices & Equipment |
Equity |
0.19% |
Preferred Series B |
232,061 | 527 | 566 | |||||||||||||
| Quanterix Corporation 113 Hartwell Avenue Lexington, MA 02421 |
Medical Devices & Equipment | Equity | 0.45% | Preferred Series D | 272,479 | 1,000 | 1,111 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Medical Devices & Equipment (1.53%)* |
|
16,512 | 12,479 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Software |
||||||||||||||||||||
| CapLinked, Inc. 2015 Manhattan Beach Blvd, #108 Redondo Beach, CA 90278 |
Software | Equity | 0.33% | Preferred Series A-3 | 53,614 | 51 | 96 | |||||||||||||
| Druva, Inc. 150 Mathilda Place, Suite 450 Sunnyvale, CA 94041 |
Software | Equity | 0.38% | Preferred Series 2 | 458,841 | 1,000 | 1,584 | |||||||||||||
| ForeScout Technologies, Inc. 900 E. Hamilton Avenue, Suite 300 Campbell, CA 95008 |
Software | Equity | 0.39% | Preferred Series D | 319,099 | 398 | 1,937 | |||||||||||||
| Software | Equity | 0.10% | Preferred Series E | 80,587 | 131 | 493 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total ForeScout Technologies, Inc. |
399,686 | 529 | 2,430 | |||||||||||||||||
| HighRoads, Inc. 3 Burlington Woods Dr Burlington, MA 01803 |
Software | Equity | 0.00% | Common Stock | 190 | 307 | — | |||||||||||||
| NewVoiceMedia Limited(4)(9) Viables Business Park, Jays Close Basingstoke, UK RG22 4BS |
Software | Equity | 0.31% | Preferred Series E | 669,173 | 963 | 1,343 | |||||||||||||
| Palantir Technologies 100 Hamilton Avenue Palo Alto, CA 94301 |
Software | Equity | 0.04% | Preferred Series E | 727,696 | 5,431 | 5,774 | |||||||||||||
| Sprinklr, Inc. 29 West 35th Street, 7th Floor New York, NY 10001 |
Software | Equity | 0.35% | Common Stock | 700,000 | 3,749 | 3,749 | |||||||||||||
| WildTangent, Inc.(15) 18578 NE 67th Court, Building 5 Redmond, WA 98052 |
Software | Equity | 0.17% | Preferred Series 3 | 100,000 | 402 | 175 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (1.85%)* |
|
12,432 | 15,151 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
150
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Surgical Devices |
||||||||||||||||||||
| Gynesonics, Inc.(15) 301 Galveston Drive Redwood City, CA 94063 |
Surgical Devices | Equity | 0.04% | Preferred Series B | 219,298 | $ | 250 | $ | 41 | |||||||||||
| Surgical Devices | Equity | 0.13% | Preferred Series C | 656,538 | 282 | 57 | ||||||||||||||
| Surgical Devices | Equity | 0.38% | Preferred Series D | 1,991,157 | 712 | 772 | ||||||||||||||
| Surgical Devices | Equity | 0.53% | Preferred Series E | 2,786,367 | 429 | 507 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Gynesonics, Inc. |
5,653,360 | 1,673 | 1,377 | |||||||||||||||||
| Transmedics, Inc. 200 Minuteman Road, Suite 302 Andover, MA 01810 |
Surgical Devices | Equity | 0.16% | Preferred Series B | 88,961 | 1,100 | 507 | |||||||||||||
| Surgical Devices | Equity | 0.22% | Preferred Series C | 119,999 | 300 | 388 | ||||||||||||||
| Surgical Devices | Equity | 0.47% | Preferred Series D | 260,000 | 650 | 1,243 | ||||||||||||||
| Surgical Devices | Equity | 0.18% | Preferred Series F | 100,200 | 500 | 600 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Transmedics, Inc. |
569,160 | 2,550 | 2,738 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (0.50%)* |
|
4,223 | 4,115 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) 4100 Alpha Road, Suite 900 Dallas, TX 75244 |
Sustainable and Renewable Technology |
Equity |
0.00% |
Common Stock |
|
19,250 |
|
|
761 |
|
|
— |
| |||||||
| Glori Energy, Inc.(3) 4315 South Drive Houston, TX 77053 |
Sustainable and Renewable Technology | Equity | 0.06% | Common Stock | 18,208 | 165 | — | |||||||||||||
| Modumetal, Inc. Northlake R&D Center,1443 N. Northlake Way Seattle, WA 98103 |
Sustainable and Renewable Technology | Equity | 0.83% | Preferred Series C | 3,107,520 | 500 | 551 | |||||||||||||
| Proterra, Inc. 1 Whitlee Ct. Greenville, SC 29607 |
Sustainable and Renewable Technology | Equity | 0.10% | Preferred Series 5 | 99,280 | 500 | 516 | |||||||||||||
| Solar Spectrum Holdings LLC (p.k.a. Sungevity, 66 Franklin Street, Suite 310 Oakland, CA 94607 |
Sustainable and Renewable Technology |
Equity |
33.30% |
Common Stock |
|
333 |
|
|
61,502 |
|
|
8,288 |
| |||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (1.14%)* |
|
63,428 | 9,355 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total: Equity Investments (9.21%)* |
|
135,080 | 75,316 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Warrant Investments |
||||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||||
| Exicure, Inc. 8045 Lamon Avenue, Suite 410 Skokie, IL 60077 |
Biotechnology Tools | Warrant | 0.20% | Preferred Series C | 104,348 | 107 | 202 | |||||||||||||
| Labcyte, Inc.(15) 1190 Borregas Avenue Sunnyvale, CA 94089 |
Biotechnology Tools | Warrant | 0.85% | Preferred Series C | 1,127,624 | 323 | 397 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
430 | 599 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Communications & Networking |
||||||||||||||||||||
| PeerApp, Inc. 29 Crafts Street, Suite 260 Newton, MA 02458 |
Communications & Networking | Warrant | 0.39% | Preferred Series B | 298,779 | 61 | 27 | |||||||||||||
| Peerless Network Holdings, Inc. 222 South Riverside Plaza, Suite 2730 Chicago, IL 60606 |
Communications & Networking | Warrant | 0.03% | Preferred Series A | 135,000 | 95 | 345 | |||||||||||||
151
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Spring Mobile Solutions, Inc. 11710 Plaza America Drive, Suite 2000 Reston, VA 20190 |
Communications & Networking | Warrant | 0.62% | Common Stock | 2,834,375 | $ | 418 | $ | — | |||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Communications & Networking (0.05%)* |
|
574 | 372 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(15) 709N 400 W #3 North Salt Lake, UT 84054 |
Consumer & Business Products | Warrant | 0.51% | Common Stock | 1,662,441 | 228 | — | |||||||||||||
| Intelligent Beauty, Inc.(15) 2301 Rosecrans Ave, Suite 4100 El Segundo, CA 90245 |
Consumer & Business Products | Warrant | 0.35% | Preferred Series B | 190,234 | 230 | 288 | |||||||||||||
| The Neat Company(15) 601 Market St., Suite 3500 Philadelphia, PA 19103 |
Consumer & Business Products | Warrant | 0.01% | Preferred Series C-1 | 540,540 | 365 | — | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Consumer & Business Products (0.04%)* |
|
823 | 288 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Delivery |
||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9)(15) 351 Galveston Drive Redwood City, CA 94063 |
Drug Delivery | Warrant | 0.39% | Common Stock | 176,730 | 785 | 36 | |||||||||||||
| Agile Therapeutics, Inc.(3) 101 Poor Farm Road Princeton, NJ 08540 |
Drug Delivery | Warrant | 0.63% | Common Stock | 180,274 | 730 | 136 | |||||||||||||
| Aprecia Pharmaceuticals Company 2010 Cabot Blvd., West Suite F Langhorne, PA 19047 |
Drug Delivery | Warrant | 0.43% | Preferred Series A-1 | 735,981 | 366 | 31 | |||||||||||||
| BIND Therapeutics, Inc.(15) 325 Vassar St. Cambridge, MA 02139 |
Drug Delivery | Warrant | 0.73% | Common Stock | 152,586 | 488 | — | |||||||||||||
| BioQ Pharma Incorporated 185 Berry St., Ste 160 San Francisco, CA 94107 |
Drug Delivery | Warrant | 1.55% | Common Stock | 459,183 | 1 | 379 | |||||||||||||
| Celsion Corporation(3) 997 Lenox Drive, Suite 100 Lawrenceville, NJ 08648 |
Drug Delivery | Warrant | 0.34% | Common Stock | 13,927 | 428 | — | |||||||||||||
| Dance Biopharm, Inc.(15) 150 North Hill Drive, Suite 24 Brisbane, CA 94005 |
Drug Delivery | Warrant | 0.40% | Common Stock | 110,882 | 74 | — | |||||||||||||
| Edge Therapeutics, Inc.(3) 300 Connell Dr., Suite 4000 Berkeley Heights, NJ 07922 |
Drug Delivery | Warrant | 0.25% | Common Stock | 78,595 | 390 | 266 | |||||||||||||
| Kaleo, Inc. (p.k.a. Intelliject, Inc.) 111 Virginia St., Ste 300 Richmond, VA 23219 |
Drug Delivery | Warrant | 0.47% | Preferred Series B | 82,500 | 594 | 306 | |||||||||||||
| Neos Therapeutics, Inc.(3)(15) 2940 N. Highway 360, Suite 400 Grand Prarie, TX 75050 |
Drug Delivery | Warrant | 0.26% | Common Stock | 70,833 | 285 | 25 | |||||||||||||
| Pulmatrix Inc.(3) 99 Hayden Avenue, Suite 390 Lexington, MA 02421 |
Drug Delivery | Warrant | 0.12% | Common Stock | 25,150 | 116 | 14 | |||||||||||||
152
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(3) 34790 Ardentech Court Fremont, CA 94555 |
Drug Delivery | Warrant | 0.18% | Common Stock | 72,379 | $ | 266 | $ | 5 | |||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Delivery (0.15%)* |
|
4,523 | 1,198 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||
| ADMA Biologics, Inc.(3) 465 Route 17 South Ramsey, NJ 07446 |
Drug Discovery & Development | Warrant | 0.35% | Common Stock | 89,750 | 295 | 15 | |||||||||||||
| Anthera Pharmaceuticals, Inc.(3)(15) 25801 Industrial Blvd., Suite B Hayward, CA 94545 |
Drug Discovery & Development | Warrant | 0.05% | Common Stock | 5,022 | 984 | — | |||||||||||||
| Audentes Therapeutics, Inc(3)(9)(15) 600 California Street, 17th Floor San Francisco, CA 94108 |
Drug Discovery & Development | Warrant | 0.04% | Common Stock | 9,914 | 62 | 82 | |||||||||||||
| Auris Medical Holding, AG(3)(4)(9) Dornacherstrasse 210 CH-4053, Basel Switzerland |
Drug Discovery & Development | Warrant | 0.35% | Common Stock | 156,726 | 249 | 21 | |||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9) One Broadway, 9th Floor Cambridge, MA 02142 |
Drug Discovery & Development | Warrant | 1.77% | Common Stock | 2,069,880 | 396 | 2,089 | |||||||||||||
| Axovant Sciences Ltd.(3)(4)(9) 2 Church Street Hamilton, Bermuda HM11 |
Drug Discovery & Development | Warrant | 0.26% | Common Stock | 274,086 | 1,269 | 3,237 | |||||||||||||
| Brickell Biotech, Inc. 5777 Central Ave, Suite 102 Boulder, CO 80301 |
Drug Discovery & Development | Warrant | 0.42% | Preferred Series C | 26,086 | 119 | 124 | |||||||||||||
| Cerecor, Inc.(3) 400 East Pratt Street, Suite 606 Baltimore, MD 21202 |
Drug Discovery & Development | Warrant | 0.16% | Common Stock | 22,328 | 70 | — | |||||||||||||
| Cerulean Pharma, Inc.(3) 35 Gatehouse Drive Waltham, MA 02451 |
Drug Discovery & Development | Warrant | 0.59% | Common Stock | 171,901 | 369 | 17 | |||||||||||||
| Chroma Therapeutics, Ltd.(4)(9) 93 Innovation Drive, Milton Park Abingdon Oxon, UK OX14 4RZ |
Drug Discovery & Development | Warrant | 0.61% | Preferred Series D | 325,261 | 490 | — | |||||||||||||
| Cleveland BioLabs, Inc.(3)(15) 73 High Street Buffalo, NY 14203 |
Drug Discovery & Development | Warrant | 0.07% | Common Stock | 7,813 | 105 | 1 | |||||||||||||
| Concert Pharmaceuticals, Inc.(3)(15) 99 Hayden Avenue, Suite 500 Lexington, MA 02421-7966 |
Drug Discovery & Development | Warrant | 0.58% | Common Stock | 132,069 | 545 | 369 | |||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(3) 3101 Western Avenue, Suite 600 Seattle, WA 98121 |
Drug Discovery & Development |
Warrant |
0.07% |
Common Stock |
|
29,239 |
|
|
165 |
|
|
4 |
| |||||||
| CytRx Corporation(3)(15) 11726 San Vicente Blvd., Suite 650 Los Angeles, CA 90049 |
Drug Discovery & Development | Warrant | 0.41% | Common Stock | 634,146 | 416 | 150 | |||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) 87 Cambridge Park Dr Cambridge, MA 02140 |
Drug Discovery & Development | Warrant | 0.00% | Common Stock | 200 | 28 | — | |||||||||||||
153
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Epirus Biopharmaceuticals, Inc. 99 High Street Boston, MA 02110-2320 |
Drug Discovery & Development | Warrant | 0.25% | Common Stock | 64,194 | $ | 276 | $ | — | |||||||||||
| Fortress Biotech, Inc. (p.k.a. Coronado Biosciences, Inc.)(3) 2 Gansevoort Street, 9th Floor New York, NY 10014 |
Drug Discovery & Development |
Warrant |
0.14% |
Common Stock |
|
73,009 |
|
|
142 |
|
|
54 |
| |||||||
| Genocea Biosciences, Inc.(3) 100 Acorn Park Drive, 5th Floor Cambridge, MA 02140 |
Drug Discovery & Development | Warrant | 0.26% | Common Stock | 73,725 | 266 | 86 | |||||||||||||
| Immune Pharmaceuticals(3) 430 East 29th St., Suite 940 New York, NY 10016 |
Drug Discovery & Development | Warrant | 0.11% | Common Stock | 10,742 | 164 | — | |||||||||||||
| Melinta Therapeutics 300 TriState International, Suite 272 Lincolnshire, IL 60069 |
Drug Discovery & Development | Warrant | 0.48% | Preferred Series 3 | 1,382,323 | 626 | 564 | |||||||||||||
| Nanotherapeutics, Inc.(15) 13200 NW Nano Court Alachua, FL 32615 |
Drug Discovery & Development | Warrant | 2.67% | Common Stock | 171,389 | 838 | 245 | |||||||||||||
| Neothetics, Inc. (p.k.a. Lithera, Inc)(3)(15) 9171 Towne Centre Drive, Suite 270 San Diego, CA 92122 |
Drug Discovery & Development | Warrant | 0.34% | Common Stock | 46,838 | 266 | 11 | |||||||||||||
| Neuralstem, Inc.(3)(15) 20271 Goldenrod Lane, 2nd floor Germantown, MD 20876 |
Drug Discovery & Development | Warrant | 0.05% | Common Stock | 5,783 | 77 | 2 | |||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3)(15) 75 Park Plaza, 4th Floor Boston, MA 02116 |
Drug Discovery & Development |
Warrant |
0.27% |
Common Stock |
|
75,214 |
|
|
178 |
|
|
477 |
| |||||||
|
PhaseRx,Inc.(3)(15) 4 10 West Harrison Street, Suite 300 Seattle, WA 98119 |
Drug Discovery & Development | Warrant | 0.54% | Common Stock | 63,000 | 125 | 4 | |||||||||||||
| Savara Inc. (p.k.a. Mast Therapeutics, Inc.) 900 S. Capital of Texas Highway, Suite 150 Austin, TX 78746 |
Drug Discovery & Development | Warrant | 0.13% | Common Stock | 32,467 | 203 | 50 | |||||||||||||
| Sorrento Therapeutics, Inc. 9380 Judicial Dr San Diego, CA 92121 |
Drug Discovery & Development | Warrant | 0.40% | Common Stock | 306,748 | 890 | 180 | |||||||||||||
| Stealth Bio Therapeutics Corp. 275 Grove Street, Suite 3-107 Newton, MA 02466 |
Drug Discovery & Development | Warrant | 0.09% | Preferred Series A | 487,500 | 116 | 116 | |||||||||||||
| uniQure B.V. Tafelbergweg 51 Amsterdam, The Netherlands 1105 BD |
Drug Discovery & Development | Warrant | 0.15% | Common Stock | 37,174 | 218 | 10 | |||||||||||||
| XOMA Corporation 2910 Seventh Street Berkeley, CA 94710 |
Drug Discovery & Development | Warrant | 0.12% | Common Stock | 9,063 | 279 | 10 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Discovery & Development (0.97%)* |
|
10,226 | 7,918 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Electronics & Computer Hardware |
| |||||||||||||||||||
| 908 DEVICES INC.(15) 27 Drydock Avenue, 7th Floor Boston, MA 02210 |
Electronics & Computer Hardware | Warrant | 0.26% | Preferred Series D | 79,856 | 100 | 114 | |||||||||||||
154
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Clustrix, Inc. 201 Mission Street, Suite 800 San Francisco, CA 94105 |
Electronics & Computer Hardware | Warrant | 0.23% | Common Stock | 50,000 | $ | 12 | $ | — | |||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.01%)* |
|
112 | 114 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Healthcare Services, Other |
| |||||||||||||||||||
| Chromadex Corporation(3)(15) 10005 Muirlands Boulevard, Suite G, First Floor Irvine, CA 92618 |
Healthcare Services, Other | Warrant | 0.30% | Common Stock | 139,673 | 157 | 155 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Healthcare Services, Other (0.02%)* |
|
157 | 155 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Information Services |
| |||||||||||||||||||
| INMOBI Inc.(4)(9) 475 Brannan St., Suite 420 San Francisco, CA 94107 |
Information Services | Warrant | 0.11% | Common Stock | 46,874 | 82 | — | |||||||||||||
| InXpo, Inc.(15) 770 N Halsted Street, Suite 6s Chicago, IL 60642 |
Information Services | Warrant | 0.61% | Preferred Series C | 648,400 | 98 | 15 | |||||||||||||
| Information Services | Warrant | 1.09% | Preferred Series C-1 | 1,165,183 | 74 | 27 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total InXpo, Inc. |
1,813,583 | 172 | 42 | |||||||||||||||||
| MDX Medical, Inc.(15) 160 Chubb Avenue, Suite 301 Lyndhurst, NJ 07071 |
Information Services | Warrant | 0.70% | Common Stock | 2,250,000 | 246 | 215 | |||||||||||||
| RichRelevance, Inc. 633 Folsom Street, 4th Floor San Francisco, CA 94107 |
Information Services | Warrant | 0.13% | Preferred Series E | 112,612 | 98 | — | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Information Services (0.03%)* |
|
598 | 257 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||||
| Aria Systems, Inc. 575 Market Street, 32nd Floor San Francisco, CA 94105 |
Internet Consumer & Business Services | Warrant | 0.11% | Preferred Series E | 239,692 | 73 | — | |||||||||||||
| Blurb, Inc.(15) 580 California St., Suite 300 San Francisco, CA 94104 |
Internet Consumer & Business Services | Warrant | 0.40% | Preferred Series C | 234,280 | 636 | 61 | |||||||||||||
| CashStar, Inc.(15) 25 Pearl Street Portland, ME 04101 |
Internet Consumer & Business Services | Warrant | 0.43% | Preferred Series C-2 | 727,272 | 130 | 30 | |||||||||||||
| ClearObject, Inc. (p.k.a. CloudOne, Inc.) 8626 E 116th Street, Suite 300 Fishers, IN 46038 |
Internet Consumer & Business Services | Warrant | 0.59% | Preferred Series E | 968,992 | 19 | 112 | |||||||||||||
| Intent Media, Inc.(15) 315 Hudson St., 9th Floor New York, NY 10013 |
Internet Consumer & Business Services | Warrant | 0.48% | Common Stock | 140,077 | 168 | 148 | |||||||||||||
| Just Fabulous, Inc. 2301 Rosecrans Avenue, Suite 5000 El Segundo, CA 90245 |
Internet Consumer & Business Services | Warrant | 0.39% | Preferred Series B | 206,184 | 1,102 | 1,850 | |||||||||||||
| Lightspeed POS, Inc.(4)(9) 700 St-Antoine Est, Suite 300 Montreal, Canada H2Y1A6 |
Internet Consumer & Business Services | Warrant | 0.10% | Preferred Series C | 245,610 | 20 | 25 | |||||||||||||
| LogicSource (15) 20 Marshall Street Sourth Norwalk, CT 06854 |
Internet Consumer & Business Services | Warrant | 0.41% | Preferred Series C | 79,625 | 30 | 32 | |||||||||||||
155
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Oportun (p.k.a. Progress Financial) 1600 Seaport Blvd., Suite 250 Redwood City, CA 94063 |
Internet Consumer & Business Services | Warrant | 0.07% | Preferred Series G | 174,562 | $ | 78 | $ | 175 | |||||||||||
| ShareThis, Inc.(15) 4005 Miranda Avenue, Suite 100 Palo Alto, CA 94304 |
Internet Consumer & Business Services | Warrant | 0.91% | Preferred Series C | 493,502 | 547 | — | |||||||||||||
| Snagajob.com, Inc. 1919 N Lynn Street, 7th Floor Arlington, VA 22209 |
Internet Consumer & Business Services | Warrant | 0.79% | Preferred Series A | 1,575,000 | 640 | 782 | |||||||||||||
| Tapjoy, Inc. 111 Sutter Street, 12th Floor San Francisco, CA 94104 |
Internet Consumer & Business Services | Warrant | 0.41% | Preferred Series D | 748,670 | 316 | 1 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.39%)* |
|
3,759 | 3,216 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Media/Content/Info |
||||||||||||||||||||
| FanDuel, Inc. 300 Park Avenue South, 14th Floor New York, NY 10005 |
Media/Content/Info | Warrant | 0.09% | Preferred Series E-1 | 4,648 | 730 | 851 | |||||||||||||
| Machine Zone, Inc.(16) 1050 Page Mill Road Palo Alto, CA 94304 |
Media/Content/Info | Warrant | 0.12% | Common Stock | 1,552,710 | 1,958 | 4,484 | |||||||||||||
| Rhapsody International, Inc.(15) 701 5th Ave., Suite 3100 Seattle, WA 98104 |
Media/Content/Info | Warrant | 0.50% | Common Stock | 715,755 | 385 | 17 | |||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9) 4950 Yonge Street, Suite 208 Toronto, ON M2M 3V5 |
Media/Content/Info | Warrant | 0.11% | Common Stock | 255,818 | 4 | 8 | |||||||||||||
| Zoom Media Group, Inc. 345 7th Avenue, Suite 1501 New York, NY 10001 |
Media/Content/Info | Warrant | 0.44% | Preferred Series A | 1,204 | 348 | 21 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (0.66%)* |
|
3,425 | 5,381 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||
| Amedica Corporation(3)(15) 1885 West 2100 South Salt Lake City, UT 84119 |
Medical Devices & Equipment | Warrant | 0.28% | Common Stock | 103,225 | 459 | 5 | |||||||||||||
| Aspire Bariatrics, Inc.(15) 3200 Horizon Drive, Suite 100 King of Prussia, PA 19406 |
Medical Devices & Equipment | Warrant | 1.03% | Preferred Series B-1 | 112,858 | 455 | 303 | |||||||||||||
| Avedro, Inc.(15) 201 Jones Rd., 5th Floor Waltham MA 02451 |
Medical Devices & Equipment | Warrant | 0.59% | Preferred Series AA | 300,000 | 401 | 316 | |||||||||||||
| Flowonix Medical Incorporated 500 International Drive, Suite 200 Mount Olive, NJ 07828 |
Medical Devices & Equipment | Warrant | 0.76% | Preferred Series AA | 155,325 | 362 | — | |||||||||||||
| Gelesis, Inc.(15) 500 Boylston Street, Suite 1600 Boston, MA 02116 |
Medical Devices & Equipment | Warrant | 0.47% | Preferred Series A-1 | 74,784 | 78 | 215 | |||||||||||||
| InspireMD, Inc.(3)(4)(9) 4 Menorat Hamaor Street, 3rd Floor Tel Aviv, Israel 67448 |
Medical Devices & Equipment | Warrant | 0.53% | Common Stock | 39,364 | 242 | 1 | |||||||||||||
| IntegenX, Inc.(15) 5720 Stoneridge Drive, Suite 300 Pleasanton, CA 94588 |
Medical Devices & Equipment | Warrant | 0.74% | Preferred Series C | 547,752 | 15 | 33 | |||||||||||||
156
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Medrobotics Corporation(15) 475 Paramount Drive Raynham, MA 02767 |
Medical Devices & Equipment | Warrant | 0.40% | Preferred Series E | 455,539 | $ | 370 | $ | 360 | |||||||||||
| Micell Technologies, Inc. 801 Capitola Drive, Suite 1 Durham, NC 27713 |
Medical Devices & Equipment | Warrant | 0.40% | Preferred Series D-2 | 84,955 | 262 | 277 | |||||||||||||
| NetBio, Inc. 266 Second Avenue Waltham, MA 02451 |
Medical Devices & Equipment | Warrant | 0.90% | Preferred Series A | 7,841 | 408 | 123 | |||||||||||||
| NinePoint Medical, Inc.(15) 2 Oak Park Dr. Bedford, MA 01730 |
Medical Devices & Equipment | Warrant | 0.30% | Preferred Series A-1 | 587,840 | 170 | 73 | |||||||||||||
| Optiscan Biomedical, Corp.(5)(15) 24590 Clawiter Road Hayward, CA 94545 |
Medical Devices & Equipment | Warrant | 0.75% | Preferred Series D | 10,535,275 | 1,252 | 180 | |||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) 1830 Bering Drive San Jose, CA 95112 |
Medical Devices & Equipment |
Warrant |
0.41% |
Preferred Series A |
500,000 | 402 | 410 | |||||||||||||
| Quanterix Corporation 113 Hartwell Avenue Lexington, MA 02421 |
Medical Devices & Equipment | Warrant | 0.29% | Preferred Series C | 173,428 | 180 | 82 | |||||||||||||
| Medical Devices & Equipment | Warrant | 0.06% | Preferred Series D | 38,828 | 25 | 16 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Quanterix Corporation |
212,256 | 205 | 98 | |||||||||||||||||
|
Sebacia(15) 2905 Premiere Parkway, Suite 150 Duluth, GA 30097 |
Medical Devices & Equipment | Warrant | 0.59% | Preferred Series D | 778,301 | 133 | 133 | |||||||||||||
| SonaCare Medical, LLC (p.k.a. US HIFU, LLC) 10130 Perimeter Parkway, Suite 250 Charlotte, NC 28216 |
Medical Devices & Equipment |
Warrant |
0.02% |
Preferred Series A |
6,464 | 188 | — | |||||||||||||
| Strata Skin Sciences, Inc. (p.k.a. MELA Sciences, Inc.)(3) 100 Lakeside Drive, Suite 100 Horsham, PA 19044 |
Medical Devices & Equipment |
Warrant |
0.57% |
Common Stock |
|
13,864 |
|
|
402 |
|
|
— |
| |||||||
| Tela Bio, Inc.(15) One Great Valley Pkwy, Suite 24 Malvern, PA 19355 |
Medical Devices & Equipment | Warrant | 0.17% | Preferred Series B | 129,310 | 20 | 12 | |||||||||||||
| ViewRay, Inc.(3)(15) 2 Thermo Fisher Way Oakwood Village, OH 44146 |
Medical Devices & Equipment | Warrant | 0.22% | Common Stock | 128,231 | 333 | 130 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Medical Devices & Equipment (0.33%)* |
|
6,157 | 2,669 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Semiconductors |
||||||||||||||||||||
| Achronix Semiconductor Corporation(15) 2953 Bunker Hill Lane, Suite 101 Santa Clara, CA 95054 |
Semiconductors | Warrant | 0.11% | Preferred Series C | 360,000 | 160 | 15 | |||||||||||||
| Semiconductors | Warrant | 0.24% | Preferred Series D-2 | 750,000 | 99 | 307 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Achronix Semiconductor Corporation |
1,110,000 | 259 | 322 | |||||||||||||||||
| Aquantia Corp. 105 E. Tasman Drive San Jose, CA 95134 |
Semiconductors | Warrant | 0.07% | Preferred Series G | 196,831 | 4 | 168 | |||||||||||||
157
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Avnera Corporation 1600 NW Compton Drive, Ste 300. Beaverton, OR 97006 |
Semiconductors | Warrant | 0.28% | Preferred Series E | 141,567 | $ | 46 | $ | 114 | |||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Semiconductors (0.07%)* |
|
309 | 604 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Software |
|
|||||||||||||||||||
| Actifio, Inc. 333 Wyman Street, Waltham Waltham, MA 02451 |
Software |
Warrant | 0.08% |
Common Stock | 73,584 | 249 | |
79 |
| |||||||||||
| Software |
Warrant | 0.03% |
Preferred Series F | 31,673 | 343 | |
60 |
| ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Actifio, Inc. |
105,257 | 592 | 139 | |||||||||||||||||
| Braxton Technologies, LLC 6 North Tejon Street, Suite 220 Colorado Springs, CO 80903 |
Software | Warrant | 0.63% | Preferred Series A | 168,750 | 188 | — | |||||||||||||
| CareCloud Corporation(15) 5200 Blue Lagoon Drive, Suite 900 Miami, FL 33126 |
Software | Warrant | 0.62% | Preferred Series B | 413,433 | 258 | 634 | |||||||||||||
| Clickfox, Inc.(15) 3445 Peachtree Road, Suite 450 Atlanta, GA 30326 |
Software |
Warrant | 1.43% |
Preferred Series B | |
1,038,563 |
|
|
330 |
|
|
152 |
| |||||||
| Software |
Warrant | 0.81% |
Preferred Series C | 592,019 | |
730 |
|
|
183 |
| ||||||||||
| Software | Warrant | 3.05% |
Preferred Series C-A | 2,218,214 | |
230 |
|
|
3,673 |
| ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Clickfox, Inc. |
3,848,796 | 1,290 | 4,008 | |||||||||||||||||
| Cloud Technology Partners, Inc. 321 Summer Street, 5th Floor Boston, MA 02210 |
Software | Warrant | 0.39% | Preferred Series C | 113,960 | 34 | 4 | |||||||||||||
| Evernote Corporation(15) 305 Walnut Street Redwood City, CA 94063 |
Software | Warrant | 0.06% | Common Stock | 62,500 | 106 | 131 | |||||||||||||
| Fuze, Inc.(15) 2 Copley Place, Floor 7 Boston, MA 02116 |
Software | Warrant | 0.18% | Preferred Series F | 256,158 | 89 | 89 | |||||||||||||
| JumpStart Games, Inc. (p.k.a 21250 Hawthorne Boulevard, Suite 380 Torrance, CA 90503 |
Software |
Warrant |
0.46% |
Preferred Series E |
|
614,333 |
|
|
16 |
|
|
— |
| |||||||
| Mattersight Corporation(3) 200 W. Madison, Suite 3100 Chicago, IL 60606 |
Software | Warrant | 1.09% | Common Stock | 357,143 | 538 | 173 | |||||||||||||
| Message Systems, Inc.(15) 9130 Guilford Road Columbia, MD 21046 |
Software | Warrant | 1.06% | Preferred Series C | 503,718 | 334 | 306 | |||||||||||||
| Mobile Posse, Inc.(15) 1010 N. Glebe Road, Suite 200 Arlington, VA 22201 |
Software | Warrant | 1.06% | Preferred Series C | 396,430 | 130 | 130 | |||||||||||||
| Neos, Inc.(15) 6210 Stoneridge Mall, Suite 450 Pleasanton, CA 94588 |
Software | Warrant | 0.10% | Common Stock | 221,150 | 22 | 18 | |||||||||||||
| NewVoiceMedia Limited(4)(9) Viables Business Park, Jays Close Basingstoke, UK RG22 4BS |
Software | Warrant | 0.10% | Preferred Series E | 225,586 | 33 | 125 | |||||||||||||
| OneLogin, Inc.(15) 150 Spear Street, Suite 1400 San Francisco, CA 94105 |
Software | Warrant | 0.41% | Common Stock | 228,972 | 150 | 348 | |||||||||||||
158
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Poplicus, Inc.(15) 19 South Park St. San Francisco, CA 94107 |
Software | Warrant | 0.55% | Preferred Series C | 2,595,230 | $ | — | $ | 5 | |||||||||||
| Quid, Inc.(15) 600 Harrison Street, Suite 400 San Francisco, CA 94107 |
Software | Warrant | 0.07% | Preferred Series D | 71,576 | 1 | 5 | |||||||||||||
| RedSeal Inc.(15) 940 Stewart Drive, Suite 101 Sunnyvale, CA 94085 |
Software | Warrant | 0.14% | Preferred Series C-Prime | 640,603 | 66 | 81 | |||||||||||||
| Signpost, Inc.(15) 127 W 26th St., Floor 2 New York, NY 10001 |
Software | Warrant | 0.82% | Preferred Series C | 324,005 | 314 | 130 | |||||||||||||
| Sonian, Inc.(15) 3 Allied Drive, Suite 155 Dedham, MA 02026 |
Software | Warrant | 0.51% | Preferred Series C | 185,949 | 106 | 109 | |||||||||||||
| Wrike, Inc. 10 Almaden Blvd, Suite 1000 San Jose, CA 95113 |
Software | Warrant | 0.98% | Common Stock | 139,751 | 462 | 691 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (0.87%)* |
|
4,729 | 7,126 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||
| Alimera Sciences, Inc.(3) 6120 Windward Parkway, Suite 290 Alpharetta, GA 30005 |
Specialty Pharmaceuticals | Warrant | 2.65% | Common Stock | 1,717,709 | 861 | 584 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.07%)* |
|
861 | 584 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Surgical Devices |
||||||||||||||||||||
| Gynesonics, Inc.(15) 301 Galveston Drive Redwood City, CA 94063 |
Surgical Devices | Warrant | 0.03% | Preferred Series C | 180,480 | 75 | 14 | |||||||||||||
| Surgical Devices | Warrant | 0.30% | Preferred Series D | 1,575,965 | 320 | 278 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Gynesonics, Inc. |
1,756,445 | 395 | 292 | |||||||||||||||||
| Transmedics, Inc. 200 Minuteman Road, Suite 302 Andover, MA 01810 |
Surgical Devices | Warrant | 0.07% | Preferred Series B | 40,436 | 225 | 12 | |||||||||||||
| Surgical Devices | Warrant | 0.32% | Preferred Series D | 175,000 | 100 | 543 | ||||||||||||||
| Surgical Devices | Warrant | 0.09% | Preferred Series F | 50,544 | 38 | 66 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Transmedics, Inc. |
265,980 | 363 | 621 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (0.11%)* |
|
758 | 913 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||||
| Agrivida, Inc.(15) 200 Boston Avenue Medford, MA 02155 |
Sustainable and Renewable Technology | Warrant | 0.44% | Preferred Series D | 471,327 | 120 | 110 | |||||||||||||
| Alphabet Energy, Inc.(15) 26225 Eden Landing Road, Suite D Hayward, CA 94545 |
Sustainable and Renewable Technology | Warrant | 0.05% | Preferred Series 1B | 13,667 | 82 | — | |||||||||||||
| American Superconductor Corporation(3) 64 Jackson Rd. Devens, MA 01434 |
Sustainable and Renewable Technology | Warrant | 0.31% | Common Stock | 58,823 | 39 | 29 | |||||||||||||
| Brightsource Energy, Inc. 1999 Harrison Street, Suite 2150 Oakland, CA 94612 |
Sustainable and Renewable Technology | Warrant | 0.22% | Preferred Series 1 | 116,666 | 104 | — | |||||||||||||
159
| Portfolio Company |
Sub-Industry |
Type of |
Percentage |
Series |
Shares | Cost(2) | Value(3) | |||||||||||||
| Calera, Inc.(15) 485 Alberto Way, #210 Los Gatos, CA 95032 |
Sustainable and Renewable Technology | Warrant | 0.17% | Preferred Series C | 44,529 | $ | 513 | $ | — | |||||||||||
| EcoMotors, Inc.(15) 17000 Federal Dr., Suite 200 Allen Park, MI 48101 |
Sustainable and Renewable Technology | Warrant | 0.68% | Preferred Series B | 437,500 | 308 | 51 | |||||||||||||
| Fluidic, Inc. 8455 North 90th Street, Suite 4 Scottsdale, AZ 85258 |
Sustainable and Renewable Technology | Warrant | 0.11% | Preferred Series D | 61,804 | 102 | 4 | |||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) 4100 Alpha Road, Suite 900 Dallas, TX 75244 |
Sustainable and Renewable Technology |
Warrant |
0.05% |
Common Stock |
|
530,811 |
|
|
181 |
|
|
— |
| |||||||
| Sustainable and Renewable Technology | Warrant | 0.00% | Preferred Series 2-A | 6,229 | 50 | — | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
537,040 | 231 | — | |||||||||||||||||
| Fulcrum Bioenergy, Inc. 4900 Hopyard Road, Suite 220 Pleasanton, CA 94588 |
Sustainable and Renewable Technology | Warrant | 0.24% | Preferred Series C-1 | 280,897 | 275 | 292 | |||||||||||||
| GreatPoint Energy, Inc.(15) 2215 W. Harrison St. Chicago, IL 60612 |
Sustainable and Renewable Technology | Warrant | 0.12% | Preferred Series D-1 | 393,212 | 548 | — | |||||||||||||
| Polyera Corporation(15) 8045 Lamon Avenue, #140 Skokie, IL 60077 |
Sustainable and Renewable Technology | Warrant | 0.97% | Preferred Series C | 311,609 | 338 | — | |||||||||||||
| Proterra, Inc. 1 Whitlee Ct. Greenville, SC 29607 |
Sustainable and Renewable Technology | Warrant | 0.46% | Preferred Series 4 | 477,517 | 41 | 548 | |||||||||||||
| Rive Technology, Inc.(15) 1 Deer Park Drive, Suite A Monmouth Junction, NJ 08852 |
Sustainable and Renewable Technology | Warrant | 0.37% | Preferred Series E | 234,477 | 12 | 4 | |||||||||||||
| Stion Corporation(5) 6321 San Ignacio Avenue San Jose, CA 95119 |
Sustainable and Renewable Technology | Warrant | 7.89% | Preferred Series Seed | 2,154 | 1,378 | — | |||||||||||||
| TAS Energy, Inc. 6110 Cullen Blvd. Houston, TX 77021 |
Sustainable and Renewable Technology | Warrant | 0.10% | Preferred Series AA | 428,571 | 299 | — | |||||||||||||
| Tendril Networks 2580 55th Street, Suite 100 Boulder, CO 80301 |
Sustainable and Renewable Technology | Warrant | 0.47% | Preferred Series 3-A | 1,019,793 | 189 | 98 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.14%)* |
|
4,579 | 1,136 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total: Warrant Investments (3.98%)* |
|
42,020 | 32,530 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total Investments (170.71%)* |
|
$ | 1,501,139 | $ | 1,395,469 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| * | Value as a percentage of net assets |
| ** | Percentage is based on the latest available information. Our portfolio companies are constantly experiencing changes to their capital structure, which the portfolio companies have no obligation to disclose and may impact our percentage of ownership. |
| (1) | Preferred and common stock, warrants, and equity interests are generally non-income producing. |
| (2) | Gross unrealized appreciation, gross unrealized depreciation, and net depreciation for U.S. federal income tax purposes totaled $22.2 million, $135.8 million and $113.6 million respectively. The tax cost of investments is $1.5 billion. |
| (3) | Except for warrants in 39 publicly traded companies and common stock in 17 publicly traded companies, all investments are restricted at June 30, 2017 and were valued at fair value as determined in good faith by the Company’s board of directors (the “Board of Directors”). No unrestricted securities of the same issuer are outstanding. The Company uses the Standard Industrial Code for classifying the industry grouping of its portfolio companies. |
160
| (4) | Non-U.S. company or the company’s principal place of business is outside the United States. |
| (5) | Affiliate investment as defined under the Investment Company Act of 1940, as amended, (the “1940 Act”) in which Hercules owns at least 5% but generally less than 25% of the company’s voting securities. |
| (6) | Control investment as defined under the 1940 Act in which Hercules owns at least 25% of the company’s voting securities or has greater than 50% representation on its board. |
| (7) | Debt is on non-accrual status at June 30, 2017, and is therefore considered non-income producing. Note that at June 30, 2017, only the $11.0 million PIK, or payment-in-kind, loan is on non-accrual for the Company’s debt investment in Tectura Corporation. |
| (8) | Denotes that all or a portion of the debt investment is convertible debt. |
| (9) | Indicates assets that the Company deems not “qualifying assets” under section 55(a) of 1940 Act. Qualifying assets must represent at least 70% of the Company’s total assets at the time of acquisition of any additional non-qualifying assets. |
| (10) | Denotes that all or a portion of the debt investment secures the notes offered in the Debt Securitization (as defined in Note 4). |
| (11) | Denotes that all or a portion of the debt investment is pledged as collateral under the Wells Facility (as defined in Note 4). |
| (12) | Denotes that all or a portion of the debt investment is pledged as collateral under the Union Bank Facility (as defined in Note 4). |
| (13) | Denotes that all or a portion of the debt investment principal includes accumulated PIK interest and is net of repayments. |
| (14) | Denotes that all or a portion of the debt investment includes an exit fee receivable. |
| A. | This fee ranges from 1.0% to 5.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| B. | This fee ranges from 5.0% to 10.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| C. | This fee ranges from 10.0% to 15.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| (15) | Denotes that all or a portion of the investment in this portfolio company is held by Hercules Technology II, L.P., or HT II, or Hercules Technology III, L.P., or HT III, the Company’s wholly owned small business investment companies, or SBIC, subsidiaries. |
| (16) | Denotes that the fair value of the Company’s total investments in this portfolio company represent greater than 5% of the Company’s total assets at June 30, 2017. |
| (17) | Denotes that there is an unfunded contractual commitment available at the request of this portfolio company at June 30, 2017. Refer to Note 10. |
| (18) | Repayment of debt investment is delinquent of the contractual maturity date as of June 30, 2017. |
| (19) | The stated PIK interest rate may be reduced to 1.45% subject to achievement of a milestone by the portfolio company. |
161
Information about our senior securities is shown in the following table for the periods as of December 31, 2016, 2015, 2014, 2013, 2012, 2011, 2010, 2009, 2008, and 2007. The information as of December 31, 2016, 2015, 2014, 2013, 2012, 2011 and 2010 has been derived from our audited financial statements for these periods, which have been audited by PricewaterhouseCoopers LLP, our independent registered public accounting firm. The report of PricewaterhouseCoopers LLP on the senior securities table as of December 31, 2016 is attached as an exhibit to the registration statement of which this prospectus is a part. The “N/A” indicates information that the SEC expressly does not require to be disclosed for certain types of senior securities.
| Class and Year |
Total Amount Outstanding Exclusive of Treasury Securities(1) |
Asset Coverage per Unit(2) |
Average Market Value per Unit(3) |
|||||||||
| Securitized Credit Facility with Wells Fargo Capital Finance |
||||||||||||
| December 31, 2007 |
$ | 79,200,000 | $ | 6,755 | N/A | |||||||
| December 31, 2008 |
$ | 89,582,000 | $ | 6,689 | N/A | |||||||
| December 31, 2009(6) |
— | — | N/A | |||||||||
| December 31, 2010(6) |
— | — | N/A | |||||||||
| December 31, 2011 |
$ | 10,186,830 | $ | 73,369 | N/A | |||||||
| December 31, 2012(6) |
— | — | N/A | |||||||||
| December 31, 2013(6) |
— | — | N/A | |||||||||
| December 31, 2014(6) |
— | — | N/A | |||||||||
| December 31, 2015 |
$ | 50,000,000 | $ | 26,352 | N/A | |||||||
| December 31, 2016 |
$ | 5,015,620 | $ | 290,234 | N/A | |||||||
| December 31, 2017 (as of June 30, 2017, unaudited)(6) |
— | — | N/A | |||||||||
| Securitized Credit Facility with Union Bank, NA |
||||||||||||
| December 31, 2009(6) |
— | — | N/A | |||||||||
| December 31, 2010(6) |
— | — | N/A | |||||||||
| December 31, 2011(6) |
— | — | N/A | |||||||||
| December 31, 2012(6) |
— | — | N/A | |||||||||
| December 31, 2013(6) |
— | — | N/A | |||||||||
| December 31, 2014(6) |
— | — | N/A | |||||||||
| December 31, 2015(6) |
— | — | N/A | |||||||||
| December 31, 2016(6) |
— | — | N/A | |||||||||
| December 31, 2017 (as of June 30, 2017, unaudited)(6) |
— | — | N/A | |||||||||
| Small Business Administration Debentures (HT II)(4) |
||||||||||||
| December 31, 2007 |
$ | 55,050,000 | $ | 9,718 | N/A | |||||||
| December 31, 2008 |
$ | 127,200,000 | $ | 4,711 | N/A | |||||||
| December 31, 2009 |
$ | 130,600,000 | $ | 3,806 | N/A | |||||||
| December 31, 2010 |
$ | 150,000,000 | $ | 3,942 | N/A | |||||||
| December 31, 2011 |
$ | 125,000,000 | $ | 5,979 | N/A | |||||||
| December 31, 2012 |
$ | 76,000,000 | $ | 14,786 | N/A | |||||||
| December 31, 2013 |
$ | 76,000,000 | $ | 16,075 | N/A | |||||||
| December 31, 2014 |
$ | 41,200,000 | $ | 31,535 | N/A | |||||||
| December 31, 2015 |
$ | 41,200,000 | $ | 31,981 | N/A | |||||||
| December 31, 2016 |
$ | 41,200,000 | $ | 35,333 | N/A | |||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
$ | 41,200,000 | $ | 38,443 | N/A | |||||||
| Small Business Administration Debentures (HT III)(5) |
||||||||||||
| December 31, 2010 |
$ | 20,000,000 | $ | 29,564 | N/A | |||||||
| December 31, 2011 |
$ | 100,000,000 | $ | 7,474 | N/A | |||||||
| December 31, 2012 |
$ | 149,000,000 | $ | 7,542 | N/A | |||||||
| December 31, 2013 |
$ | 149,000,000 | $ | 8,199 | N/A | |||||||
| December 31, 2014 |
$ | 149,000,000 | $ | 8,720 | N/A | |||||||
| December 31, 2015 |
$ | 149,000,000 | $ | 8,843 | N/A | |||||||
| December 31, 2016 |
$ | 149,000,000 | $ | 9,770 | N/A | |||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
$ | 149,000,000 | $ | 10,630 | N/A | |||||||
162
| Class and Year |
Total Amount Outstanding Exclusive of Treasury Securities(1) |
Asset Coverage per Unit(2) |
Average Market Value per Unit(3) |
|||||||||
| 2016 Convertible Notes |
||||||||||||
| December 31, 2011 |
$ | 75,000,000 | $ | 10,623 | $ | 885 | ||||||
| December 31, 2012 |
$ | 75,000,000 | $ | 15,731 | $ | 1,038 | ||||||
| December 31, 2013 |
$ | 75,000,000 | $ | 16,847 | $ | 1,403 | ||||||
| December 31, 2014 |
$ | 17,674,000 | $ | 74,905 | $ | 1,290 | ||||||
| December 31, 2015 |
$ | 17,604,000 | $ | 74,847 | $ | 1,110 | ||||||
| December 31, 2016 |
— | — | — | |||||||||
| April 2019 Notes |
||||||||||||
| December 31, 2012 |
$ | 84,489,500 | $ | 13,300 | $ | 986 | ||||||
| December 31, 2013 |
$ | 84,489,500 | $ | 14,460 | $ | 1,021 | ||||||
| December 31, 2014 |
$ | 84,489,500 | $ | 15,377 | $ | 1,023 | ||||||
| December 31, 2015 |
$ | 64,489,500 | $ | 20,431 | $ | 1,017 | ||||||
| December 31, 2016 |
$ | 64,489,500 | $ | 22,573 | $ | 1,022 | ||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
— | — | — | |||||||||
| September 2019 Notes |
||||||||||||
| December 31, 2012 |
$ | 85,875,000 | $ | 13,086 | $ | 1,003 | ||||||
| December 31, 2013 |
$ | 85,875,000 | $ | 14,227 | $ | 1,016 | ||||||
| December 31, 2014 |
$ | 85,875,000 | $ | 15,129 | $ | 1,026 | ||||||
| December 31, 2015 |
$ | 45,875,000 | $ | 28,722 | $ | 1,009 | ||||||
| December 31, 2016 |
$ | 45,875,000 | $ | 31,732 | $ | 1,023 | ||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
— | — | — | |||||||||
| 2024 Notes |
||||||||||||
| December 31, 2014 |
$ | 103,000,000 | $ | 12,614 | $ | 1,010 | ||||||
| December 31, 2015 |
$ | 103,000,000 | $ | 12,792 | $ | 1,014 | ||||||
| December 31, 2016 |
$ | 252,873,175 | $ | 5,757 | $ | 1,016 | ||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
$ | 258,509,600 | $ | 6,127 | $ | 1,019 | ||||||
| 2017 Asset-Backed Notes |
||||||||||||
| December 31, 2012 |
$ | 129,300,000 | $ | 8,691 | $ | 1,000 | ||||||
| December 31, 2013 |
$ | 89,556,972 | $ | 13,642 | $ | 1,004 | ||||||
| December 31, 2014 |
$ | 16,049,144 | $ | 80,953 | $ | 1,375 | ||||||
| December 31, 2015 |
— | — | — | |||||||||
| 2021 Asset-Backed Notes |
||||||||||||
| December 31, 2014 |
$ | 129,300,000 | $ | 10,048 | $ | 1,000 | ||||||
| December 31, 2015 |
$ | 129,300,000 | $ | 10,190 | $ | 996 | ||||||
| December 31, 2016 |
$ | 109,205,263 | $ | 13,330 | $ | 1,002 | ||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
$ | 87,677,604 | $ | 18,064 | $ | 1,002 | ||||||
| 2022 Convertible Notes |
||||||||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
$ | 230,000,000 | $ | 6,886 | $ | 1,028 | ||||||
| Total Senior Securities(7) |
||||||||||||
| December 31, 2007 |
$ | 134,250,000 | $ | 3,985 | N/A | |||||||
| December 31, 2008 |
$ | 216,782,000 | $ | 2,764 | N/A | |||||||
| December 31, 2009 |
$ | 130,600,000 | $ | 3,806 | N/A | |||||||
| December 31, 2010 |
$ | 170,000,000 | $ | 3,478 | N/A | |||||||
| December 31, 2011 |
$ | 310,186,830 | $ | 2,409 | N/A | |||||||
| December 31, 2012 |
$ | 599,664,500 | $ | 1,874 | N/A | |||||||
| December 31, 2013 |
$ | 559,921,472 | $ | 2,182 | N/A | |||||||
| December 31, 2014 |
$ | 626,587,644 | $ | 2,073 | N/A | |||||||
| December 31, 2015 |
$ | 600,468,500 | $ | 2,194 | N/A | |||||||
| December 31, 2016 |
$ | 667,658,558 | $ | 2,180 | N/A | |||||||
| December 31, 2017 (as of June 30, 2017, unaudited) |
$ | 766,387,204 | $ | 2,067 | N/A | |||||||
| (1) | Total amount of each class of senior securities outstanding at the end of the period presented. |
163
| (2) | The asset coverage ratio for a class of senior securities representing indebtedness is calculated as our consolidated total assets, less all liabilities and indebtedness not represented by senior securities, including senior securities not subject to asset coverage requirements under the 1940 Act due to exemptive relief from the SEC, divided by senior securities representing indebtedness. This asset coverage ratio is multiplied by $1,000 to determine the Asset Coverage per Unit. |
| (3) | Not applicable because senior securities are not registered for public trading. |
| (4) | Issued by HT II, one of our SBIC subsidiaries, to the SBA. These categories of senior securities were not subject to the asset coverage requirements of the 1940 Act as a result of exemptive relief granted to us by the SEC. |
| (5) | Issued by HT III, one of our SBIC subsidiaries, to the SBA. These categories of senior securities were not subject to the asset coverage requirements of the 1940 Act as a result of exemptive relief granted to us by the SEC. |
| (6) | The Company’s Wells Facility and Union Bank Facility had no borrowings outstanding during the periods noted above. |
| (7) | The total senior securities and Asset Coverage per Unit shown for those securities do not represent the asset coverage ratio requirement under the 1940 Act because the presentation includes senior securities not subject to the asset coverage requirements of the 1940 Act as a result of exemptive relief granted to us by the SEC. As of June 30, 2017 our asset coverage ratio under our regulatory requirements as a business development company was 241.9% excluding our SBA debentures as a result of our exemptive order from the SEC which allows us to exclude all SBA leverage from our asset coverage ratio. |
164
Our business and affairs are managed under the direction of our Board of Directors. Our Board of Directors elects our officers who serve at the discretion of the Board of Directors. Our Board of Directors currently consists of seven members, one who is an “interested person” of the Company as defined in Section 2(a)(19) of the 1940 Act and six who are not interested persons and who we refer to as our independent directors.
Directors, Executive Officers and Key Employees
Our executive officers, directors and key employees and their positions are set forth below. Information regarding our current Board of Directors is set forth below as of June 30, 2017. The address for each executive officer, director and key employee is c/o Hercules Capital, Inc., 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301.
| Name |
Age | Positions | ||||
| Interested Director: |
||||||
| Manuel A. Henriquez(1) |
53 | Chairman of the Board of Directors, President and Chief Executive Officer | ||||
| Independent Directors: |
||||||
| Robert P. Badavas |
64 | Director | ||||
| Allyn C. Woodward, Jr. |
76 | Director | ||||
| Thomas J. Fallon |
56 | Director | ||||
| Susanne D. Lyons |
60 | Director | ||||
| Joseph F. Hoffman |
68 | Director | ||||
| Doreen Woo Ho |
70 | Director | ||||
| Executive Officers: |
||||||
| Mark Harris |
47 | Chief Financial Officer and Chief Accounting Officer | ||||
| Melanie Grace |
48 | General Counsel and Chief Compliance Officer | ||||
| Scott Bluestein |
39 | Chief Investment Officer | ||||
| Andrew Olson |
34 | Vice President of Finance and Senior Controller(2) | ||||
| (1) | Mr. Henriquez is an interested person, as defined in section 2(a)(19) of the 1940 Act, of the Company due to his position as an executive officer of the Company. |
| (2) | Mr. Olson announced his resignation, effective July 21, 2017, from his position as Vice President of Finance and Senior Controller. Gerard R. Waldt, Jr., the Company’s current Assistant Controller, will assume the position of Controller. |
Set forth below is information regarding our current directors, including each director’s (i) name and age; (ii) a brief description of their recent business experience, including present occupations and employment during at least the past five years; (iii) directorships, if any, that each director holds and has held during the past five years; and (iv) the year in which each person became a director of the Company. As the information that follows indicates, the nominee and each continuing director brings strong and unique experience, qualifications, attributes, and skills to the Board of Directors. This provides the Board of Directors, collectively, with competence, experience, and perspective in a variety of areas, including: (i) corporate governance and Board service; (ii) executive management, finance, and accounting; (iii) venture capital financing with a technology-related focus; (iv) business acumen; and (v) an ability to exercise sound judgment.
Moreover, the nominating and corporate governance committee believes that it is important to seek a broad diversity of experience, professions, skills, geographic representation and backgrounds. The nominating and corporate governance committee does not assign specific weights to particular criteria and no particular criterion is necessarily applicable to all prospective nominees. We believe that the backgrounds and qualifications of the directors, considered as a group, should provide a significant composite mix of experience, knowledge and abilities that will allow the Board of Directors to fulfill its responsibilities. Our Board of Directors does not have
165
a specific diversity policy, but considers diversity of race, religion, national origin, gender, sexual orientation, disability, cultural background and professional experiences in evaluating candidates for Board membership.
For each director, we have highlighted certain key areas of experience that qualify him or her to serve on the Board of Directors in each of their respective biographies below.
| Name, Address, and Age |
Position(s) |
Term of Office |
Principal Occupation(s) During Past |
Other Directorships | ||||
| Independent Directors |
||||||||
| Susanne D. Lyons (60) |
Director | Class I Director since 2015 | Retired. Chief Marketing Officer, VISA from 2005-2007. | None. | ||||
| Robert P. Badavas (64) |
Director | Class I Director since 2006 | Retired. Chairman and Chief Executive Officer of PlumChoice, provider of remote technical services and support, from 2011-2016. | Constant Contract, Inc., an online marketing company, from 2007-2016. | ||||
| Thomas J. Fallon (56) |
Director | Class II Director since 2014 | Chief Executive Officer of Infinera Corporation, manufacturer of high capacity optical transmission equipment, since 2010. | Infinera Corporation since 2014. | ||||
| Allyn C. Woodward, Jr. (76) |
Director | Class II Director since 2004 | Retired. Vice Chairman and Director of Adams Harkness Financial Group, an institutional investment bank, from 2001-2006. | None. | ||||
| Joseph F. Hoffman (68) |
Director | Class III Director since 2015 | Retired. SEC Reviewing Partner and Silicon Valley Professional for KPMG from 1998-2009. | None. | ||||
| Doreen Woo Ho (70) |
Director | Class III Director since 2016 | Commissioner of the San Francisco Port Commission since May, 2011 and served as President from 2012 to 2014. | U.S. Bank since 2012. | ||||
| Interested Director |
||||||||
| Manuel A. Henriquez (53)(3) |
Director, Chief Executive Officer and Chairman of the Board of Directors | Class III since 2004 | Hercules Capital, Inc. since 2004. | None. | ||||
| (1) | The address for each officer and director is c/o Hercules Capital, Inc., 400 Hamilton Avenue., Suite 310, Palo Alto, California 94301. |
| (2) | No director otherwise serves as a director of an investment company subject to the 1940 Act. |
| (3) | Mr. Henriquez is an interested director due to his position as an officer of the Company. |
166
Interested Director
| Manuel A. Henriquez |
Board Committee: |
Independent: | ||
| N/A |
No |
Mr. Henriquez, age 53, is a co-founder of Hercules and has been our Chairman and Chief Executive Officer since 2004 and our President (since 2005) and his term expires in 2019.
| Prior Business Experience: |
• Partner, VantagePoint Venture Partners, a $2.5 billion multi-stage technology venture fund (2000-2003) • President and Chief Investment Officer, Comdisco Ventures, a division of Comdisco, Inc., a leading technology and financial services company (1999-2000) • Managing Director, Comdisco Ventures (1997-1999) • Senior Member, Investment Team, Comdisco Ventures (1997-2000) | |
| Private Directorships/ Memberships: |
• Northeastern University, a global, experiential research university • Vice Chairman of the board of directors of Lucile Packard Foundation for Children’s Health, the sole fundraising entity for Lucile Packard Children’s Hospital and the child health programs at Stanford University School of Medicine, and Chairman of the Compensation Committee, Member of the Investment Committee, and Member of the Executive Committee of the board of directors • Children’s Health Council, a diagnostic and treatment center for children and adolescents facing developmental and behavioral challenges, Chairman of the Finance Committee and Chairman of the Investment Committee, and Corporate Treasurer and Member of the Executive Committee of the board of directors | |
| Education: |
• Bachelor’s degree in Business Administration from Northeastern University | |
| Skills/ Qualifications: |
In particular, Mr. Henriquez’ key areas of skills/qualifications include, but are not limited to:
• Client Industries—vast array of knowledge in venture capital financing, including software, life sciences and clean tech • Banking/Financial Services—extensive experience with equity and debt financings as well SEC rules and regulations and business development companies • Leadership/Strategy—current role as chairman and CEO as well as officer and director experience in several private and public companies and knowledge of financial risk assessment • Finance/IT and Other Business Processes—extensive experience in IT and supervising IT internal control and procedures | |
167
Independent Directors
| Joseph F. Hoffman |
Board Committee: |
Independent: | ||
| • Nominating, Chair • Audit |
Yes |
Mr. Hoffman, age 68, is retired from KPMG LLP after 26 years as a partner and senior executive with that firm. He has served as a director on our Board of Directors since April 2015 and his term expires in 2019.
| Prior Business Experience: | • SEC Reviewing Partner and Silicon Valley Professional Practice Partner, KPMG LLP (1998-2009) • Audit Partner and Business Unit Partner in Charge, KPMG LLP (1983-1998) | |
| Private Directorships: | • LiveOps, Inc., a cloud based contact center (since 2013) • KPMG LLP, an audit, tax, and advisory professional services firm. (2005-2009) | |
| Audit Committees: | • LiveOps, Inc. (since 2013) • KPMG LLP (2005-2009) • Willamette University (since 2014) | |
| Non-Profit Leadership: | • Board of Trustees, Willamette University (since 2011) | |
| Memberships: | • California Society of Certified Public Accountants • National Association of Corporate Directors • American College of Corporate Directors • Association of Governing Boards of Universities and Colleges | |
| Education: | • Bachelor’s degree in Mathematics and Economics, Willamette University • Master’s degree in Business Administration, Stanford Graduate School of Business • Certified public accountant, State of California | |
| Skills/ Qualifications: | In particular, Mr. Hoffman’s key areas of skill/qualifications include, but are not limited to:
• Client Industries—extensive experience in the technology, manufacturing, and financial services industries • Finance and Enterprise Risk Management—extensive experience as an advisor to senior management and audit committees on complex accounting, financial reporting, internal controls, and enterprise risk management • Leadership/Strategy—significant experience as a business executive and director • Governance—experience as the chairman of the governance committee with corporate governance issues, particularly in a publicly-traded company • Banking/Financial Services—experience with banking, mutual funds, or other financial services industries, including regulatory experience and specific knowledge of the Securities Act of 1933, as amended | |
168
| Allyn C. Woodward, Jr. |
Board Committee: |
Independent: | ||
| • Audit • Compensation |
Yes—Lead Director |
Mr. Woodward, age 76, has extensive experience and qualifications in banking and financial services. He has served as a director on our Board of Directors since February 2004 and his term expires in 2018.
| Business Experience: |
• Vice Chairman and Director, Adams Harkness Financial Group (formerly Adams, Harkness & Hill), an independent institutional research, brokerage and investment banking firm (2001-2006) • President and Director, Adams Harkness Financial Group (1995-2001) • Silicon Valley Bank • Vice President, Founder, Wellesley, Massachusetts office • Senior Vice President (1990-1992) • Chief Operating Officer (California) (1992-1995) • Senior Vice President and Group Manager of Technology Group, Bank of New England (1963-1990) | |
| Private Directorships: |
• Union Specialties, manufacturer of water-based polyurethane dispersions and specialty products (1990-present) | |
| Current Advisory Board Directorships: |
• Fletcher Spaght Venture Capital (2005-present) • Boston Millennia Partners (2000-present) • Ampersand Venture Capital (2013-present) | |
| Prior Directorships: |
• AH&H Venture Capital • Square 1 Bank • Lecroy Corporation, Chairman • Viewlogic Systems • Cayenne Software, Inc. | |
| Non-Profit Leadership: |
• Member of Finance Committee and Board of Overseers, Newton Wellesley Hospital (2000-present)
• Babson College, Member of: • Investment Committee • Finance Committee • Private Equity Committee (co-founder) (2000-present) | |
| Education: |
• Bachelor’s degree in Finance and Accounting from Babson College • Banking degree, Stonier Graduate School of Banking at Rutgers University | |
| Memberships |
• National Association of Corporate Directors • Board Leaders Group | |
| Certifications: |
• Executive Masters Professional Director Certification, American College of Corporate Directors | |
| Skills/ Qualifications: |
• In particular, Mr. Woodward’s key areas of skill/qualifications include, but are not limited to: • Client Industries and Banking/Financial Services—extensive leadership, management and director experience in financial services, banking and technology-related companies • Leadership/Strategy—significant executive and board experience for both private and public companies in business, finance and investments with a special emphasis on best policies regarding compensation and governance and service as Lead Independent Director • Finance, IT and Other Business Processes—extensive experience related to finance, accounting, IT, treasury, human resources or other key business processes • Governance—as lead director extensive experience with corporate governance issues, particularly in a publicly-traded company | |
169
| Robert P. Badavas |
Board Committee: |
Independent: | ||
| • Audit, Chair |
Yes |
Mr. Badavas, aged 64, retired in August 2016 as Chairman and Chief Executive Officer of PlumChoice, a venture-backed technology, software and services company (since December 2011). He has served as a director on our Board of Directors since March 2006 and his term expires in 2017.
| Business Experience: |
• President, Petros Ventures, Inc., a management and advisory services firm (2009-2011 and 2016-present) • President and Chief Executive Officer of TAC Worldwide, a multi-national technical workforce management and business services company (2005-2009) • Executive Vice President and Chief Financial Officer, TAC Worldwide (2003-2005) • Senior Partner and Chief Operating Officer, Atlas Venture, an international venture capital firm (2001-2003) • Chief Executive Officer at Cerulean Technology, Inc., a venture capital backed wireless application software company (1995-2001) • Certified Public Accountant, PwC (1974-1983) | |
| Public Directorships: |
• Constant Contact, Inc., including chairman of the audit committee, a provider of email and other engagement marketing products and services for small and medium sized organizations, acquired by Endurance International Group Holdings, Inc., (2007-2016) | |
| Prior Directorships: |
• PlumChoice • Arivana, Inc.; a telecommunications infrastructure company—publicly traded until its acquisition by SAC Capital • RSA Security; an IT security company—publicly traded until its acquisition by EMC • On Technology; an IT software infrastructure company—publicly traded until its acquisition by Symantec • Renaissance Worldwide; an IT services and solutions company—publicly traded until its acquisition by Aquent | |
| Other Experience: |
• Vice-Chairman, Board of Trustees. Bentley University (since 2005) • Board of Trustees Executive Committee and Corporate Treasurer, Hellenic College/Holy Cross Orthodox School of Theology, including positions on the executive committee and corporate treasurer (since 2002) • Chairman Emeritus, The Learning Center for the Deaf (1995-2005) • Master Professional Director Certification, American College of Corporate Directors • National Association of Corporate Directors • Annunciation Greek Orthodox Cathedral of New England, Parish Council President (since 2016) | |
| Education: |
• Bachelor’s degree in Accounting and Finance from Bentley University | |
| Skills/ Qualifications: |
In particular, Mr. Badavas’ key areas of skill/qualifications include, but are not limited to:
• Client Industries—extensive experience in software, business and technology enabled services and venture capital • Leadership/Strategy—significant experience as a senior corporate executive in private and public companies, including tenure as chief executive officer, chief financial officer and chief operating officer • Finance, IT and Other Business Strategy and Enterprise Risk Management—prior experience as a CEO directing business strategy and as a CFO directing IT, financing and accounting, strategic alliances and human resources and evaluation of enterprise risk in such areas • Governance—extensive experience as an executive and director of private and public companies with governance matters | |
170
| Thomas J. Fallon |
Board Committee: |
Independent: | ||
| • Nominating |
Yes |
Mr. Fallon, aged 56, currently serves as Chief Executive Officer of Infinera Corporation (since 2010) and a member of Infinera’s board of directors (since 2009). He has served as a director on our Board of Directors since July 2014 and his term expires in 2018.
| Infinera Corporation Experience: |
• President and Chief Executive Officer, Infinera Corporation (2010-Current) • Chief Operating Officer, Infinera Corporation (2006-2009) • Vice President of Engineering and Operations, Infinera Corporation (2004-2006) | |
| Other Business Experience |
• Vice President, Corporate Quality and Development Operations of Cisco Systems, Inc. (2003-2004) • General Manager of Cisco Systems’ Optical Transport Business Unit, VP Operations, VP Supply, various executive positions (1991-2003) | |
| Prior Directorships: |
• Piccaro, a leading provider of solutions to measure greenhouse gas concentrations, trace gases and stable isotopes (2010-2016) | |
| Other Experience: |
• Member, Engineering Advisory Board of the University of Texas at Austin • Member, President’s Development Board University of Texas | |
| Education: |
• Bachelor’s degree in Mechanical Engineering from the University of Texas at Austin • Master’s degree in Business Administration from the University of Texas at Austin | |
| Skills/ Qualifications: |
In particular, Mr. Fallon’s key areas of skill/qualifications include, but are not limited to:
• Client Industries—significant experience in venture capital and technology • Leadership/Strategy—extensive experience as a director and executive in both public and private companies • Finance, IT and Other Business Processes—extensive experience as a manager and CEO related to finance, accounting, IT, treasury, human resources, or other key business processes. • Enterprise Risk Management—experience in managing enterprise risk • Governance—experienced in both corporate governance and executive compensation for both public and private companies | |
171
| Susanne D. Lyons |
Board Committee: | Independent: | ||
| • Compensation, Chair |
Yes | |||
| • Nominating |
Ms. Lyons, aged 60, is a retired senior executive who has held top marketing and general management roles at some of the largest financial services companies in America. She has served as a director on our Board of Directors since March 2015 and her term expires in 2017.
| Prior Business Experience: |
• Chief Marketing Officer, VISA (USA) (2004-2007) • Various marketing and general management positions, including enterprise president of retail client service, Charles Schwab & Co., Inc. (1992-2001) • Chief Marketing Officer, Charles Schwab & Co., Inc. (2000-2001) • Senior positions in marketing, product development and business strategy, Fidelity Investments (1982-1992) | |
| Private Directorships: |
• U.S. Olympic Committee (since December 2010) • Wildcare, a non-for-profit organization (since 2008) | |
| Prior Directorships: |
• CNET Networks until its acquisition by CBS Corp. (2007-2008) • Gain Capital Holdings, Inc. (2008-2013) | |
| Other Experience: |
• Advisory Board, Marketo, Inc., a marketing automation software company (2008-2011) | |
| Education: |
• Bachelor’s degree in French from Vassar College • Master’s degree in Business Administration from Boston University | |
| Skills/ Qualifications: |
In particular, Ms. Lyons’ key areas of skill/qualifications include, but are not limited to:
• Banking/Financial Services—held a variety of key executive and management positions at large global financial institutions, including 1940-Act regulated companies • Leadership/Strategy—extensive experience as a director and executive with broad operational experience in investments, finance, human resources, and marketing • Finance, IT and Other Business Processes—expertise in Human Resources, including extensive experience in public company compensation governance • Governance—experienced executive and director for public companies, including extensive experience in public company compensation and governance | |
172
| Doreen Woo Ho |
Board Committee: | Independent: | ||
| • Compensation |
Yes |
Ms. Woo Ho, aged 70, is a retired senior executive who has held top management roles at some of the largest commercial banks in America, including Wells Fargo Bank, Citibank and United Commercial Bank. She has served as a director on our Board of Directors since October 2016 and her term expires in 2019.
| Business Experience: |
• President and Chief Executive Officer of United Commercial Bank (2009) • Executive Vice President, Student Loans and Corporate Trust, Wells Fargo & Company (2008) • President of the Consumer Credit Group, Wells Fargo Bank (1998-2007) • Senior Vice President of National Business Banking, US Consumer Bank, Citibank (1974-1998) | |
| Public Directorships: |
• U.S. Bank (since 2012) | |
| Prior Directorships: |
• United Commercial Bank (2009) | |
| Private Directorships: |
• San Francisco Opera (since 1992) | |
| Other Experience: |
• Commissioner of the Port of San Francisco (since 2011) • Wells Fargo Management Committee member (1999-2008) | |
| Education: |
• Bachelor’s in History from Smith College • Masters in East Asian Studies from the School of International and Public Affairs at Columbia University | |
| Skills/ Qualifications: |
In particular, Ms. Woo Ho’s key areas of skill/qualifications include, but are not limited to:
• Banking/Financial Services—held a variety of key executive and management positions at large global financial institutions • Leadership/Strategy—extensive experience as a director and executive with broad operational experience in investments and finance • Finance, IT and other Business Processes—extensive experience in commercial lending, sales marketing as well as other key business processes • Enterprise Risk Management—extensive experience in risk management and regulatory compliance in banking services • Governance—gained extensive experience as CEO of a banking institution in corporate governance and executive management | |
173
Executives
Our executive officers perform policy-making functions for us within the meaning of applicable SEC rules. They may also serve as officers of our other subsidiaries. There are no family relationships among our directors or executive officers.
The following information, as of June 30, 2017 outlines the name and age of our executive officers (as of the date of this prospectus) and his or her principal occupation with the Company, followed by the biographical information of each of such executive officer:
| Name |
Age | Principal Occupation | ||||
| Manuel A. Henriquez |
53 | Chairman and Chief Executive Officer | ||||
| Mark R. Harris |
47 | Chief Financial Officer and Chief Accounting Officer | ||||
| Scott Bluestein |
39 | Chief Investment Officer | ||||
| Melanie Grace |
48 | General Counsel, Chief Compliance Officer and Secretary | ||||
| Andrew Olson |
34 | Vice President of Finance and Senior Controller* | ||||
| * | Mr. Olson announced his resignation, effective July 21, 2017, from his position as Vice President of Finance and Senior Controller. Gerard R. Waldt, Jr., the Company’s current Assistant Controller, will assume the position of Controller. |
Executive Biographies
Manuel A. Henriquez’ biography can be found under “Interested Director” above.
Mark Harris joined us in 2015 as Chief Financial Officer and Chief Accounting Officer. Mr. Harris has over 20 years of experience working with public companies, as well as the mezzanine and direct lending space. Mr. Harris oversees the financial and accounting functions of the Company.
| Other Prior Experience | • Chief Financial Officer, Asia Strategy and Senior Managing Director/Head of Asia, Avenue Capital, where he lead the Asia strategy (2006-2015) • Corporate Financial Controller, Hutchinson Telecommunication International Limited (a NYSE and Stock Exchange of Hong Kong company) (2004-2006) • Vice President of Finance, Vsource (a NASDAQ listed company) (2001-2004) • Manager, Global Capital Markets Group, PricewaterhouseCoopers (1995-2001) | |
| Education/Other: | • Master’s of Business Administration from the University of Chicago, Booth School of Business • Bachelor’s in Business Administration with an emphasis in Accounting from California Polytechnic State University, San Luis Obispo • Active Certified Public Accountant in California • Member, Foundation Board of California Polytechnic State University, San Luis Obispo | |
Scott Bluestein joined us in 2010 as Chief Credit Officer. He was promoted to Chief Investment Officer in 2014. Mr. Bluestein is responsible for managing the investment teams and investments made by the Company.
| Other Prior Experience | • Founder and Partner, Century Tree Capital Management (2009-2010) • Managing Director, Laurus-Valens Capital Management, an investment firm specializing in financing small and microcap growth-oriented businesses through debt and equity securities (2003-2010) • Member of Financial Institutions Coverage Group focused on Financial Technology, UBS Investment Bank (2000-2009) | |
| Education/Other: | • Bachelor’s in Business Administration from Emory University | |
174
Melanie Grace joined us in 2015 as General Counsel, Chief Compliance Officer and Secretary. She has over 17 years of experience representing public and private companies in securities, compliance and transactional matters. Ms. Grace oversees the legal and compliance function for the Company and serves as secretary for the Company and select subsidiaries.
| Other Prior Experience |
• Chief Legal Officer and Corporate Secretary, WHV Investments, Inc. where she also served as interim Chief Compliance Officer (2011-2015) • Member, Management, Operations and Proxy Committees, WHV Investments, Inc. (2013-2015) • Chair, Ethics Committee, WHV Investments, Inc. (2013-2015) • Chief Counsel, Corporate, NYSE Euronext (2005-2008) • Associate, Fenwick & West LLP (2000-2005) | |
| Education/Other: | • Bachelor’s and Master’s in History from the University of California, Riverside • Juris Doctor from Boston University School of Law • Member, State Bar of California • Registered In-House Counsel, New York • Designated Investment Adviser Certified Compliance Professional® | |
Andrew Olson joined us in 2014 as Corporate Controller. He served as our Interim Chief Financial Officer (June 9, 2015 to August 1, 2015). Mr. Olson was our Vice President of Finance and Senior Controller and is responsible for financial and regulatory reporting, financial planning and analysis, and financial systems design and implementation. Mr. Olson announced his resignation, effective July 21, 2017, from his position as Vice President of Finance and Senior Controller. Gerard R. Waldt, Jr., our current Assistant Controller, will assume the position of Controller.
| Other Prior Experience | • Senior Manager in Financial Services practice of PricewaterhouseCoopers, LLP San Francisco and Hong Kong where he developed extensive experience providing audit and consulting services to both regional and international institutions (2006-2014) | |
| Education/Other: | • Bachelor’s in Business Economics from the University of California, Santa Barbara • Active Certified Public Accountant in California | |
Board of Directors
The number of directors is currently fixed at seven directors.
Our Board of Directors is divided into three classes. Class I directors hold office for a term expiring at the annual meeting of stockholders to be held in 2017, Class II directors hold office for a term expiring at the annual meeting of stockholders to be held in 2018 and Class III directors hold office for a term expiring at the annual meeting of stockholders to be held in 2019. Each director holds office for the term to which he or she is elected and until his or her successor is duly elected and qualifies. Messrs. Woodward and Fallon’s terms expire in 2018, Messrs. Henriquez and Hoffman and Ms. Woo Ho’s terms expire in 2019 and Mr. Badavas and Ms. Lyons’ terms expire in 2017. At each annual meeting of our stockholders, the successors to the class of directors whose terms expire at such meeting will be elected to hold office for a term expiring at the annual meeting of stockholders held in the third year following the year of their election and until their successors are duly elected and qualify.
175
Our business, property and affairs are managed under the direction of our Board of Directors. Members of our Board of Directors are kept informed of our business through discussions with our chairman and chief executive officer, our chief financial officer, our chief investment officer, our secretary, and our other officers and employees, and by reviewing materials provided to them and participating in meetings of our Board of Directors and its committees.
Because our Board of Directors is committed to strong and effective corporate governance, it regularly monitors our corporate governance policies and practices to ensure we meet or exceed the requirements of applicable laws, regulations and rules, and the NYSE’s listing standards. The Board of Directors has approved corporate governance guidelines that provide a framework for the operation of the Board of Directors and address key governance practices. The Board of Directors has adopted a number of policies to support our values and good corporate governance, including corporate governance guidelines, Board of Directors’ committee charters, insider trading policy, code of ethics, code of business conduct and ethics, and related person transaction approval policy.
During 2016, as part of its on-going review of our corporate governance policies, our Board of Directors undertook the following relating to our corporate governance practices:
| • | reviewed our compliance manual and made changes, where required, with the approval of our Board of Directors; and |
| • | as a result of the ongoing plan to integrate our comprehensive compliance program, conducted training sessions in 2016 to remind employees of their obligations as employees and officers of a business development company and the specific policies and procedures that have been designed by us to reasonably ensure that the our employees are in compliance with federal securities laws and other laws. |
Our Board of Directors will continue to review and update the corporate governance guidelines, corporate governance practices, and our corporate governance framework, including the potential expansion of the size of our Board of Directors.
Board Leadership Structure
Chairman and Chief Executive Officer
Our Board of Directors currently combines the role of chairman of the Board of Directors with the role of chief executive officer, coupled with a lead independent director position to further strengthen our governance structure. Our Board of Directors believes this provides an efficient and effective leadership model for our company. Combining the chairman and chief executive officer roles fosters clear accountability, effective decision-making, and alignment on corporate strategy. Since 2004, Mr. Henriquez has served as both chairman of the Board of Directors and as our chief executive officer. Mr. Henriquez is an interested director.
No single leadership model is right for all companies at all times. Our Board of Directors recognizes that depending on the circumstances, other leadership models, such as a separate independent chairman of the Board of Directors, might be appropriate. Accordingly, our Board of Directors periodically reviews its leadership structure.
Moreover, our Board of Directors believes that its governance practices provide adequate safeguards against any potential risks that might be associated with having a combined chairman and chief executive officer. Specifically:
| • | six of our seven current directors are independent directors; |
176
| • | all of the members of our Audit Committee, Compensation Committee, and NCG Committee are independent directors; |
| • | our Board of Directors and its committees regularly conduct scheduled meetings in executive session, out of the presence of Mr. Henriquez and other members of management; |
| • | our Board of Directors and its committees regularly conduct meetings which specifically include Mr. Henriquez; |
| • | our Board of Directors and its committees remain in close contact with, and receive reports on various aspects of Hercules’s management and enterprise risk directly from our senior management and independent auditors. |
Lead Independent Director
Our Board of Directors has instituted the lead independent director position to provide an additional measure of balance, ensure our Board of Directors’ independence, and enhance its ability to fulfill its management oversight responsibilities. Allyn C. Woodward, Jr. currently serves as our lead independent director. The lead independent director:
| • | presides over all meetings of the independent directors at which our chairman is not present, including executive sessions of the independent directors; |
| • | has the authority to call meetings of the independent directors; |
| • | frequently consults with our chairman and chief executive officer about strategic policies; |
| • | provides our chairman and chief executive officer with input regarding Board of Directors meetings; |
| • | serves as a liaison between the chairman and chief executive officer and the independent directors; and |
| • | otherwise assumes such responsibilities as may be assigned to him by the independent directors. |
Having a combined chairman and chief executive officer, coupled with a substantial majority of independent, experienced directors, including a lead independent director with specified responsibilities on behalf of the independent directors, provides the right leadership structure for our company and is best for us and our stockholders at this time.
Board Oversight of Risk
While risk management is primarily the responsibility of our management team, our Board of Directors is responsible for oversight of the material risks faced by us at both the full board level and at the committee level.
Our Audit Committee has oversight responsibility not only for financial reporting with respect to our major financial exposures and the steps management has taken to monitor and control such exposures, but also for the effectiveness of management’s enterprise risk management process that monitors and manages key business risks facing our company. In addition to our Audit Committee, the other committees of our Board of Directors consider the risks within their areas of responsibility. For example, our Compensation Committee considers the risks that may be posed by our executive compensation program.
Management provides regular updates throughout the year to our Board of Directors regarding the management of the risks they oversee at each regular meeting of our Board of Directors. Also, our Board of Directors receives presentations throughout the year from various department and business group heads that include discussion of significant risks as necessary. Additionally, our full Board of Directors reviews our short and long-term strategies, including consideration of significant risks facing our business and their potential impact.
177
During 2016, in addition to unanimous written consents, the Board of Directors held the following meetings:
| Type of Meeting |
Number | |||
| Regular Meetings to address regular, quarterly business matters |
4 | |||
| Other Meetings to address business matters that arise between quarters |
9 | |||
Each director makes a diligent effort to attend all Board of Directors and committee meetings, as well as our annual meeting of stockholders. All directors attended at least 75% of the aggregate number of meetings of the Board of Directors and of the respective committees on which they served. Each of our then-serving directors attended our 2016 annual meeting of stockholders in person.
Board Committees
Our Board of Directors has established an Audit Committee, a Compensation Committee, and a NCG Committee. A brief description of each committee is included in this prospectus and the charters of the Audit, Compensation, and NCG Committees are available on the Investor Relations section of our website at http://investor.htgc.com/corporate-governance.cfm.
As of the date of this prospectus, the members of each of our Board of Directors committees are as follows (the names of the respective committee chairperson are bolded):
| Audit |
Compensation |
Nominating and Governance | ||
| Robert Badavas Joseph Hoffman Allyn Woodward, Jr. |
Susanne Lyons Allyn Woodward, Jr. Doreen Woo Ho |
Joseph Hoffman Susanne Lyons Thomas Fallon |
Each of our directors who sits on a committee satisfies the independence requirements for purposes of the rules promulgated by the NYSE and the requirements to be a non-interested director as defined in Section 2(a)(19) of the 1940 Act. Messrs. Badavas and Hoffman, Chairman and member of the Audit Committee, respectively, are each an “audit committee financial expert” as defined by applicable SEC rules.
Committee Governance
Each committee is governed by a charter that is approved by the Board of Directors, which sets forth each committee’s purpose and responsibilities. The Board of Directors reviews the committees’ charters, and each committee reviews its own charter, on at least an annual basis, to assess the charters’ content and sufficiency, with final approval of any proposed changes required by the full Board of Directors.
Committee Responsibilities and Meetings
The key oversight responsibilities of the Board of Directors committees, and the number of meetings held by each committee during 2016, are as follows:
| Audit Committee |
Number of meetings held in 2016: 4 |
| • | Appointing, overseeing and replacing, if necessary, our independent auditor. |
| • | Overseeing the accounting and financial reporting processes and the integrity of the financial statements. |
| • | Establishing procedures for complaints relating to accounting, internal accounting controls or auditing matters. |
| • | Examining the independence qualifications of our auditors. |
178
| • | Assisting our Board of Directors’ oversight of our compliance with legal and regulatory requirements and enterprise risk management. |
| • | Assisting our Board of Directors in fulfilling its oversight responsibilities related to the systems of internal controls and disclosure controls which management has established regarding finance, accounting, and regulatory compliance. |
| • | Reviewing and recommending to the Board of Directors the valuation of the Company’s portfolio. |
| Compensation Committee |
Number of meetings held in 2016: 5 |
| • | Oversees our overall compensation strategies, plans, policies and programs. |
| • | The approval of director and executive compensation. |
| • | The assessment of compensation-related risks. |
| Nominating and Corporate Governance Committee |
Number of meetings held in 2016: 6 |
| • | Our general corporate governance practices, including review of our Corporate Governance Guidelines. |
| • | The annual performance evaluation of our Board of Directors and its committees. |
| • | The identification and nomination of director candidates. |
| • | Succession planning for management. |
| • | Criteria considered by the NCG Committee in evaluating qualifications of individuals for election as members of the Board of Directors consist of the independence and other applicable NYSE corporate governance requirements; the 1940 Act and all other applicable laws, rules, regulations and listing standards; and the criteria, polices and principles set forth in the NCG Committee charter. |
| • | Considers nominees properly recommended by a stockholder. Nominations for directors may be made by stockholders if notice is timely given and if the notice contains the information required in our Bylaws. Proposals must comply with the other requirements contained in our Bylaws, including supporting documentation and other information. |
Director Independence
The NYSE’s listing standards and Section 2(a)(19) of the 1940 Act require that a majority of our Board of Directors and every member of our Audit, Compensation, and NCG Committees are “independent.” Under the NYSE’s listing standards and our corporate governance guidelines, no director will be considered to be independent unless and until our Board of Directors affirmatively determines that such director has no direct or indirect material relationship with our company or our management. Our Board of Directors reviews the independence of its members annually.
In determining that Ms. Lyons and Ho and Messrs. Badavas, Woodward, Fallon and Hoffman are independent, our Board of Directors, through the NCG Committee, considered the financial services, commercial, family and other relationships between each director and his or her immediate family members or affiliated entities, on the one hand, and Hercules and its subsidiaries, on the other hand.
Communication with the Board
We believe that communications between our Board of Directors, our stockholders and other interested parties are an important part of our corporate governance process. Stockholders with questions about Hercules
179
are encouraged to contact our Investor Relations department at (650) 289-3060. However, if stockholders believe that their questions have not been addressed, they may communicate with our Board of Directors by sending their communications to Hercules Capital, Inc., c/o Melanie Grace, Secretary, 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301. All stockholder communications received in this manner will be delivered to one or more members of our Board of Directors.
Mr. Woodward currently serves as the lead independent director, and he presides over executive sessions of the independent directors. Parties may communicate directly with Mr. Woodward by sending their communications to Hercules Capital, Inc., c/o Melanie Grace, Secretary at the above address. All communications received in this manner will be delivered to Mr. Woodward.
All communications involving accounting, internal accounting controls and auditing matters, possible violations of, or non-compliance with, applicable legal and regulatory requirements or our code of ethics, or retaliatory acts against anyone who makes such a complaint or assists in the investigation of such a complaint, will be referred to Melanie Grace, Secretary. The communication will be forwarded to the chair of our Audit Committee if our secretary determines that the matter has been submitted in conformity with our whistleblower procedures or otherwise determines that the communication should be so directed.
The acceptance and forwarding of a communication to any director does not imply that the director owes or assumes any fiduciary duty to the person submitting the communication, all such duties being only as prescribed by applicable law.
Code of Business Conduct and Ethics
Our code of business conduct and ethics requires that our directors and executive officers avoid any conflict, or the appearance of a conflict, between an individual’s personal interests and the interests of Hercules. Pursuant to our code of business conduct and ethics, which is available on our website at http://investor.htgc.com/corporate-governance.cfm, each director and executive officer must disclose any conflicts of interest, or actions or relationships that might give rise to a conflict, to our Audit Committee. Certain actions or relationships that might give rise to a conflict of interest are reviewed and approved by our Board of Directors.
Availability of Corporate Governance Documents
To learn more about our corporate governance and to view our corporate governance guidelines, code of business conduct and ethics, and the charters of our Audit Committee, Compensation Committee, and NCG Committee, please visit the Investor Relations page of our website at http://investor.htgc.com/corporate-governance.cfm, under “Corporate Governance.” Copies of these documents are also available in print free of charge by writing to Hercules Capital, Inc., c/o Melanie Grace, secretary, 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301.
Compensation Committee Interlocks and Insider Participation
All members of our Compensation Committee are independent directors and none of the members are present or past employees of the Company. No member of our Compensation Committee: (i) has had any relationship with the Company requiring disclosure under Item 404 of Regulation S-K under the Securities Exchange Act of 1934, as amended, referred to as the Exchange Act; or (ii) is an executive officer of another entity, at which one of our executive officers serves on our Board of Directors.
180
Compensation Discussion and Analysis
The Compensation Discussion and Analysis discusses our 2016 executive compensation program, as it relates to the following executive officers:
| Manuel A. Henriquez |
Chairman of the Board of Directors and Chief Executive Officer | |
| Mark R. Harris |
Chief Financial Officer and Chief Accounting Officer | |
| Scott Bluestein |
Chief Investment Officer | |
| Melanie Grace |
General Counsel, Chief Compliance Officer and Secretary | |
| Andrew Olson |
Vice President of Finance and Senior Controller* |
| * | Mr. Olson announced his resignation, effective July 21, 2017, from his position as Vice President of Finance and Senior Controller. Gerard R. Waldt, Jr., the Company’s current Assistant Controller, will assume the position of Controller. |
We refer to Messrs. Henriquez, Harris, Bluestein and Olson and Ms. Grace as our “named executive officers,” or “NEOs”.
Executive Summary
Under the oversight of our Compensation Committee, the Company’s executive compensation program is designed to attract, incent and retain talented individuals who are critical to our continued success and our corporate growth and who will deliver sustained strong performance over the longer term. Our executive compensation program is designed to motivate the Company’s executive officers to maintain the financial strength of the Company while avoiding any inappropriate focus on short-term profits that would impede the Company’s long-term growth and encourage excessive risk-taking.
In 2016, the Company continued to review and enhance our compensation practices in accordance with our executive compensation philosophy. The review considered both compensation levels and company performance over a one-, three-, and five-year period from 2012 to 2016 (the “Performance Periods”). (See “Compensation Philosophy and Objectives” below). The Company believes that compensation paid to our NEOs for 2016 was commensurate with the Company’s overall absolute performance as well as our performance relative to peers during the relevant Performance Periods. The 2016 compensation decisions made by the Compensation Committee considered the fact that our performance relative to a peer group of companies was above the median, and in most cases above the 75th percentile, measured using Return on Average Assets (“ROAA”), Return on Equity (“ROE”), Return on Investment Capital (“ROIC”), and Total Shareholder Return (“TSR”) during the trailing one-, three-, and five-years.
The Company’s incentive compensation practices are significantly limited by the requirements imposed on us as an internally managed business development company pursuant to the 1940 Act. (See “Limitations Imposed by the 1940 Act Relating to Implementation of Non-Equity Incentive Plans” below). These are regulatory limitations related to our corporate structure that are relatively unique and do not apply to most other publicly-traded companies. As discussed further below, our NEOs were compensated to reflect the Company’s performance during the relevant Performance Periods (See “Performance Highlights and Assessment of Company Performance” below) as well as individual performance.
In addition to key factors involved in the 2016 decisions made by the Compensation Committee, we continue to maintain the enhancements to our executive officer compensation program that we adopted in 2016, such as our clawback policy for all Section 16 officers and consideration of a mix of corporate and individual performance factors for our NEOs. In addition, the Compensation Committee did not grant restricted stock awards in 2017. Rather, the Compensation Committee granted restricted stock units with an additional one-year deferral period following the last vesting date. We believe these restricted stock unit awards assist the Company in retaining NEOs.
181
Compensation Philosophy and Objectives
As an internally managed business development company, the Company’s compensation program is designed to encourage the NEOs to think and act like stockholders. The structure of the NEOs’ compensation program is designed to encourage and reward the following factors, among other things:
| • | sourcing and pursuing attractively priced investment opportunities to venture-backed companies; |
| • | achieving the Company’s dividend objectives (which focus on stability and potential growth); |
| • | maintaining credit quality, monitoring financial performance and ultimately managing a successful exit of the Company’s investment portfolio; |
| • | providing compensation and incentives necessary to attract, motivate and retain key executives critical to our continued success and growth; |
| • | focusing management behavior and decision-making on goals that are consistent with the overall strategy of the business; |
| • | ensuring a linkage between NEO compensation and individual contributions to our performance; and |
| • | risk management. |
We believe that our continued success during 2016, despite strong competition for top-quality executive talent in the venture debt industry, was attributable to our ability to attract, motivate and retain the Company’s outstanding executive team through the use of both short- and long-term incentive compensation programs.
The Company’s compensation objectives are achieved through its executive compensation program, which for 2016 consisted of the following:
| • | Annual Base Salary: Cash paid on a regular basis throughout the year. This provides a level of fixed income that is competitive to allow the Company to retain and attract executive talent. |
| • | Annual Cash Bonus Awards: Cash awards paid on an annual basis following year-end. This rewards NEOs who contribute to our financial performance and strategic success during the year, and rewards individual achievements. |
| • | Long-Term Equity Incentive Awards: Equity incentive awards vest 1/3 on a one-year cliff with remaining 2/3 vesting quarterly over two years based on continued employment with the Company. This rewards NEOs who contribute to our success through the creation of shareholder value, provides meaningful retention incentives, and rewards individual achievements. |
The compensation program is designed to reflect best practices in executive compensation:
| • | No employment agreements for NEOs. |
| • | No guaranteed retirement benefits. |
| • | No cash severance payments. |
| • | No change in control benefits. |
| • | No tax gross ups for NEOs. |
| • | No pension. |
| • | No executive perquisite allowances beyond the benefit programs offered to all employees. |
| • | No repricing of stock options without stockholder approval, as required under applicable NYSE rules (and subject to other requirements under the 1940 Act). |
| • | Routine engagement of an independent compensation consultant to review NEO compensation. |
182
| • | Maintenance of stock ownership guidelines for NEOs to own at least two times his or her salary. |
| • | Clawback policy for all Section 16 officers. |
Executive Compensation Governance
The Company’s executive compensation program is supported by strong corporate governance and Board-level oversight. The Compensation Committee provides primary oversight of our compensation programs, including the design and administration of executive compensation plans, assessment and setting of corporate performance goals, as well as individual performance metrics, and the approval of executive compensation. In addition, the Compensation Committee retains an independent compensation consultant, and where appropriate, discusses compensation-related matters with our CEO, as it relates to the other NEOs. The Compensation Committee developed our 2016 compensation program, and the compensation paid to our NEOs during and in respect of 2016 was approved by the Compensation Committee as well as all of our independent directors.
| • | Role of Compensation Committee: The Compensation Committee is comprised entirely of independent directors who are also non-employee directors as defined in Rule 16b-3 under the Exchange Act, independent directors as defined by the NYSE rules, and are not “interested persons” of the Company, as defined by Section 2(a)(19) of the 1940 Act. Ms. Lyons, Ms. Woo Ho and Mr. Woodward comprise the Compensation Committee. Ms. Lyons chairs the Compensation Committee. |
The Compensation Committee operates pursuant to a charter that sets forth its mission, specific goals and responsibilities. A key component of the Compensation Committee’s goals and responsibilities is to evaluate, approve and/or make recommendations to our Board of Directors regarding the compensation of our NEOs, and to review their performance relative to their compensation to assure that they are compensated in a manner consistent with the compensation philosophy discussed above. In addition, the Compensation Committee evaluates and makes recommendations to our Board of Directors regarding the compensation of the directors for their services. Annually, the Compensation Committee:
| • | evaluates our CEO’s performance, |
| • | reviews our CEO’s evaluation of the other NEOs’ performance, |
| • | determines and approves the compensation paid to our CEO, and |
| • | with input from our CEO, reviews and approves the compensation of the other NEOs. |
The Compensation Committee periodically reviews our compensation programs and equity incentive plans to ensure that such programs and plans are consistent with our corporate objectives and appropriately align our NEOs’ interests with those of our stockholders. The Compensation Committee also administers our stock incentive program. The Compensation Committee may not delegate its responsibilities discussed above.
| • | Role of Compensation Consultant: The Compensation Committee has engaged Frederic W. Cook & Co., Inc., or F.W. Cook, as an independent outside compensation consultant to assist the Compensation Committee and provide advice on a variety of compensation matters relating to CEO compensation, compensation paid to our other NEOs, peer group selection, compensation program design, market and industry compensation trends, director compensation levels and regulatory developments. F.W. Cook was hired by and reports directly to the Compensation Committee. Our compensation consultant does not provide any other services to the Company. The Compensation Committee has assessed the independence of F.W. Cook pursuant to the NYSE rules, and it has been concluded that the consultant’s work for the Compensation Committee does not raise any conflict of interest. |
| • | Role of Chief Executive Officer: From time to time and at the Compensation Committee’s request, our CEO will attend the Compensation Committee’s meetings to discuss the Company’s performance and compensation-related matters. Our CEO does not attend executive sessions of the Compensation |
183
| Committee, unless invited by the Compensation Committee. While our CEO does not participate in any deliberations relating to his own compensation, our CEO reviews on at least an annual basis the performance of each of the other NEOs and other executive officers. Based on these performance reviews and the Company’s overall absolute and relative performance, our CEO makes recommendations to the Compensation Committee on any changes to base salaries, annual bonuses and equity awards. The Compensation Committee considers the recommendations submitted by our CEO, as well as data and analysis provided by management and F.W. Cook, but retains full discretion to approve and/or recommend for the Board of Directors approval all executive and director compensation. |
Competitive Benchmarking Against Peers
To determine the competitiveness of executive compensation levels, the Compensation Committee analyzes a group of internally managed business development companies, financial services companies and real estate investment trusts (“REITs”) as set forth below (the “Peer Group”). The Peer Group is viewed as reflecting the labor market for our officer and employee talent, has a similar investor base, and, like the Company, the business development companies and REITs in the Peer Group are pass-through entities with the majority of earnings required to be distributed to shareholders as a dividend. The Compensation Committee does not specifically benchmark the compensation of our NEOs against that paid by other companies. During 2016, the Compensation Committee, based on the advice of F.W. Cook, reviewed the peer group used in connection with prior compensation decisions. Based on this review, and the advice of F.W. Cook, the Compensation Committee updated our Peer Group to better align it to our business. Our Peer Group was used as a factor in determining the annual cash bonus awards made with respect to 2016 (but paid in 2017), along with the various performance metrics outlined below under “Performance Highlights and Assessment of Company Performance,” as well as the further considerations further described below under “Annual Cash Bonus Awards.”
Our current Peer Group includes:
Internally Managed Business Development Companies: American Capital, KCAP Financial, Main Street Capital and Triangle Capital
Financial Services: Alliance Bernstein, BGC Partners, Cowen Group, Evercore Partners, Fortress Investment Group, Greenhill & Co., Houlihan Lokey, LPL Financial Holdings, On Deck Capital and WisdomTree Investments
Real Estate Investment Trusts: Capstead Mortgage, CYS Investments, Hannon Armstrong, iStar Inc., Ladder Capital, MFA Financial, Redwood Trust, Sabra Health Care and Seritage Growth.
As of October 31 2016, which is the period the Compensation Committee reviewed our Peer Group, the Company outperformed most of its Peer Group over the one-, three- and five-years as follows*:
| Return on Average Assets (excl. cash) |
Return on Equity | Return on Invested Capital |
Total Shareholder Returns |
|||||||||||||||||||||||||||||
| Performance |
HTGC | % Rank of Peer Group |
HTGC | % Rank of Peer Group |
HTGC | % Rank of Peer Group |
HTGC | % Rank of Peer Group |
||||||||||||||||||||||||
| 1-year |
6.1 | % | 100 | % | 10.5 | % | 93 | % | 6.2 | % | 93 | % | 36.2 | % | 100 | % | ||||||||||||||||
| 3-year |
6.2 | % | 99 | % | 10.2 | % | 89 | % | 6.3 | % | 89 | % | 5.3 | % | 64 | % | ||||||||||||||||
| 5-year |
6.3 | % | 96 | % | 10.3 | % | 86 | % | 6.4 | % | 87 | % | 17.2 | % | 88 | % | ||||||||||||||||
| * | The data are from S&P Capital IQ. Data reflects most recent four quarters and TSR available as of 10/31/16. |
The Company believes that compensation paid to our NEOs for 2016 was commensurate with the Company’s overall absolute performance as well as our performance relative to the Peer Group during the
184
relevant Performance Periods. The 2016 compensation decisions made by the Compensation Committee considered the fact that our performance relative to the Peer Group was above the median, and in most cases above the 75th percentile, measured using ROAA, ROE, ROIC and TSR during the trailing one-, three-, and five-years as indicated in the chart above.
Limitations Imposed by the 1940 Act Relating to Implementation of Non-Equity Incentive Plans
We are an internally-managed, non-diversified, closed-end investment company that has elected to be regulated as a business development company under the 1940 Act. As a business development company, we are required to comply with certain regulatory requirements, including the 1940 Act, rules promulgated under the 1940 Act, and exemptive orders issued to us by the SEC. We refer to these requirements, rules, and exemptive orders as the “1940 Act Requirements.” The 1940 Act Requirements provide that the Company may maintain either an equity incentive plan or a “profit sharing plan.” A “profit sharing plan” as defined under the 1940 Act is any written or oral plan, contract, authorization or arrangement, or any practice, understanding or undertaking whereby amounts payable under the compensation plan are dependent upon or related to the profits of the company. The SEC has stated that compensation plans possess profit-sharing characteristics if an investment company is obligated to make payments under such a plan based on the level of income, realized gains or loss on investments or unrealized appreciation or depreciation of assets of such investment company.
The Company believes that equity incentives strongly align the interests of our stockholders with our NEOs, and, accordingly, an equity incentive plan was adopted in 2004. Since the Company has adopted the 2004 Equity Incentive Plan (the “Equity Plan”), the 1940 Act Requirements prohibit us from also implementing a “profit sharing plan.”
Why is this important to the Company’s executive compensation? The 1940 Act Requirements that restrict the Company to sponsoring either an equity incentive plan or a “profit sharing plan” limit the Company’s use of formulas or non-discretionary objective performance goals or criteria in its incentive plans. This means that the Compensation Committee is not permitted to use a nondiscretionary formulaic application of any performance criteria for corporate and individual goals to determine compensation. Rather, the Compensation Committee must take into consideration all factors and use its discretion to determine the appropriate amount of compensation for our NEOs. The Compensation Committee’s objective is to work within this regulatory framework to maintain and motivate pay-for-performance alignment, to establish appropriate compensation levels relative to our Peer Group and to implement compensation best practices.
2016 Advisory Vote on Executive Compensation
At our 2016 annual meeting of stockholders, our advisory vote on say-on-pay received support from our stockholders (89.4% of votes cast). The Company believes that the continuing dialogue with our stockholders on company performance, compensation and other governance matters is important. In advance of our 2016 annual meeting of stockholders, management engaged in numerous direct dialogues with our largest institutional shareholders, as well as a number of other institutional shareholders, to gain broad-based and/or specific insights into the Company’s overall performance, operating expenses, including executive compensation and corporate governance practices. In addition, we invited each of our institutional stockholders holding more than 1% of the Company’s stock to speak directly with management specifically on executive compensation and corporate governance practices.
The Company anticipates continuing our stockholder engagement efforts following the 2017 annual meeting and in advance of our future annual meetings.
Performance Highlights and Assessment of Company Performance
In determining the compensation for our NEOs, the Compensation Committee evaluates our performance relative to our Peer Group (See “Competitive Benchmarking Against Peers” above), as well as Company-specific
185
absolute performance factors over the relevant Performance Periods. In 2016, relative and company-specific factors included:
Key Performance Indicators
| Performance Period Outcomes | ||||||||||||||||||||
| Metric |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
| Total of New Fundings (in $ millions) |
680.7 | 712.3 | 621.3 | 500.7 | 554.9 | |||||||||||||||
| Total Investments at Cost (in $ millions) |
1,511.5 | 1,252.3 | 1,035.3 | 906.3 | 914.3 | |||||||||||||||
| Net Interest Margin (in $ million) |
138.0 | 120.2 | 108.1 | 104.6 | 73.8 | |||||||||||||||
| • | Total New Fundings: Debt and equity fundings grew from $554.9 million in 2012 to $680.7 million in 2016 or a CAGR of 5.2%, as we continue to expand our origination team, increase our market share and organically grow our business via a record funding year for Hercules. |
| • | Total Investments: Total investments at cost increased to $1,511.5 billion in 2016 from $914.3 million in 2012, a CAGR of 13.4% due to record new fundings, combined with the monetization of our warrants and equity positions. |
| • | Net Interest Margin: We continue to grow our net interest margin due to strong portfolio growth and effectively managing our weighted average cost of debt. |
Execution Across Performance Metrics
| Performance Period Outcomes | ||||||||||||||||||||
| Metric |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
| Liquidity Levels (in $ millions) |
203.0 | 195.2 | 377.1 | 373.4 | 288.0 | |||||||||||||||
| Available Unfunded Commitments (in $ millions) |
59.7 | 75.4 | 147.7 | 69.1 | 19.3 | |||||||||||||||
| Cumulative Net Realized Losses (in $ millions) |
2.3 | 6.9 | 12.0 | 32.1 | 47.0 | |||||||||||||||
| Distribution Yield (%)* |
8.8 | 10.2 | 8.3 | 6.8 | 8.5 | |||||||||||||||
| * | Distribution Yield: Distribution Yield is a financial ratio that indicates the amount of distributions paid by the Company relative to its share price and is calculated as annual distributions per share divided by price per share as of measurement date. Distribution yield does not reflect a return of capital to the Company’s stockholders nor does it reflect the total return on a stockholder’s investment in the Company. |
| • | Liquidity Levels: The use of our credit facilities has been an integral component of our treasury management as we minimize our cash drag on our assets via the use of our warehouse facilities. These facilities have a low interest cost and allow us to build up our asset base for future offerings at competitive rates. |
| • | Available Unfunded Commitments: We have done an outstanding job on managing our Available Unfunded Commitments. Our Available Unfunded Commitments was 4.5% of our loan portfolio at the end of 2016, where as in 2015, it was 6.8%. |
| • | Cumulative Net Realized Losses: We continue to demonstrate strong credit management and nothing shows this more than our cumulative net losses, where we finished in 2016 at $2.3 million on commitments of $6.5 billion. In 2012, our cumulative net realized losses were $47 million since inception, demonstrating our ability to manage our portfolio effectively over the last 5 years. |
| • | Distribution Yield: We saw our Distribution Yield decline to 8.8% at the end of 2016. We believe that our continued strong performance will be recognized and our Distribution Yields will reduce further to the range we believe is representative of our stock price. |
Assessment of Company Performance
In determining annual compensation for our NEOs, the Compensation Committee analyzes and evaluates the individual achievements and performance of our NEOs as well as the overall relative and absolute operating
186
performance and achievements of the Company. We believe that the alignment of (i) our business plan, (ii) stockholder expectations and (iii) our employee compensation is essential to long-term business success and the interests of our stockholders and employees and to our ability to attract and retain executive talent, especially in a competitive environment for top-quality executive talent in the venture debt industry.
Our business plan involves taking on credit risk over an extended period of time, and a premium is placed on our ability to maintain stability and growth of net asset values as well as continuity of earnings growth to pass through to stockholders in the form of recurring dividends over the long term. Our strategy is to generate income and capital gains from our investments in the debt with warrant securities, and to a lesser extent direct equity, of our portfolio companies. This income supports the anticipated payment of dividends to our stockholders. Therefore, a key element of our return to stockholders is current income through the payment of dividends. This recurring payout requires a methodical asset acquisition analyses as well as highly active monitoring and management of our investment portfolio over time. To accomplish these functions, our business requires implementation and oversight by management and key employees with highly specialized skills and experience in the venture debt industry. A substantial part of our employee base is dedicated to the generation of new investment opportunities to allow us to sustain dividends and to the maintenance of asset values in our portfolio. In addition to the performance factors above, the Company considered the following Company-specific performance factors over the relevant Performance Periods: overall credit performance, performance against annual gross funding goals, overall yields, efficiency ratios, total and net investment income and realized and unrealized gains and losses.
Elements of Executive Compensation and 2016 Compensation Determinations
Base Salary
We believe that base salaries are a fundamental element of our compensation program. The Compensation Committee establishes base salaries for each NEO to reflect (i) the scope of the NEO’s industry experience, knowledge and qualifications, (ii) the NEO’s position and responsibilities and contributions to our business growth and (iii) salary levels and pay practices of those companies with whom we compete for executive talent.
The Compensation Committee considers base salary levels at least annually as part of its review of the performance of NEOs and from time to time upon a promotion or other change in job responsibilities. During its review of base salaries for our executives, the Compensation Committee primarily considers: individual performance of the executive, including leadership and execution of strategic initiatives and the accomplishment of business results for our company; market data provided by our compensation consultant; our NEOs total compensation, both individually and relative to our other NEOs; and for NEOs other than the CEO, the base salary recommendations of our CEO.
| NEO |
2016 Base Salary |
|||
| Manuel Henriquez |
$ | 803,154 | ||
| Mark Harris |
$ | 412,000 | ||
| Scott Bluestein |
$ | 432,600 | ||
| Melanie Grace |
$ | 283,250 | ||
| Andrew Olson |
$ | 211,150 | ||
Annual Cash Bonus Awards
The Compensation Committee, together with input from our CEO, developed a specific bonus pool for the 2016 operating year to be available for our annual cash bonus program. The amount determined to be available for our annual cash program was dependent upon many factors, including those outlined previously under “Performance Highlights and Assessment of Company Performance.”
187
The Compensation Committee designs our annual cash bonuses to motivate our NEOs to achieve financial and non-financial objectives consistent with our operating plan. The Compensation Committee generally targets cash bonuses to 50% to 100% of an NEO’s base salary; however, such bonus amounts may exceed these targets in the event of exceptional company and individual performance.
Bonuses are not formulaic to comply with the 1940 Act regulations that govern our business. As a result, the Compensation Committee considers overall business performance factors and individual factors, including CEO feedback, when determining the size of individual NEO bonuses. Accordingly, should actual company and NEO performance exceed expectations, the Compensation Committee may adjust individual cash bonuses to take such superior performance into account. Conversely, if company and NEO performance is below expectations, the Compensation Committee will consider such performance in determining the NEO’s actual cash bonus.
In evaluating the performance of our NEOs to arrive at their 2016 cash bonus awards, the Compensation Committee considered the performance factor achievements discussed above under “Performance Highlights and Assessment of Company Performance,” and the Compensation Committee specifically compared our performance and the returns of our stockholders against the performance and shareholder returns of other business development companies. In particular, the Committee considered our high relative total shareholder return and return on invested capital relative to peer group benchmarks, which was above the 75th percentile over the last year, as this shows the success for shareholders and of the core business mission of allocating equity and debt capital efficiently for a high risk-adjusted return.
When sizing our cash bonus pool and allocating bonus awards, the total compensation paid to our NEOs and other employees is evaluated against the expense ratios of other business development companies. With respect to 2016, company-wide compensation expense as a percentage of average assets among the peers in the Peer Group was considered. For the fiscal year ended December 31, 2016, the ratio of our compensation expense divided by total revenue was below the median of the Peer Group.
Based on the foregoing considerations and analysis, and after due deliberation, the Compensation Committee awarded our current NEOs the following annual cash bonuses with respect to 2016.
| NEO |
2016 Cash Bonus Award |
|||
| Manuel Henriquez |
$ | 1,200,000 | ||
| Mark Harris |
$ | 400,000 | ||
| Scott Bluestein |
$ | 650,000 | ||
| Melanie Grace |
$ | 145,000 | ||
| Andrew Olson |
$ | 150,000 | ||
Long-Term Equity Incentive Compensation
2004 Equity Incentive Plan
Our long-term equity incentive compensation is designed to develop a strong linkage between pay and our strategic goals and performance, as well as to align the interests of our NEOs, and other executives and key employees, with those of our stockholders by awarding long-term equity incentives in the form of stock options, restricted stock and/or restricted stock units. These awards are made pursuant to our Equity Plan, which permits options, restricted stock and restricted stock unit awards.
We believe that annual equity grants, in the form of restricted stock awards or restricted stock units, to our NEOs are a critical part of our compensation program as they allow us to:
| • | align our business plan, stockholder interests and employee concerns, |
| • | manage dilution associated with equity-based compensation, |
188
| • | match the return expectations of the business more closely with our equity-based compensation plan, and |
| • | retain key management talent. |
We believe that these annual equity grants motivate performance that is more consistent with the type of return expectations that we have established for our stockholders. Accordingly, the Company awards restricted stock award grants to our NEOs. These grants typically vest over three years.
Grant Practices for Executive Officers
Annual equity compensation grants to executive officers have typically been granted in the first quarter of the year. The Company does not grant stock options to executive officers. As a result, there were no option grants to our NEOs in 2016.
Restricted Stock Units
In 2017, the Compensation Committee did not grant restricted stock awards to NEOs. Rather, in January 2017, the Compensation Committee granted restricted stock units to the NEOs. With respect to the restricted stock units, the Compensation Committee assessed each current NEO’s individual performance for 2016, our overall company performance in 2016 (including the performance factors detailed above under “Performance Highlights and Assessment of Company Performance” and “Annual Cash Bonus Awards”) and the levels of equity compensation paid by other companies with whom we compete for executive talent. Based on this assessment, the Compensation Committee determined that the following restricted stock units be granted to our current NEOs with respect to 2016, in the amounts and on the dates set forth below to reward them for services performed in 2016. These restricted stock units vest as to one-third of the shares underlying the awards on the first anniversary of the grant date, and they vest as to the remaining shares in equal quarterly installments over the next two years. Settlement of the restricted stock units is deferred following vesting and the restricted stock units will not be settled until the earliest to occur of (1) January 24, 2021, (2) the death or disability of the NEO, (3) the separation from service of the NEO, or (4) a change in control of the Company. Each restricted stock unit will entitle the holder to dividend equivalents in the form of the Company’s common stock, which dividend equivalent payments will be settled on the date the related restricted stock unit is settled. We believe these restricted stock unit awards assist the Company in retaining NEOs.
| NEO |
Grant Date |
Restricted Stock Units |
Fair Value of Restricted Stock Awards(1) |
|||||||||
| Manuel Henriquez |
1/24/2017 | 351,865 | $ | 5,000,000 | ||||||||
| Scott Bluestein |
1/24/2017 | 123,153 | $ | 1,750,000 | ||||||||
| Mark Harris |
1/24/2017 | 35,187 | $ | 500,000 | ||||||||
| Melanie Grace |
1/24/2017 | 21,112 | $ | 300,000 | ||||||||
| Andrew Olson |
1/24/2017 | 17,593 | $ | 250,000 | ||||||||
| (1) | Based on the closing price per share of our common stock of $14.21 on January 24, 2017. |
Other Elements of Compensation
| • | Severance: No NEO or employee of the Company has a written severance agreement or other arrangement providing for payments or benefits upon a termination of employment. |
| • | Benefits and Perquisites: Our NEOs receive the same benefits and perquisites as other full-time employees. Our benefits program is designed to provide competitive benefits and is not based on performance. Our NEOs and other full-time employees receive health and welfare benefits, which consist of life, long-term and short-term disability, health, dental, vision insurance benefits and the |
189
| opportunity to participate in our defined contribution 401(k) plan. During 2016, our 401(k) plan provided for a match of contributions by the company for up to $18,000 per full-time employee. Other than the benefits set forth immediately above, our NEOs are not entitled to any other benefits or perquisites. |
| • | Potential Payments Upon Termination or Change of Control: No NEO or employee of the Company has a written employment agreement, or other agreement, providing for payments or other benefits in connection with a change of control of the Company. Further, no NEO or any other employee is entitled to any tax gross-up payments. |
Corporate Goals
For 2016, the Compensation Committee developed corporate goals that were required to be achieved for executive officers to receive up to 50% of their incentive compensation. These goals included operational performance as well as performance relative to the Peer Group. While the criteria may not be weighted, the Compensation Committee took into consideration each of these factors to determine whether the executive officers are eligible for up to 50% of the proposed incentive compensation. The Compensation Committee believes that the corporate goals applicable to all executive officers create an alignment not only with shareholders but also to the Company’s business strategy and performance goals.
Defined Individual Goals
For 2016, the Compensation Committee developed individual goals for the CEO. In addition, the CEO and each NEO developed individual goals for the NEOs and such goals were approved by the Compensation Committee. Each set of individual goals are unique to the executive officer’s responsibilities and position within the Company. While each of the factors may not be weighted, the Compensation Committee took into consideration each of these factors to determine whether the executive officers are eligible for up to 50% of the executive officer’s incentive compensation.
Pay-for-Performance Alignment
The Company believes that there exists an alignment between the compensation of our NEOs and our performance over the relevant Performance Periods. As noted above, a broad range of individual performance factors and company performance factors are analyzed each year, including total shareholder return relative to our Peer Group, and, in 2016, analysis of relative ROAA, ROE, and ROIC versus the compensation peers over one-, three-, and five-years to measure short-, medium-, and long-term performance. The objective in analyzing these key performance factors is to align NEO compensation to our performance relative to our Peer Group and our absolute corporate performance.
The Company’s annual bonus and equity awards constitute an effective mix of short- and long-term compensation components and reflect key measures of our performance and the returns enjoyed by our stockholders. Consistent with our pay-for-performance philosophy, the Compensation Committee will make future compensation decisions taking into account our absolute and relative performance, and, if our future performance were to fall significantly below our peers, the Compensation Committee would consider adjusting NEO compensation prospectively.
Total Compensation Expense Relative to other Internally Managed Business Development Companies
In determining annual bonus awards, the total compensation paid to our NEOs and other employees against the expense ratios of other internally managed business development companies was considered.
190
Internal Pay Equity Analysis
Our compensation program is designed with the goal of providing compensation to our NEOs that is fair, reasonable, and competitive. To achieve this goal, the Company believes it is important to compare compensation paid to each NEO not only with compensation in our comparative group companies, as discussed above, but also with compensation paid to each of our other NEOs. Such an internal comparison is important to ensure that compensation is equitable among our NEOs.
As part of the Compensation Committee’s review, we made a comparison of our CEO’s total compensation paid for the period ending October 31, 2016 against that paid to our other NEOs during the same year. Upon review, the Compensation Committee determined that our CEO’s compensation relative to that of our other NEOs was appropriate because of his level and scope of responsibilities, expertise and performance history, and other factors deemed relevant by the Compensation Committee. The Compensation Committee also reviewed the mix of the individual elements of compensation paid to our NEOs for this period, the individual performance of each NEO and any changes in responsibilities of the NEO.
Stock Ownership Guidelines
The Company maintains stock ownership guidelines, which are outlined in our corporate governance guidelines, because we believe that material stock ownership by our executives plays a role in effectively aligning the interests of these employees with those of our stockholders and strongly motivates our executives to build long-term shareholder value. Pursuant to our stock ownership guidelines, each member of senior management is required to beneficially own at least two times the individual’s annual salary in Company common stock, based on market value, within three years of joining the Company. Our Board of Directors may make exceptions to this requirement based on particular circumstances; however, no exceptions have been made for our current NEOs. Messrs. Henriquez, Bluestein and Harris have met their minimum guidelines.
The Compensation Committee’s review of the CEO’s stock ownership in the fourth quarter of 2016 showed that he owns shares worth more than 20x his annual base salary.
Tax and Accounting Matters
Stock-Based Compensation. We account for stock-based compensation, including options and shares of restricted stock granted pursuant to our Equity Plan and 2006 Non-Employee Director Plan in accordance with the requirements of (“ASC”) Topic 718, “Compensation—Stock Compensation”. Under the ASC Topic 718, we estimate the fair value of our option awards at the date of grant using the Black-Scholes-Merton option-pricing model, which requires the use of certain subjective assumptions. The most significant of these assumptions are our estimates on the expected term, volatility and forfeiture rates of the awards. Forfeitures are not estimated due to our limited history but are reversed in the period in which forfeiture occurs. As required under the accounting rules, we review our valuation assumptions at each grant date and, as a result, are likely to change our valuation assumptions used to value stock-based awards granted in future periods. We estimate the fair value of our restricted stock awards based on the grant date market closing price.
Deductibility of Executive Compensation. When analyzing both total compensation and individual elements of compensation paid to our NEOs, the Company considers the income tax consequences to the Company of its compensation policies and procedures. In particular, the Company considers Section 162(m) of the Code, which limits the deductibility of non-performance-based compensation paid to certain of the NEOs to $1,000,000 per affected NEO. The Compensation Committee intends to balance its objective of providing compensation to our NEOs that is fair, reasonable, and competitive with the Company’s ability to claim compensation expense deductions. Our Board of Directors believes that the best interests of the Company and our stockholders are served by executive compensation programs that encourage and promote our principal compensation philosophy,
191
enhancement of shareholder value, and permit the Compensation Committee to exercise discretion in the design and implementation of compensation packages. Accordingly, we may from time to time pay compensation to our NEOs that may not be fully tax deductible, including certain bonuses and restricted stock. Stock options granted under our stock plan are intended to qualify as performance-based compensation under Section 162(m) of the Code. The Company will continue to review its executive compensation plans periodically to determine what changes, if any, should be made as a result of any deduction limitations.
Clawback Policy for Section 16 Officers
In 2016, the Board of Directors adopted a clawback policy for all Section 16 officers. This was an enhancement to the Company’s then-existing clawback policy for the CEO and CFO pursuant to Section 304 of the Sarbanes-Oxley Act. With respect to the Company’s clawback policy, the Company has
| • | broadened its clawback policy to apply to all Section 16 officers; and |
| • | broadened the scope of its clawback policy beyond financial restatements. |
Pursuant to our clawback policy, for payments that are predicated on financial results augmented by fraud, embezzlement, gross negligence or deliberate disregard of applicable rules resulting in significant monetary loss, damage or injury to the Company (“Excess Compensation”), the Compensation Committee has the authority to seek repayment of any Excess Compensation, including (1) cancellation of unvested, unexercised or unreleased equity incentive awards; and (2) repayment of any compensation earned on previously exercised or released equity incentive awards whether or not such activity resulted in a financial restatement.
The Compensation Committee will have sole discretion under this policy, consistent with any applicable statutory requirements, to seek reimbursement of any Excess Compensation paid or received by the Section 16 officer for up to a 12-month period prior to the date of the Compensation Committee action to require reimbursement of the Excess Compensation. Any clawback of Excess Compensation must be based upon fraud adjudicated by a court of competent jurisdiction or a financial restatement. Further, following a restatement of our financial statements, we will recover any compensation received by the CEO and CFO that is required to be recovered by Section 304 of the Sarbanes-Oxley Act.
For purposes of this policy, Excess Compensation will be measured as the positive difference, if any, between the compensation earned by a Section 16 officer and the compensation that would have been earned by the Section 16 officer had the fraud, embezzlement, gross negligence or deliberate disregard of applicable rules resulting from significant monetary loss, damage or injury to the Company not occurred.
Risk Assessment of the Compensation Programs
Our Board of Directors believes that risks arising from our compensation policies and practices for our employees are not reasonably likely to have a material adverse effect on the Company. The Company has designed our compensation programs, including our incentive compensation plans, with specific features to address potential risks while rewarding employees for achieving long-term financial and strategic objectives through prudent business judgment and appropriate risk taking. We use common variable compensation designs, with a significant focus on individual contributions to our performance and the achievement of absolute and relative corporate objectives, as generally described in this Compensation Discussion and Analysis.
The Compensation Committee and the Board of Directors reviewed our compensation programs to assess whether any aspect of the programs would encourage any of our employees to take any unnecessary or inappropriate risks that could threaten the value of the Company. The Company has designed our compensation programs to reward our employees for achieving annual profitability and long-terms increase shareholder value.
192
Our Board of Directors recognizes that the pursuit of corporate objectives possibly leads to behaviors that could weaken the link between pay and performance, and, therefore, the correlation between the compensation delivered to employees and the long-term return realized by stockholders. Accordingly, our executive compensation program is designed to mitigate these possibilities and to ensure that our compensation practices are consistent with our risk profile. These features include the following:
| • | bonus payouts and equity incentive awards that are not based solely on corporate performance objectives, but are also based on individual performance levels, |
| • | the financial opportunity in our long-term equity incentive program that is best realized through long-term appreciation of our stock price, which mitigates excessive short-term risk-taking, |
| • | annual cash bonuses that are paid after the end of the fiscal year to which the bonus payout relates, |
| • | the engagement and use of a compensation consultant, |
| • | the institution of stock ownership guidelines applicable to our executive officers, and |
| • | final decision making by our Compensation Committee and our Board of Directors on all awards. |
Additionally, the Company performed an assessment of compensation-related risks for all of our employees. Based on this assessment, we concluded that our compensation programs do not create risks that are reasonably likely to have a material adverse effect on the Company. In making this evaluation, the Company reviewed the key design elements of our compensation programs in relation to industry “best practices,” as well as the means by which any potential risks may be mitigated. In addition, management completed an inventory of incentive programs below the executive level and reviewed the design of these incentives and concluded that such incentive programs do not encourage excessive risk-taking.
Compensation Committee Report
We have reviewed and discussed the foregoing Compensation Discussion and Analysis with management. Based on our review and discussions with management, we recommend to the Board of Directors that the Compensation Discussion and Analysis be included in the Annual Report on Form 10-K/A for the year ended December 31, 2016.
COMPENSATION COMMITTEE MEMBERS
Susanne D. Lyons, Chair
Allyn C. Woodward, Jr.
Doreen Woo Ho
193
Summary Compensation Table
| Name and Principal Position |
Year | Salary ($)(1) | Bonus ($)(2) | Stock Awards ($)(3) |
Option Awards ($)(3) |
All Other Compensation ($)(4) |
Total ($) | |||||||||||||||||||||
| Manuel Henriquez |
2016 | $ | 803,154 | $ | 1,200,000 | $ | 4,005,335 | — | $ | 771,425 | $ | 6,779,914 | ||||||||||||||||
| Chairman & Chief Executive Officer |
2015 | $ | 779,762 | $ | 1,000,000 | $ | 4,472,142 | $ | 1,635,353 | $ | 7,887,257 | |||||||||||||||||
| 2014 | $ | 779,762 | $ | 692,500 | $ | 5,992,250 | — | $ | 804,675 | $ | 8,269,187 | |||||||||||||||||
| Mark R. Harris |
2016 | $ | 412,000 | $ | 400,000 | $ | 396,330 | — | $ | 95,624 | $ | 1,303,954 | ||||||||||||||||
| Chief Financial Officer |
2015 | $ | 166,667 | $ | 200,000 | $ | 400,001 | — | $ | 26,404 | $ | 793,072 | ||||||||||||||||
| Scott Bluestein |
2016 | $ | 432,600 | $ | 650,000 | $ | 1,249,040 | $ | 200,555 | $ | 2,532,195 | |||||||||||||||||
| Chief Investment Officer |
2015 | $ | 420,000 | $ | 525,000 | $ | 670,212 | $ | 193,370 | $ | 1,808,582 | |||||||||||||||||
| 2014 | $ | 420,000 | $ | 233,750 | $ | 967,100 | — | $ | 144,396 | $ | 1,765,246 | |||||||||||||||||
| Melanie Grace |
2016 | $ | 283,250 | $ | 145,000 | $ | 112,894 | $ | 40,726 | $ | 581,870 | |||||||||||||||||
| General Counsel, Chief Compliance Officer and Secretary |
2015 | $ | 79,167 | $ | 50,000 | $ | 112,500 | — | $ | 36,466 | $ | 278,133 | ||||||||||||||||
| Andrew Olson |
2016 | $ | 211,150 | $ | 150,000 | $ | 72,060 | $ | 28,684 | $ | 461,894 | |||||||||||||||||
| Vice President of Finance and Senior Controller |
2015 | $ | 186,250 | $ | 195,000 | $ | 53,332 | — | $ | 22,717 | $ | 457,299 | ||||||||||||||||
| Jessica Baron. |
2016 | — | — | — | — | — | — | |||||||||||||||||||||
| Former Chief Financial Officer |
2015 | $ | 130,096 | — | $ | 267,838 | — | $ | 63,168 | $ | 461,102 | |||||||||||||||||
| 2014 | $ | 293,550 | $ | 123,750 | $ | 517,825 | — | $ | 109,841 | $ | 1,044,966 | |||||||||||||||||
| (1) | Salary column amounts represent base salary compensation received by each named executive officer (“NEO”) for the listed fiscal year. |
| (2) | Bonus column amounts represent the annual cash bonus earned during the fiscal year and awarded and paid out during the first quarter of the following fiscal year. |
| (3) | The amounts reflect the aggregate grant date fair value of restricted stock and stock option awards made to our NEOs and former NEOs during the applicable year computed in accordance with ASC Topic 718. The grant date fair value of each restricted stock award is measured based on the closing price of our common stock on the date of grant. |
| (4) | All Other Compensation column includes the following: |
| • | We made matching contributions under our 401(k) plan of (a) $18,000 in 2016 to Messrs. Henriquez, Bluestein, Harris and Olson and $17,703 to Ms. Grace (b) $18,000 in 2015 to Messrs. Henriquez, Bluestein and Olson and Ms. Baron; and (c) $17,000 in 2014 to Messrs. Henriquez and Bluestein and Ms. Baron. |
| • | Distributions to Messrs. Henriquez, Harris, Bluestein and Olson and Ms. Grace in the amount of $753,425, $77,624, $182,555, $10,684 and $23,023, respectively, were paid on unvested restricted stock awards during 2016. |
| • | Distributions to Messrs. Henriquez, Harris, Bluestein and Olson and Ms. Grace in the amount of $845,550, $22,587, $134,985, $4,717 and $3,100, respectively, were paid on unvested restricted stock awards during 2015. |
| • | Distributions to Messrs. Henriquez and Bluestein and Ms. Baron in the amount of $787,675, $127,396, and $92,841, respectively, were paid on unvested restricted stock awards during 2014. |
| • | Due to a change in the vacation policy of NEOs, Messrs. Henriquez, Harris, Bluestein and Ms. Grace were each paid out of all of their accrued vacation through August 30, 2015 in the amount of $771,803, $3,817, $40,385 and $1,007, respectively. NEOs no longer accrue vacation effective September 1, 2015. |
| • | Ms. Grace began as a contractor on August 3, 2015 until she was approved by the Board of Directors as an executive officer on September 17, 2015. During this period, Ms. Grace earned $32,359 in compensation. |
194
Grants of Plan Based Awards in 2016
| NEO |
Grant Date | All
Other Stock Awards: Number of Shares of Stock or Units(1) |
All
Other Option Awards: Number of Securities Underlying Options(1) |
Grant Date Fair Value of Stock and Option Awards(2) |
||||||||||||
| Manuel Henriquez |
01/10/2016 | 333,500 | — | $ | 4,005,335 | |||||||||||
| Mark Harris |
01/10/2016 | 33,000 | — | $ | 396,330 | |||||||||||
| Scott Bluestein |
01/10/2016 | 104,000 | — | $ | 1,249,040 | |||||||||||
| Andrew Olson |
01/10/2016 | 6,000 | — | $ | 72,060 | |||||||||||
| Melanie Grace |
01/10/2016 | 9,400 | — | $ | 112,894 | |||||||||||
| (1) | Restricted stock awards vest as to one-third of the award on the one year anniversary of the date of the grant and quarterly over the succeeding 24 months. When payable, distributions are paid on a current basis on the unvested shares. |
| (2) | The amounts reflect the aggregate grant date fair value of computed in accordance with ASC Topic 718. |
Outstanding Equity Awards at Fiscal Year End, December 31, 2016
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Name and Principal Position |
Number of Securities Underlying Unexercised Options Exercisable |
Number of Securities Underlying Unexercised Options Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested |
Market Value of Shares or Units of Stock That Have Not Vested(1) |
||||||||||||||||||
| Manuel Henriquez |
— | — | — | — | 12,284 | (2) | $ | 173,327 | ||||||||||||||||
| — | — | — | — | 132,917 | (3) | $ | 1,875,459 | |||||||||||||||||
| — | — | — | — | 333,500 | (6) | $ | 4,705,685 | |||||||||||||||||
| Mark Harris |
— | — | — | — | 21,252 | (4) | $ | 299,866 | ||||||||||||||||
| — | — | — | — | 33,000 | (6) | $ | 465,630 | |||||||||||||||||
| Scott Bluestein |
— | — | — | — | 2,457 | (2) | $ | 34,668 | ||||||||||||||||
| — | — | — | — | 19,920 | (3) | $ | 281,071 | |||||||||||||||||
| — | — | — | — | 104,000 | (6) | $ | 1,467,440 | |||||||||||||||||
| Melanie Grace |
— | — | — | — | 5,834 | (5) | $ | 82,318 | ||||||||||||||||
| — | — | — | — | 9,400 | (6) | $ | 132,634 | |||||||||||||||||
| Andrew Olson |
13,332 | (7) | 6,668 | $ | 15.12 | 12/03/2021 | 1,586 | (3) | $ | 22,378 | ||||||||||||||
| — | — | — | — | 6,000 | (6) | $ | 84,660 | |||||||||||||||||
| (1) | Market value is computed by multiplying the closing market price of the Company’s stock at December 31, 2016 by the number of shares. |
| (2) | Restricted stock granted on 3/4/13 that vests as to one-fourth of the total award on the one-year anniversary of the date of the grant and quarterly over the succeeding 36 months |
| (3) | Restricted stock granted on 3/10/15 that vests as to one-third of the total award on the one-year anniversary of the date of the grant and quarterly over the succeeding 24 months. |
| (4) | Restricted stock granted on 8/6/15 that vests as to one-third of the total award on the one-year anniversary of the date of the grant and quarterly over the succeeding 24 months |
| (5) | Restricted stock granted on 9/17/15 that vests as to one-third of the total award on the one-year anniversary of the date of the grant and quarterly over the succeeding 24 months |
| (6) | Restricted stock granted on 1/10/2016 that vests as to one-third of the total award on the one-year anniversary of the date of the grant and quarterly over the succeeding 24 months |
| (7) | Options granted on 12/03/2014 that vest as to one-third of the total underlying shares on the one-year anniversary of the date of the grant and on a monthly basis over the succeeding 24 months |
195
Options Exercised and Stock Vested in 2016
| Option Awards | Stock Awards | |||||||||||||||
| Name and Principal Position |
Number of Shares Acquired on Exercise |
Value Realized on Exercise |
Number of Shares Acquired on Vesting |
Value Realized on Vesting |
||||||||||||
| Manuel Henriquez |
— | — | 359,264 | $ | 4,347,348 | |||||||||||
| Mark Harris |
— | — | 15,178 | $ | 205,146 | |||||||||||
| Scott Bluestein |
— | — | 57,399 | $ | 692,290 | |||||||||||
| Melanie Grace |
— | — | 4,166 | $ | 55,499 | |||||||||||
| Andrew Olson |
— | — | 2,218 | $ | 26,908 | |||||||||||
COMPENSATION OF DIRECTORS
Our Compensation Committee has the authority from our Board of Directors for the appointment, compensation and oversight of our outside compensation consultant. Our Compensation Committee generally engages a compensation consultant every other year to assist it with its responsibilities related to our director compensation program.
The following table discloses the cash, equity awards and other compensation earned, paid or awarded, as the case may be, to each of our current directors during the fiscal year ended December 31, 2016. We provide further information relating to equity awards made to our non-employee directors below under “—2006 Non-Employee Director Plan.”
| Name |
Fees Earned or Paid in Cash ($)(1) |
Stock Awards ($)(2) |
Option Awards ($)(3) |
All
Other Compensation ($)(4) |
Total ($) | |||||||||||||||
| Robert P. Badavas |
$ | 175,000 | — | — | $ | 3,099 | $ | 178,099 | ||||||||||||
| Thomas J. Fallon |
$ | 150,000 | $ | — | $ | — | $ | 6,199 | $ | 156,199 | ||||||||||
| Joseph F. Hoffman |
$ | 165,000 | $ | 62,350 | $ | 8,499 | $ | 5,683 | $ | 241,532 | ||||||||||
| Susanne D. Lyons |
$ | 175,000 | $ | — | $ | — | $ | 2,066 | $ | 177,066 | ||||||||||
| Allyn C. Woodward, Jr. |
$ | 175,000 | $ | — | $ | — | $ | 5,166 | $ | 180,166 | ||||||||||
| Doreen Woo Ho |
$ | — | $ | 45,362 | $ | 6,415 | $ | 1,033 | $ | 52,810 | ||||||||||
| Manuel A. Henriquez(5) |
— | — | — | — | — | |||||||||||||||
| (1) | Messrs. Badavas, Fallon, Hoffman, Woodward and Ms. Lyons earned $125,000, $100,000, $115,000, $125,000 and $125,000, respectively, and each elected to receive an additional retainer fee of 3,720 shares of our common stock in lieu of cash. The total value of the shares issued to each of Messrs. Badavas, Fallon, Hoffman and Woodward and Ms. Lyons services in fiscal 2016 was $50,000. Ms. Woo Ho did not receive any cash compensation during 2016. |
| (2) | During 2016, in connection his re-election to our Board of Directors, we granted Mr. Hoffman a restricted stock award for 5,000 shares of common stock, and we granted Ms. Woo Ho a restricted stock award for 3,333 shares of common stock upon her appointment to our Board of Directors. The amounts presented reflect the aggregate grant date fair value of the stock awards, as computed in accordance with ASC Topic 718. The grant date fair value of each restricted stock award is measured based on the closing price of our common stock on the date of grant. |
| (3) | During 2016, in connection with his re-election to our Board of Directors, we granted Mr. Hoffman a stock option award with respect to 15,000 shares of our common stock, and, in connection with her appointment to our Board of Directors, we granted Ms. Woo Ho a stock option award with respect to 10,000 shares of our common stock. The amounts presented reflect the aggregate grant date fair value of option awards computed in accordance with ASC Topic 718. The fair value of each stock option grant is estimated based on the fair market value of the option on the date of grant using the Black-Scholes-Merton option pricing model. For a further discussion on the valuation model and the assumptions used to calculate the fair value of our stock options, please see Note 7 to the consolidated financial statements included in our annual report on Form 10-K for the 2016 fiscal year. |
| (4) | Represents distributions paid during 2016 on unvested common stock under restricted stock awards. |
| (5) | As an employee director, Mr. Henriquez does not receive any compensation for his service as a director. The compensation Mr. Henriquez receives as our chief executive officer is disclosed in the Summary Compensation Table and elsewhere under “EXECUTIVE COMPENSATION.” |
196
As of December 31, 2016, Messrs. Badavas, Fallon, Hoffman and Woodward and Ms. Lyons and Ms. Woo Ho had outstanding options in the amount of 20,000, 25,000, 25,000, 25,000, 10,000 and 10,000, respectively. As of December 31, 2016, Messrs. Badavas, Fallon, Hoffman and Woodward and Ms. Lyons and Ms. Woo Ho held unvested shares of restricted stock in the amount of 1,666, 3,333, 6,666, 3,333, 1,666 and 3,333, respectively.
Upon her appointment to our Board of Directors in October 2016, Ms. Woo Ho received a restricted stock award with respect to 3,333 shares of our common stock and a stock option to purchase 10,000 shares of our common stock.
During 2016, the compensation for serving on our Board of Directors as an independent director included the following:
| Annual Director Retainer Fee |
$100,000 | |
| Annual Chairperson Fee |
$25,000, Audit Committee | |
| $25,000, Compensation Committee | ||
| $15,000, NCG Committee | ||
| Annual Lead Director Fee |
$25,000 |
In 2016, we granted each independent director an additional retainer of $50,000, which was distributed as shares of common stock in lieu of cash. In addition, upon re-election to the Board of Directors, each independent director is granted an option to purchase 15,000 shares and an additional award of 5,000 shares of restricted stock. Employee directors do not receive compensation for serving on our Board of Directors. In addition, we reimburse our directors for their reasonable out-of-pocket expenses incurred in attending Board of Directors meetings.
Under current SEC rules and regulations applicable to business development companies, a business development company may not grant options or restricted stock to non-employee directors unless it receives exemptive relief from the SEC. We filed an exemptive relief request with the SEC to allow options and restricted stock to be issued to our non-employee directors, which was approved on October 10, 2007. On June 22, 2010, we received approval from the SEC regarding our exemptive relief request permitting its employees to exercise their stock options and restricted stock and pay any related income taxes using a cashless exercise program.
On June 21, 2007, our stockholders approved amendments to the Equity Plan and the 2006 Non-Employee Director Plan allowing for the grant of restricted stock. The Equity Plan and 2006 Non-Employee Director Plan limit the combined maximum amount of restricted stock that may be issued under both of the Equity Plan and 2006 Non-Employee Director Plan to 10% of the outstanding shares of our common stock on the effective date of the Equity Plan and 2006 Non-Employee Director Plan plus 10% of the number of shares of common stock issued or delivered by us during the terms of the Equity Plan and 2006 Non-Employee Director Plan.
197
EQUITY COMPENSATION PLAN INFORMATION
The following table sets forth information as of December 31, 2016, with respect to compensation plans under which the Company’s equity securities are authorized for issuance:
| Plan Category |
(a) Number of Securities to be issued upon exercise of outstanding options, restricted stock and warrants |
(b) Weighted-average exercise price of outstanding options, restricted stock and warrants |
(c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| Equity compensation plans approved by stockholders: |
||||||||||||
| 2004 Equity Incentive Plan |
553,171 | $ | 13.85 | 3,772,736 | ||||||||
| 2006 Non-Employee Director Plan |
115,000 | $ | 13.18 | 713,333 | ||||||||
| Equity compensation plans not approved by stockholders: |
||||||||||||
| Total |
668,171 | $ | 13.52 | 4,486,069 | ||||||||
2004 Equity Incentive Plan
Our board and our stockholders have approved our Equity Plan to align our employees’ interest with the performance of our Company and to attract and retain the services of executive officers and other key employees. Under our Equity Plan our Compensation Committee may award incentive stock options, referred to as ISOs, within the meaning of Section 422 of the Code, and non-qualified stock options to employees and employee directors. The following is a summary of the material features of our Equity Plan.
Under our Equity Plan, we had 3,200,605 shares of common stock available for issuance as of August 30, 2017. Participants in our Equity Plan may receive awards of options to purchase our common stock and/or restricted shares, as determined by our Compensation Committee. Options granted under our Equity Plan generally may be exercised for a period of no more than ten years from the date of grant unless the option agreement provides for an earlier expiration. Unless sooner terminated by our Board of Directors, our Equity Plan will terminate on the tenth anniversary of the date it was last approved by our stockholders. Such approval was last given by our stockholders on July 7, 2015. Our Equity Plan provides that all awards granted under the plan are subject to modification as required to ensure that such awards do not conflict with the requirements of the 1940 Act applicable to us.
Options granted under our Equity Plan will entitle the optionee, upon exercise, to purchase shares of common stock from us at a specified exercise price per share. ISOs must have a per share exercise price of no less than the fair market value of a share of stock on the date of the grant or, if the optionee owns or is treated as owning (under Section 424(d) of the Code) more than 10% of the total combined voting power of all classes of our stock, 110% of the fair market value of a share of stock on the date of the grant. Nonstatutory stock options granted under our Equity Plan must have a per share exercise price of no less than the fair market value of a share of stock on the date of the grant. Options will not be transferable other than by laws of descent and distribution, or in the case of nonstatutory stock options, by gift, and will generally be exercisable during an optionee’s lifetime only by the optionee.
Under our Equity Plan, we are permitted to issue shares of restricted stock to all key employees of the Company and its affiliates consistent with such terms and conditions as the Board of Directors shall deem appropriate. Our Board of Directors determines the time or times at which such shares of restricted stock will become exercisable and the terms on which such shares will remain exercisable. Any shares of restricted stock for which forfeiture restrictions have not vested at the point at which the participant terminates his employment will terminate immediately and such shares will be returned to Hercules and will be available for future awards under this plan.
198
Our Board of Directors administers our Equity Plan and has the authority, subject to the provisions of the Equity Plan, to determine who will receive awards under the Equity Plan and the terms of such awards. Our Board of Directors has the authority to adjust the number of shares available for awards, the number of shares subject to outstanding awards and the exercise price for awards following the occurrence of events such as stock splits, dividends, distributions and recapitalizations. The exercise price of an option may be paid in the form of shares of stock that are already owned by such option holder.
Upon specified covered transactions (as defined in the Equity Plan), all outstanding awards under our Equity Plan may either be assumed or substituted for by the surviving entity. If the surviving entity does not assume or substitute similar awards, the awards held by the participants will be accelerated in full and then terminated to the extent not exercised prior to the covered transaction.
2006 Non-Employee Director Plan
Our Board of Directors and our stockholders approved our 2006 Non-Employee Director Plan. Under current SEC rules and regulations applicable to business development companies, absent exemptive relief, a business development company may not grant options or shares of restricted stock to non-employee directors. On February 15, 2007, we received exemptive relief from the SEC to permit us to grant options to non-employee directors as a portion of their compensation for service on our Board of Directors. On May 23, 2007, we received exemptive relief from the SEC to permit us to grant shares of restricted stock to non-employee directors as a portion of their compensation for service on our Board of Directors. Our 2006 Non-Employee Director Plan terminated in accordance with its terms on June 21, 2017 and no additional awards may be made under our 2006 Non-Employee Director Plan. The following is a summary of the material features of the 2006 Non-Employee Director Plan.
We instituted our 2006 Non-Employee Director Plan for the purpose of advancing our interests by providing for the grant of awards under our 2006 Non-Employee Director Plan to eligible non-employee directors. Under our 2006 Non-Employee Director Plan, we authorized for issuance up to 1,000,000 shares of common stock.
Our 2006 Non-Employee Director Plan authorized the issuance to non-employee directors of non-statutory stock options, referred to as NSOs, to purchase shares of our common stock at a specified exercise price per share and/or restricted stock. NSOs granted under our 2006 Non-Employee Director Plan had a per share exercise price of no less than the current market value of a share of stock as determined in good faith by our Board of Directors on the date of the grant. The amount of the options that may be granted were limited by the terms of our 2006 Non-Employee Director Plan, which prohibited any grant that would cause us to be in violation of Section 61(a)(3) of the 1940 Act.
Under our 2006 Non-Employee Director Plan, non-employee directors each received an initial grant of an option to purchase 10,000 shares of stock upon initial election to such position. The options granted vest over two years, in equal installments on each of the first two anniversaries of the date of grant, provided that the non-employee director remains in service on such dates. In addition, each non-employee director was automatically granted an option to purchase 15,000 shares of stock on the date of such non-employee director’s re-election to our Board of Directors and such grant vests over three years, in equal installments on each of the first three anniversaries of the date of grant, provided that the non-employee director remains in service on such dates. Our Compensation Committee had, subject to SEC approval, the authority to determine from time to time which of the persons eligible under our 2006 Non-Employee Director Plan was granted awards; when and how each award was granted, including the time or times when a person was permitted to exercise an award; and the number of shares of stock with respect to which an award was granted to such person. The exercise price of options granted under our 2006 Non-Employee Director Plan was set at the closing price of our common stock on the NYSE as of the date of grant and was not be adjusted unless we received an exemptive order from the SEC or written confirmation from the staff of the SEC that we may do so (except for adjustments resulting from changes in our capital structure, such as stock dividends, stock splits and reverse stock splits).
199
Our 2006 Non-Employee Director Plan provided that all awards granted under our 2006 Non-Employee Director Plan were subject to modification as required to ensure that such awards did not conflict with the requirements of the 1940 Act. Our Compensation Committee determined the period during which any options granted under our 2006 Non-Employee Director Plan remained exercisable, provided that no option will be exercisable after the expiration of ten years from the date on which it was granted. Options granted under our 2006 Non-Employee Director Plan were not transferable other than by will or the laws of descent and distribution, or by gift, and were generally exercisable during a non-employee director’s lifetime only by such non-employee director. In general, any portion of any options that were not then exercisable terminated upon the termination of the non-employee director’s services to Hercules. Generally, any portion of any options that were exercisable at the time of the termination of the non-employee director’s services to Hercules remained exercisable for the lesser of (i) a period of three months (or one year if the non-employee director’s services to Hercules terminated by reason of the non-employee director’s death) or (ii) the period ending on the latest date on which such options could have been exercised had the non-employee director’s services to Hercules not terminated. In addition, if our Board of Directors determines that a non-employee director’s service to Hercules terminated for reasons that cast such discredit on the non-employee director as to justify immediate termination of the non-employee director’s options, then all options then held by the non-employee director immediately terminate.
Under our 2006 Non-Employee Director Plan, we also were permitted to issue shares of restricted stock to our non-employee directors. Upon initial election to such position, non-employee directors were automatically granted 3,333 shares of restricted stock. The forfeiture restrictions for such initial shares of restricted stock vest as to one-half of such shares on the first anniversary of the date of grant and as to an additional one-half of the restricted stock on the second anniversary of the date of grant. In addition, each non-employee director was automatically granted 5,000 shares of restricted stock on the date of such non-employee director’s re-election to our Board of Directors and the forfeiture restrictions on such shares vest as to one-third of such shares on the anniversary of such grant over three years, provided that the non-employee director remains in service on such dates.
Our Compensation Committee administered our 2006 Non-Employee Director Plan. If there was a change in our capital structure by reason of a stock dividend, stock split or combination of shares (including a reverse stock split), recapitalization or other change in our capital structure, our Board of Directors would make appropriate adjustments to the number and class of shares of stock subject to our 2006 Non-Employee Director Plan and each option outstanding under it. In the event of a consolidation, merger, stock sale, a sale of all or substantially all of our assets, our dissolution or liquidation or other similar events, referred to as a Covered Transaction, our Board of Directors would provide for the assumption of some or all outstanding options or for the grant of new substitute options by the acquirer or survivor. If no such assumption or substitution occurs, all outstanding options become exercisable prior to the Covered Transaction and terminate upon consummation of the Covered Transaction.
200
CONTROL PERSONS AND PRINCIPAL STOCKHOLDERS
The following table sets forth, as of August 30, 2017, the beneficial ownership of each current director, each nominee for director, our NEOs, each person known to us to beneficially own 5% or more of the outstanding shares of our common stock, and our executive officers and directors as a group.
Beneficial ownership is determined in accordance with the rules of the SEC. Common stock subject to options or warrants that are currently exercisable or exercisable within 60 days of August 30, 2017 are deemed to be outstanding and beneficially owned by the person holding such options or warrants. Such shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. Percentage of ownership is based on 82,864,802 shares of common stock outstanding as of August 30, 2017.
Unless otherwise indicated, to our knowledge, each stockholder listed below has sole voting and investment power with respect to the shares beneficially owned by the stockholder, except to the extent authority is shared by their spouses under applicable law. Unless otherwise indicated, the address of all executive officers and directors is c/o Hercules Capital, Inc., 400 Hamilton Avenue, Suite 310, Palo Alto, California 94301.
Our directors are divided into two groups—interested directors and independent directors. Interested directors are “interested persons” as defined in Section 2(a)(19) of the 1940 Act, and independent directors are all other directors.
| Name and Address of Beneficial Owner |
Type of Ownership |
Number of Shares Owned Beneficially(1) |
Percentage of Class |
|||||||
| Interested Director |
||||||||||
| Manuel A. Henriquez(2) |
Record/Beneficial | 1,896,569 | 2.3 | % | ||||||
| Independent Directors |
||||||||||
| Robert B. Badavas(3) |
Beneficial | 148,565 | * | |||||||
| Allyn C. Woodward, Jr.(4) |
Record/Beneficial | 277,105 | * | |||||||
| Thomas J. Fallon(5) |
Record/Beneficial | 51,836 | * | |||||||
| Susanne D. Lyons(6) |
Beneficial | 21,575 | * | |||||||
| Joseph F. Hoffman(7) |
Record/Beneficial | 35,478 | * | |||||||
| Doreen Woo Ho(8) |
Record/Beneficial | 12,236 | * | |||||||
| Other Named Executive Officers |
||||||||||
| Mark R. Harris(9) |
Record/Beneficial | 53,605 | * | |||||||
| Scott Bluestein(10) |
Record/Beneficial | 213,458 | * | |||||||
| Melanie Grace(11) |
Record/Beneficial | 15,247 | * | |||||||
| Executive officers and directors as a group (10 persons)(12) |
3.3 | % | ||||||||
| (1) | Beneficial ownership has been determined in accordance with Rule 13d-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). |
| (2) | Includes 246,509 shares of restricted stock. 1,583,488 shares of common stock held by the Manuel A. Henriquez and Elizabeth H. Henriquez TTEE The Henriquez Family Trust U/A 5/11/99 of which 861,058 shares are pledged as a security; 27,174 shares of common stock held in the Isabelle Irrev Trust, EH Trustee; 27,174 shares of common stock held in the Natalie Irrev Trust, EH Trustee; and 12,224 shares of common stock held in the Manuel Henriquez-Roth IRA. Mr. Henriquez disclaims any beneficial ownership interest of such shares except to the extent of his pecuniary interest therein. |
| (3) | Includes 20,000 shares of common stock that can be acquired upon the exercise of outstanding options. All shares are held of record by the Robert P. Badavas Trust of 2007, and Mr. Badavas disclaims any beneficial ownership interest of such shares except to the extent of his pecuniary interest therein. |
| (4) | Includes 20,000 shares of common stock that can be acquired upon the exercise of outstanding options, 1,666 shares of restricted common stock, and 35,000 shares of common stock held by Mr. Woodward’s spouse in her name. Mr. Woodward disclaims any beneficial ownership interest of such shares held by his spouse except to the extent of his pecuniary interest therein. |
| (5) | Includes 20,000 shares of common stock that can be acquired upon the exercise of outstanding options and 1,666 shares of restricted common stock. All shares are held of record by the Fallon Family Revocable Trust, and Mr. Fallon disclaims any beneficial ownership interest of such shares except to the extent of his pecuniary interest therein. |
201
| (6) | Includes 10,000 shares of common stock that can be acquired upon the exercise of outstanding options. All shares are held of record by the Lyons Family Trust, and Ms. Lyons disclaims any beneficial ownership interest of such shares except to the extent of her pecuniary interest therein. |
| (7) | Includes 15,000 shares of common stock that can be acquired upon the exercise of outstanding options and 3,333 shares of restricted common stock. All shares are held of record by the Hoffman Trust, and Mr. Hoffman disclaims any beneficial ownership interest of such shares except to the extent of his pecuniary interest therein. |
| (8) | Includes 5,000 shares of common stock that can be acquired upon the exercise of outstanding options and includes 3,333 shares of restricted common stock. |
| (9) | Includes 28,645 shares of restricted common stock. |
| (10) | Includes 63,955 shares of restricted common stock. |
| (11) | Includes 8,868 shares of restricted common stock. |
| (12) | Includes 90,000 shares of common stock that can be acquired upon the exercise of outstanding options and 357,975 shares of restricted common stock. |
| * | Less than 1%. |
The following table sets forth as of August 30, 2017, the dollar range of our securities owned by our directors and executive officers.
| Name |
Dollar Range of Equity Securities Beneficially Owned |
|||
| Interested Director |
||||
| Manuel A. Henriquez |
Over $100,000 | |||
| Independent Directors |
||||
| Robert B. Badavas |
Over $100,000 | |||
| Allyn C. Woodward, Jr. |
Over $100,000 | |||
| Thomas J. Fallon |
Over $100,000 | |||
| Susanne D. Lyons |
Over $100,000 | |||
| Joseph F. Hoffman |
Over $100,000 | |||
| Doreen Woo Ho |
Over $100,000 | |||
| Other Named Executive Officers |
||||
| Mark R. Harris |
Over $100,000 | |||
| Scott Bluestein |
Over $100,000 | |||
| Melanie Grace |
Over $100,000 | |||
202
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
We have established a written policy to govern the review, approval and monitoring of transactions involving the Company and certain persons related to Hercules. As a business development company, the 1940 Act restricts us from participating in transactions with any persons affiliated with Hercules, including our officers, directors, and employees and any person controlling or under common control with us.
In order to ensure that we do not engage in any prohibited transactions with any persons affiliated with Hercules, our officers screen each of our transactions for any possible affiliations, close or remote, between the proposed portfolio investment, Hercules, companies controlled by us and our employees and directors.
We will not enter into any agreements unless and until we are satisfied that no affiliations prohibited by the 1940 Act exist or, if such affiliations exist, we have taken appropriate actions to seek Board of Directors’ review and approval or exemptive relief from the SEC for such transaction.
203
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a general summary of certain material U.S. federal income tax considerations relating to our qualification and taxation as a RIC and the acquisition, ownership and disposition of our preferred stock or common stock, but does not purport to be a complete description of the income tax considerations relating thereto. Except as otherwise noted, this discussion assumes you are a taxable U.S. person (as defined for U.S. federal income tax purposes) and that you hold your shares of our stock as capital assets for U.S. federal income tax purposes (generally, assets held for investment). This discussion is based upon current provisions of the Code, the regulations promulgated thereunder and judicial and administrative authorities, all of which are subject to change or differing interpretations by the courts or the IRS, possibly with retroactive effect. No attempt is made to present a detailed explanation of all U.S. federal income tax concerns affecting the Company and its shareholders (including shareholders subject to special rules under U.S. federal income tax law).
The discussions set forth herein do not constitute tax advice. We have not sought and will not seek any ruling from the IRS regarding any matters discussed herein. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to those set forth below. This summary does not discuss any aspects of foreign, state or local tax. Prospective investors must consult their own tax advisers as to the U.S. federal income tax consequences (including the alternative minimum tax consequences) of acquiring, holding and disposing of shares of our stock, as well as the effects of state, local and non-U.S. tax laws.
Election to be Subject to Tax as a RIC
Through December 31, 2005, we were subject to U.S. federal income tax as an ordinary corporation under Subchapter C of the Code. Effective beginning on January 1, 2006 we met the criteria specified below to qualify as a RIC, and elected to be treated as a RIC under Subchapter M of the Code with the filing of our U.S. federal income tax return for 2006. To qualify as a RIC we must, among other things, meet certain source of income and asset diversification requirements (as described below). In addition, we must distribute to our stockholders, in respect of each taxable year, dividends for U.S. federal income tax purposes of an amount generally at least equal to 90% of our “investment company taxable income,” which is generally equal to the sum of our net ordinary income plus the excess of our realized net short-term capital gains over our realized net long-term capital losses, determined without regard to any deduction for distributions paid, or the “Annual Distribution Requirement.” Upon satisfying these requirements in respect of a taxable year, we generally will not be subject to corporate taxes on any income we distribute to our stockholders as dividends for U.S. federal income tax purposes, which will allow us to reduce or eliminate our liability for corporate-level income tax.
On December 31, 2005, immediately before the effective date of our RIC election, we held assets with “built-in gains,” which are assets whose fair market value as of the effective date of the election exceeded their tax basis as of such date. We elected to recognize all of our net built-in gains on such assets at the time of the conversion and paid tax on the built-in gain with the filing of our 2005 U.S. federal income tax return. In making this election, we marked our portfolio investments and other assets to market at the time of our RIC election and paid approximately $294,000 in income tax on the resulting gains.
Taxation as a Regulated Investment Company
For any taxable year in which we:
| • | qualify as a RIC; and |
| • | distribute dividends for U.S. federal income tax purposes to our shareholders of an amount at least equal to the Annual Distribution Requirement; |
We generally will not be subject to U.S. federal income tax on the portion of our investment company taxable income and net capital gain (i.e., net realized long-term capital gains in excess of net realized short-term
204
capital losses) we distribute (or are deemed to distribute) as dividends for U.S. federal income tax purposes to stockholders with respect to that taxable year.
As described above, we made the election to recognize built-in gains as of the effective date of our election to be treated as a RIC and therefore were not subject to built-in gains tax when we sold those assets. However, if we subsequently acquire built-in gain assets from a C corporation in a carryover basis transaction, then we may be subject to tax on the gains recognized by us on dispositions of such assets unless we make a special election to pay corporate-level tax on such built-in gain at the time the assets are acquired. We will be subject to U.S. federal income tax at the regular corporate rates on any income or capital gains not distributed (or deemed distributed) as dividends for U.S. federal income tax purposes to our stockholders.
In order to qualify as a RIC for U.S. federal income tax purposes and obtain the tax benefits of RIC status, in addition to satisfying the Annual Distribution Requirement, we must, among other things:
| • | have in effect at all times during each taxable year an election to be regulated as a business development company under the 1940 Act; |
| • | derive in each taxable year at least 90% of our gross income from (a) dividends, interest, payments with respect to certain securities loans, gains from the sale of stock or other securities, or other income derived with respect to our business of investing in such stock or securities and (b) net income derived from an interest in a “qualified publicly traded partnership” (the “90% Income Test”); |
| • | diversify our holdings so that at the end of each quarter of the taxable year: |
| • | at the close of each quarter of each taxable year, at least 50% of the value of our assets consists of cash, cash equivalents, U.S. government securities, securities of other RICs, and other securities if such other securities of any one issuer do not represent more than 5% of the value of our assets or more than 10% of the outstanding voting securities of such issuer; and |
| • | at the close of each quarter of each taxable year, no more than 25% of the value of our assets is invested in (i) securities (other than U.S. government securities or securities of other RICs) of one issuer, (ii) securities of two or more issuers that are controlled, as determined under applicable tax rules, by us and that are engaged in the same or similar or related trades or businesses or (iii) securities of one or more “qualified publicly traded partnerships” (the “Diversification Tests”). |
We may invest in partnerships which may result in our being subject to state, local or foreign income, franchise or other tax liabilities. In addition, some of the income and fees that we may recognize will not satisfy the 90% Income Test. In order to mitigate the risk that such income and fees would disqualify us as a RIC as a result of a failure to satisfy the 90% Income Test, we may be required to recognize such income and fees indirectly through one or more entities classified as corporations for U.S. federal income tax purposes. Such corporations generally will be subject to corporate income taxes on their earnings, which ultimately will reduce our return on such income and fees.
As a RIC, we will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income and gains unless we make distributions treated as dividends for U.S. federal income tax purposes in a timely manner to our stockholders in respect of each calendar year of an amount at least equal to the sum of (1) 98% of our ordinary income for each calendar year (subject to certain deferrals and elections), (2) 98.2% of our capital gain net income (adjusted for certain ordinary losses) for the 1-year period ending October 31 in that calendar year and (3) any income realized, but not distributed, in the preceding years (the “Excise Tax Avoidance Requirement”). We are not subject to this excise tax on any amount on which we incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains).
Depending on the level of taxable income earned in a taxable year, we may choose to carry over taxable income in excess of current taxable year distributions treated as dividends for U.S. federal income tax purposes
205
from such taxable income into the next taxable year and incur a 4% excise tax on such taxable income, as required. The maximum amount of excess taxable income that may be carried over for distribution in the next taxable year under the Code is the total amount of distributions treated as dividends for U.S. federal income tax purposes paid in the following taxable year, subject to certain declaration and payment guidelines. To the extent we choose to carry over taxable income into the next taxable year, distributions declared and paid by us in a taxable year may differ from our taxable income for that taxable year as such distributions may include the distribution of current taxable year taxable income, the distribution of prior taxable year taxable income carried over into and distributed in the current taxable year, or returns of capital.
Under applicable Treasury regulations and other administrative guidance issued by the IRS, we are permitted to treat certain distributions payable in our stock as taxable distributions that will satisfy the Annual Distribution Requirement as well as the Excise Tax Avoidance Requirement provided that shareholders have the opportunity to elect to receive the distribution in cash. Taxable stockholders receiving such distributions will be required to include the full amount of such distributions as ordinary income (or as long-term capital gain to the extent such distribution is properly designated as a capital gain dividend) to the extent of our current and accumulated earnings and profits for U.S. federal income tax purposes. As a result, a U.S. stockholder may be subject to tax with respect to such distributions in excess of any cash received. If a U.S. stockholder sells the stock it receives as a distribution in order to pay this tax, the sales proceeds may be less than the amount included in income with respect to the distribution, depending on the market price of our stock at the time of the sale. Furthermore, with respect to non-U.S. stockholders, we may be required to withhold U.S. tax with respect to such distributions, including in respect of all or a portion of such distribution that is payable in stock. In addition, if a significant number of our stockholders determine to sell shares of our stock in order to pay taxes owed on distributions, then such sales may put downward pressure on the trading price of our stock. We may in the future determine to make taxable distributions that are payable in part in our common stock.
We may be required to recognize taxable income in circumstances in which we do not receive a corresponding payment in cash. For example, if we hold debt obligations that are treated under applicable tax rules as having OID (such as debt instruments with PIK interest provisions or, in certain cases, increasing interest rates or debt instruments that were issued with warrants), we must include in income each taxable year a portion of the OID that accrues over the life of the obligation, regardless of whether cash representing such income is received by us in the same taxable year. Because any OID accrued is generally required to be included in our investment company taxable income for the taxable year of accrual, we may be required to make a distribution to our stockholders in order to satisfy the Annual Distribution Requirement and the Excise Tax Avoidance Requirement, even though we will not have received any corresponding cash amount.
Gain or loss realized by us from the sale or exchange of warrants acquired by us as well as any loss attributable to the lapse of such warrants generally will be treated as capital gain or loss. Such gain or loss generally will be long-term or short-term, depending on how long we held a particular warrant.
We are authorized to borrow funds and to sell assets in order to satisfy the Annual Distribution Requirement and the Excise Tax Avoidance Requirement (collectively, the “Distribution Requirements”). However, under the 1940 Act, we are not permitted to make distributions to our stockholders while our debt obligations and other senior securities are outstanding unless certain “asset coverage” tests are met. See “Regulation—Senior Securities; Coverage Ratio.” We may be restricted from making distributions under the terms of our debt obligations themselves unless certain conditions are satisfied. Moreover, our ability to dispose of assets to meet the Distribution Requirements may be limited by (1) the illiquid nature of our portfolio, or (2) other requirements relating to our status as a RIC, including the Diversification Tests. If we dispose of assets in order to meet the Distribution Requirements, we may make such dispositions at times that, from an investment standpoint, are not advantageous. If we are prohibited from making distributions or are unable to obtain cash from other sources to make the distributions, we may fail to be subject to tax as a RIC, which would result in us becoming subject to corporate-level income taxes.
206
In addition, we will be partially dependent on our SBIC subsidiaries for cash distributions to enable us to meet the RIC Distribution Requirements. Our SBIC subsidiaries may be limited by the Small Business Investment Act of 1958, as amended, and SBA regulations governing SBICs, from making certain distributions to us that may be necessary to maintain our status as a RIC. We may have to request a waiver of the SBA’s restrictions for our SBIC subsidiaries to make certain distributions to maintain our RIC status. We cannot assure you that the SBA will grant such waiver. If our SBIC subsidiaries are unable to obtain a waiver, compliance with the SBA regulations may cause us to fail to be subject to tax as a RIC, which would result in us becoming subject to corporate-level income taxes.
Certain of our investment practices are subject to special and complex U.S. federal income tax provisions that may, among other things, (i) convert distributions that would otherwise constitute qualified dividend income into ordinary income, (ii) treat distributions that would otherwise be eligible for deductions available to certain U.S. corporations under the Code as ineligible for such treatment, (iii) disallow, suspend or otherwise limit the allowance of certain losses or deductions, (iv) convert long-term capital gains into short-term capital gains or ordinary income, (v) convert short-term capital losses into long-term capital losses, (vi) convert an ordinary loss or deduction into a capital loss (the deductibility of which is more limited), (vii) cause us to recognize income or gain without a corresponding receipt of cash, (viii) adversely alter the characterization of certain complex financial transactions, and (ix) produce gross income that will not constitute qualifying gross income for purposes of the 90% Income Test. These rules also could affect the amount, timing and character of distributions to stockholders.
A RIC is limited in its ability to deduct expenses in excess of its “investment company taxable income.” If our otherwise deductible expenses in a given taxable year exceed our ordinary taxable gross income (e.g., as the result of large amounts of equity-based compensation), we would incur a net operating loss for that taxable year. However, a RIC is not permitted to carry back or carry forward net operating losses, respectively, to prior and subsequent taxable years, and such net operating losses do not pass through to the RIC’s stockholders. In addition, deductible expenses can be used only to offset investment company taxable income, not net capital gain. A RIC may not use any net capital losses (that is, realized capital losses in excess of realized capital gains) to offset the RIC’s investment company taxable income, but may carry forward such net capital losses, and generally use them to offset capital gains indefinitely. Due to these limits on the deductibility of expenses and net capital losses, we may for tax purposes have aggregate taxable income for several taxable years that we are required to distribute and that is taxable to our stockholders even if such taxable income is greater than the aggregate net income we actually earned during those taxable years. Such required distributions may be made from our cash assets or by liquidation of investments, if necessary. We may realize gains or losses from such liquidations. In the event we realize net capital gains from such transactions, you may receive a larger capital gain distribution than you would have received in the absence of such transactions.
Investment income received from sources within foreign countries, or capital gains earned by investing in securities of foreign issuers, may be subject to foreign income taxes withheld at the source. In this regard, withholding tax rates in countries with which the United States does not have a tax treaty are often as high as 35% or more. The United States has entered into tax treaties with many foreign countries that may entitle us to a reduced rate of tax or exemption from tax on this related income and gains. The effective rate of foreign tax cannot be determined at this time since the amount of our assets to be invested within various countries is not now known. We do not anticipate being eligible for the special election that allows a RIC to treat foreign income taxes paid by such RIC as having been paid by its shareholders.
If we acquire the equity securities of certain foreign corporations that earn at least 75% of their annual gross income from passive sources (such as interest, dividends, rents, royalties or capital gain) or hold at least 50% of their total assets in investments producing such passive income (“passive foreign investment companies” or “PFICs”), we could be subject to U.S. federal income tax and additional interest charges on “excess distributions” received from such companies or gain from the sale of stock in such companies, even if all income or gain actually received by us is timely distributed to our shareholders. We would not be able to pass through to
207
our shareholders any credit or deduction for such a tax. Certain elections may, if available, ameliorate these adverse tax consequences, but any such election could require us to recognize taxable income or gain without the concurrent receipt of cash. Furthermore, under recently proposed Treasury Regulations, certain income derived by us from PFICs with respect to which we have made certain U.S. tax elections would generally constitute qualifying income for purposes of the 90% Income Test only to the extent such PFICs make distributions of that income to us. As such, we intend to limit and/or manage our holdings in passive foreign investment companies to minimize our liability for any such taxes and related interest charges.
If we hold greater than 10% of the interests treated as equity for U.S. federal income tax purposes in a foreign corporation that is treated as a controlled foreign corporation (“CFC”), we may be treated as receiving a deemed distribution (taxable as ordinary income) each taxable year from such foreign corporation in an amount equal to our pro rata share of the corporation’s income for such taxable year (including both ordinary earnings and capital gains), whether or not the corporation makes an actual distribution during such taxable year. We would be required to include the amount of a deemed distribution from a CFC when computing our investment company taxable income as well as in determining whether we satisfy the distribution requirements applicable to RICs, even to the extent the amount of our income deemed recognized from the CFC exceeds the amount of any actual distributions from the CFC and our proceeds from any sales or other dispositions of CFC stock during a taxable year. In general, a foreign corporation will be considered a CFC if greater than 50% of the shares of the corporation, measured by reference to combined voting power or value, is owned (directly, indirectly or by attribution) by U.S. Shareholders. A “U.S. Shareholder,” for this purpose, is any U.S. person that possesses (actually or constructively) 10% or more of the combined voting power of all classes of shares of a foreign corporation. Furthermore, under recently proposed Treasury Regulations, certain income derived by us from a CFC would generally constitute qualifying income for purposes of determining our ability to be subject to tax as a RIC only to the extent the CFC makes distributions of that income to us. As such, we may limit and/or manage our holdings in issuers that could be treated as CFCs in order to limit our tax liability or maximize our after-tax return from these investments.
Our functional currency, for U.S. federal income tax purposes, is the U.S. dollar. Under the Code, foreign exchange gains and losses realized by us in connection with certain transactions involving foreign currencies, or payables or receivables denominated in a foreign currency, as well as certain non-U.S. dollar denominated debt securities, certain foreign currency futures contracts, foreign currency option contracts, foreign currency forward contracts, and similar financial instruments are subject to Code provisions that generally treat such gains and losses as ordinary income and losses and may affect the amount, timing and character of distributions to our stockholders. Any such transactions that are not directly related to our investment in securities (possibly including speculative currency positions or currency derivatives not used for hedging purposes) also could, under future Treasury regulations, produce income not among the types of “qualifying income” from which a RIC must derive at least 90% of its annual gross income.
Taxation of U.S. Stockholders
A “U.S. stockholder” generally is a beneficial owner of shares of our common stock who is for U.S. federal income tax purposes:
| • | a citizen or individual resident of the United States including an alien individual who is a lawful permanent resident of the United States or meets the “substantial presence” test under Section 7701(b) of the Code; |
| • | a corporation or other entity taxable as a corporation, for U.S. federal income tax purposes, created or organized in or under the laws of the United States or any political subdivision thereof; |
| • | a trust if (1) a court in the United States has primary supervision over its administration and one or more U.S. persons has the authority to control all substantial decisions of such trust or (2) if such trust validly elects to be treated as a U.S. person for U.S. federal income tax purposes; or |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source. |
208
For U.S. federal income tax purposes, distributions by us generally are taxable to U.S. stockholders as ordinary income or capital gains. Distributions of our “investment company taxable income” (which is, generally, our ordinary income plus net realized short-term capital gains in excess of net realized long-term capital losses) will be taxable as ordinary income to U.S. stockholders to the extent of our current or accumulated earnings and profits, whether paid in cash or reinvested in additional common stock. To the extent such distributions are attributable to dividends from certain U.S. corporations and certain qualified foreign corporations, such distributions may be reported by us as “qualified dividend income” eligible to be taxed in the hands of U.S. non-corporate stockholders (including individuals) at the rates applicable to long-term capital gains, provided certain holding period and other requirements are met at both the stockholder and corporate levels. In this regard, it is anticipated that distributions paid by us generally will not be attributable to dividends and, therefore, generally will not be qualified dividend income. Distributions of our net capital gains (which is generally our realized net long-term capital gains in excess of realized net short-term capital losses) properly reported by us as “capital gain dividends” will be taxable to a U.S. stockholder as long-term capital gains (currently at a maximum rate of 20%, in the case of individuals, trusts or estates), regardless of the U.S. stockholder’s holding period for his, her or its common stock and regardless of whether paid in cash or reinvested in additional common stock. Provided that certain holding period and other requirements are met, ordinary income dividends (if properly reported by us) may qualify (i) for the dividends received deduction available to certain corporations, but only to the extent that our income consists of certain qualifying dividend income from U.S. corporations and (ii) in the case of U.S. noncorporate stockholders, as qualified dividend income eligible to be taxed at long-term capital gain rates to the extent that we earn qualified dividend income (generally, dividend income from taxable U.S. resident corporations and certain qualified foreign corporations). There can be no assurance as to what portion of our distributions will be eligible for the corporate dividends received deduction or for the reduced rates applicable to qualified dividend income. Distributions in excess of our current and accumulated earnings and profits first will reduce a U.S. stockholder’s adjusted tax basis in such stockholder’s common stock and, after the adjusted basis is reduced to zero, will constitute capital gains to such U.S. stockholder.
We currently intend to retain some or all of our realized net long-term capital gains in excess of realized net short-term capital losses. In that case, among other consequences, we will pay tax on the retained amount, each U.S. stockholder will be required to include his, her or its share of the deemed distribution in income as if it had been actually distributed to the U.S. stockholder, and the U.S. stockholder will be entitled to claim a tax credit equal to his, her or its allocable share of the tax paid thereon by us. Since we expect to pay tax on any retained net capital gains at our regular corporate tax rate, and since that rate is in excess of the maximum rate currently payable by non-corporate stockholders on long-term capital gains, the amount of tax that non-corporate stockholders will be treated as having paid and for which they will receive a credit will exceed the tax they owe on the retained net capital gain. Such excess generally may be claimed as a credit against the U.S. stockholder’s other U.S. federal income tax obligations or may be refunded to the extent it exceeds a stockholder’s liability for U.S. federal income tax. A stockholder that is not subject to U.S. federal income tax or otherwise required to file a U.S. federal income tax return would be required to file a U.S. federal income tax return on the appropriate form in order to claim a refund for the taxes we paid. For U.S. federal income tax purposes, the tax basis of shares owned by a U.S. stockholder will be increased by an amount equal under current law to the difference between the amount of undistributed capital gains included in the U.S. stockholder’s gross income and the tax deemed paid by the U.S. stockholder as described in this paragraph. In order to utilize the deemed distribution approach, we must provide written notice to our stockholders prior to the expiration of 60 days after the close of the relevant taxable year. We cannot treat any of our investment company taxable income as a “deemed distribution.”
Under applicable Treasury regulations and certain administrative guidance issued by the IRS, RICs are permitted to treat certain distributions payable in part in shares of their stock, as taxable dividends that will satisfy their Distribution Requirements provided that shareholders have the opportunity to elect to receive the distribution in cash. Taxable stockholders receiving such dividends will be required to include the full amount of the dividend as ordinary income (or as long-term capital gain to the extent such distribution is properly
209
designated as a capital gain dividend) to the extent of our current and accumulated earnings and profits for U.S. federal income tax purposes. As a result, a U.S. stockholder may be required to pay tax with respect to such dividends in excess of any cash received. If a U.S. stockholder sells the stock it receives as a dividend in order to pay this tax, the sales proceeds may be less than the amount included in income with respect to the dividend, depending on the market price of our stock at the time of the sale. Furthermore, with respect to non-U.S. stockholders, we may be required to withhold U.S. tax with respect to such dividends, including in respect of all or a portion of such dividend that is payable in stock. In addition, if a significant number of our stockholders determine to sell shares of our stock in order to pay taxes owed on dividends, then such sales may put downward pressure on the trading price of our stock. We previously determined to pay a portion of our first quarter 2009 dividend in shares of newly issued common stock, and we may in the future determine to distribute taxable dividends that are payable in part in our common stock.
For purposes of determining (1) whether the Annual Distribution Requirement is satisfied for any taxable year and (2) the amount of the deduction for ordinary income and capital gain dividends paid for that taxable year, we may, under certain circumstances, elect to treat a dividend that is paid during the following taxable year as if it had been paid during the taxable year in question. If we make such an election, the U.S. stockholder will still be treated as receiving the dividend in the taxable year in which the distribution is made. However, any dividend declared by us in October, November or December of any calendar year, payable to stockholders of record on a specified date in such a month and actually paid during January of the following calendar year, will be treated as if it had been received by our U.S. stockholders on December 31 of the calendar year in which the dividend was declared.
If an investor acquires shares of our or common stock shortly before the record date of a distribution, the price of the shares will include the value of the distribution and the investor will be subject to tax on the distribution even though economically it may represent a return of his, her or its investment.
A U.S. stockholder generally will recognize taxable gain or loss if the U.S. stockholder sells or otherwise disposes of his, her or its shares of our common stock. Any gain arising from such sale or disposition generally will be treated as long-term capital gain or loss if the U.S. stockholder has held his, her or its shares for more than one year. Otherwise, it will be classified as short-term capital gain or loss. However, any capital loss arising from the sale or disposition of shares of our common stock held for six months or less will be treated as long-term capital loss to the extent of the amount of capital gain dividends received, or undistributed capital gain deemed received, with respect to such shares. In addition, all or a portion of any loss recognized upon a disposition of shares of our common stock may be disallowed if other shares of our common stock are purchased (whether through reinvestment of distributions or otherwise) within 30 days before or after the disposition. In such a case, the basis of the newly purchased shares will be adjusted to reflect the disallowed loss. Reporting of adjusted cost basis information is required for covered securities, which generally include shares of a RIC acquired after January 1, 2012, to the IRS and to taxpayers. Stockholders should contact their intermediaries with respect to reporting of cost basis and available elections for their accounts.
If a Stockholder recognizes losses with respect to Shares of $2 million or more for an individual Stockholder or $10 million or more for a corporate Stockholder, the Treasury Regulations require the Stockholder to file a disclosure statement with the IRS on IRS Form 8886. Direct Stockholders of portfolio securities are in many cases excepted from this reporting requirement, but under current guidance, stockholders of a RIC are not excepted. Future guidance may extend the current exception from this reporting requirement to stockholders of most or all RICs. The fact that a loss is reportable under these Treasury Regulations does not affect the legal determination of whether the taxpayer’s treatment of the loss is proper. Stockholders should consult their tax advisors to determine the applicability of these regulations in light of their individual circumstances.
In general, individual U.S. stockholders currently are subject to a reduced maximum U.S. federal income tax rate of 20% on their net capital gain (i.e., the excess of realized net long-term capital gain over realized net short-
210
term capital loss for a taxable year) including any long-term capital gain derived from an investment in our shares. Such rate is lower than the maximum rate on ordinary income currently payable by individuals. In addition, individuals with income in excess of certain threshold amounts and certain estates and trusts are subject to an additional 3.8% tax on their “net investment income,” which generally includes net income from interest, dividends, annuities, royalties, and rents, and net capital gains (other than certain amounts earned from trades or businesses). Corporate U.S. stockholders currently are subject to U.S. federal income tax on net capital gain at the maximum 35% rate also applied to ordinary income. Non-corporate U.S. stockholders with net capital losses for a taxable year (i.e., capital losses in excess of capital gains) generally may deduct up to $3,000 of such losses against their ordinary income each taxable year; any net capital losses of a non-corporate stockholder in excess of $3,000 generally may be carried forward and used in subsequent taxable years as provided in the Code. Corporate U.S. stockholders generally may not deduct any net capital losses for a taxable year, but may carry back such losses for three taxable years or carry forward such losses for five taxable years.
We or the applicable withholding agent will send to each of our U.S. stockholders, as promptly as possible after the end of each calendar year, a notice reporting the amounts includible in such U.S. stockholder’s taxable income for such calendar year as ordinary income and as long-term capital gain. In addition, the U.S. federal tax status of each calendar year’s distributions generally will be reported to the IRS (including the amount of dividends, if any, eligible for the 20% “qualified dividend income” rate). Distributions may also be subject to additional state, local, and foreign taxes depending on a U.S. stockholder’s particular situation. Dividends distributed by us generally will not be eligible for the corporate dividends-received deduction or the preferential rate applicable to “qualified dividend income.” The Code requires reporting of adjusted cost basis information for covered securities, which generally include shares of our stock, acquired after January 1, 2012, to the IRS and to taxpayers. U.S. stockholders should contact their financial intermediaries with respect to reporting of cost basis and available elections for their accounts.
In some taxable years, we may be subject to the alternative minimum tax (“AMT”). If we have tax items that are treated differently for AMT purposes than for regular tax purposes, we may apportion those items between us and our stockholders, and this may affect our stockholder’s AMT liabilities. Although regulations explaining the precise method of apportionment have not yet been issued by the IRS, we may apportion these items in the same proportion that dividends paid to each stockholder bear to our taxable income (determined without regard to the dividends paid deduction), unless we determine that a different method for a particular item is warranted under the circumstances. You should consult your own tax advisor to determine how an investment in our stock could affect your AMT liability.
We or the applicable withholding agent may be required to withhold U.S. federal income tax (“backup withholding”) from all distributions to any non-corporate U.S. stockholder (1) who fails to furnish us with a correct taxpayer identification number or a certificate that such stockholder is exempt from backup withholding, or (2) with respect to whom the IRS notifies us or the applicable withholding agent that such stockholder has failed to properly report certain interest and dividend income to the IRS and to respond to notices to that effect. An individual’s taxpayer identification number is his or her social security number. Any amount withheld under backup withholding is allowed as a credit against the U.S. stockholder’s U.S. federal income tax liability, provided that proper information is timely provided to the IRS.
Dividend Reinvestment Plan We have adopted a dividend reinvestment plan through which all distributions are paid to our common stockholders in the form of additional shares of our common stock, unless a stockholder elects to receive cash in accordance with the terms of the plan. See “Dividend Reinvestment Plan.” Any distributions made to a U.S. stockholder that are reinvested under the plan will nevertheless remain generally taxable to the U.S. stockholder. The U.S. stockholder will have an adjusted tax basis in the additional shares of our common stock purchased through the plan equal to the amount of the reinvested distribution. The additional shares will have a new holding period commencing on the day following the day on which the shares are credited to the U.S. stockholder’s account.
211
Taxation of Non-U.S. Stockholders
A “Non-U.S. stockholder” is a beneficial owner of shares of our common stock that is not a U.S. stockholder or a partnership (including an entity treated as a partnership) for U.S. federal income tax purposes.
Whether an investment in our shares is appropriate for a Non-U.S. stockholder will depend upon that person’s particular circumstances. An investment in the shares by a Non-U.S. stockholder may have adverse tax consequences. Non-U.S. stockholders should consult their tax advisors before investing in our common stock.
Distributions (other than certain distributions derived from net long-term capital gains) paid by us to a Non-U.S. stockholder are generally subject to U.S. federal withholding tax at a rate of 30% (or lower applicable treaty rate) even if they are funded by income or gains (such as portfolio interest, short-term capital gains, or foreign-source dividend and interest income) that, if paid to a Non-U.S. stockholder directly, would not be subject to withholding. If the distributions are effectively connected with a U.S. trade or business of the Non-U.S. stockholder (and, if an income tax treaty applies, attributable to a permanent establishment maintained by the Non-U.S. stockholder in the United States), we will not be required to withhold tax if the Non-U.S. stockholder complies with applicable certification and disclosure requirements, although the distributions will be subject to U.S. federal income tax at the rates applicable to U.S. stockholders. (Special certification requirements apply to a Non-U.S. stockholder that is a foreign partnership or a foreign trust, and such entities are urged to consult their own tax advisors.)
However, no withholding is required with respect to certain distributions if (i) the distributions are properly reported to our stockholders as “interest-related dividends” or “short-term capital gain dividends” in written statements to our stockholders, (ii) the distributions are derived from sources specified in the Code for such dividends and (iii) certain other requirements are satisfied. Currently, we do not anticipate that any significant amount of our distributions would be reported as eligible for this exemption from withholding. In the case of shares of our stock held through an intermediary, the intermediary may withhold even if we report all or a portion of any of our distributions as “interest-related dividends” or “short-term capital gain dividends.” Non-U.S. stockholders should contact their intermediaries with respect to the application of these rules to their accounts. No assurance can be provided as to whether any amount of our distributions will be eligible for this exemption from withholding or if eligible, will be reported as such by us.
Actual or deemed distributions of our net capital gains to a Non-U.S. stockholder, and gains realized by a Non-U.S. stockholder upon the sale of our common stock, will not be subject to U.S. federal withholding tax and generally will not be subject to U.S. federal income tax unless the distributions or gains, as the case may be, are effectively connected with a U.S. trade or business of the Non-U.S. stockholder (and, if an income tax treaty applies, are attributable to a permanent establishment maintained by the Non-U.S. stockholder in the United States), or in the case of an individual stockholder, the stockholder is present in the United States for a period or periods aggregating 183 days or more during the year of the sale or capital gain dividend and certain other conditions are met.
If we distribute our net capital gains in the form of deemed rather than actual distributions, a Non-U.S. stockholder will be entitled to a federal income tax credit or tax refund equal to the stockholder’s allocable share of the tax we pay on the capital gains deemed to have been distributed. In order to obtain the refund, the Non-U.S. stockholder must obtain a U.S. taxpayer identification number and file a U.S. federal income tax return even if the Non-U.S. stockholder would not otherwise be required to obtain a U.S. taxpayer identification number or file a U.S. federal income tax return. For a corporate Non-U.S. stockholder, distributions (both actual and deemed), and gains realized upon the sale of our common stock that are effectively connected to a U.S. trade or business may, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate (or at a lower rate if provided for by an applicable treaty). Accordingly, investment in the shares may not be appropriate for a Non-U.S. stockholder.
212
A Non-U.S. stockholder who is a non-resident alien individual, and who is not otherwise subject to withholding of U.S. federal income tax, may be subject to information reporting and backup withholding of U.S. federal income tax on dividends unless the Non-U.S. stockholder provides us or the dividend paying agent with an IRS Form W-8BEN or IRS Form W-8BEN-E, (or an acceptable substitute or successor form) or otherwise meets documentary evidence requirements for establishing that it is a Non-U.S. stockholder or otherwise establishes an exemption from backup withholding.
The “Foreign Account Tax Compliance Act,” or “FATCA,” provisions of the Code, generally imposes a 30% withholding tax on payments of certain types of income to foreign financial institutions that fail to enter into an agreement with the U.S. Treasury to report certain required information with respect to accounts held by U.S. persons (or held by foreign entities that have U.S. persons as substantial owners). The types of income subject to the tax include U.S. source interest and dividends and the gross proceeds from the sale of any property that could produce U.S.-source interest or dividends paid after December 31, 2018. The information required to be reported includes the identity and taxpayer identification number of each account holder that is a U.S. person and transaction activity within the holder’s account. In addition, subject to certain exceptions, this legislation also imposes a 30% withholding on payments to foreign entities that are not financial institutions unless the foreign entity certifies that it does not have a greater than 10% U.S. owner or provides the withholding agent with identifying information on each greater than 10% U.S. owner. Depending on the status of a Non-U.S. Holder and the status of the intermediaries through which they hold their shares, Non-U.S. Holders could be subject to this 30% withholding tax with respect to distributions on their shares and proceeds from the sale of their shares. Under certain circumstances, a Non-U.S. Holders might be eligible for refunds or credits of such taxes.
Non-U.S. persons should consult their own tax advisors with respect to the U.S. federal income tax and withholding tax, and state, local and foreign tax consequences of an investment in the shares.
Failure to Qualify as a Regulated Investment Company
If we fail to satisfy the 90% Income Test or the Diversification Tests for any taxable year, we may nevertheless continue to qualify as a RIC for such taxable year if certain relief provisions are applicable (which may, among other things, require us to pay certain corporate-level federal taxes or to dispose of certain assets).
If we were unable to qualify for treatment as a RIC and the foregoing relief provisions are not applicable, we would be subject to tax on all of our taxable income at regular corporate rates. We would not be able to deduct distributions to stockholders, nor would they be required to be made. Such distributions would be taxable to our stockholders and provided certain holding period and other requirements were met, could qualify for treatment as “qualified dividend income” eligible for the 20% maximum U.S. federal income tax rate if earned by certain U.S. resident non-corporate stockholders to the extent of our current and accumulated earnings and profits. Subject to certain limitations under the Code, corporate distributions generally would be eligible for the dividends-received deduction with respect to distributions current and accumulated earnings and profits if earned by certain U.S. resident corporate stockholders. Distributions in excess of our current and accumulated earnings and profits would be treated first as a return of capital to the extent of the stockholder’s tax basis, and any remaining distributions would be treated as a capital gain. To requalify as a RIC in a subsequent taxable year, we would be required to satisfy the RIC qualification requirements for that taxable year and dispose of any earnings and profits from any taxable year in which we failed to qualify as a RIC. Subject to a limited exception applicable to a corporation that qualified as a RIC under Subchapter M of the Code for at least one taxable year prior to disqualification and that requalify as a RIC no later than the second taxable year following the nonqualifying taxable year, we also could be subject to tax on any unrealized net built-in gains in the assets held by us during the period in which we failed to qualify as a RIC that are recognized within the subsequent five taxable years, unless we made a special election to incur a corporate-level income tax on such built-in gain at the time of our requalification as a RIC.
213
The following discussion is a general summary of the material prohibitions and descriptions governing business development companies. It does not purport to be a complete description of all of the laws and regulations affecting business development companies.
A business development company primarily focuses on investing in or lending to private companies and making managerial assistance available to them, while providing its stockholders with the ability to retain the liquidity of a publicly-traded stock. The 1940 Act contains prohibitions and restrictions relating to transactions between business development companies and their directors and officers and principal underwriters and certain other related persons and requires that a majority of the directors be persons other than “interested persons,” as that term is defined in the 1940 Act. In addition, the 1940 Act provides that we may not change the nature of our business so as to cease to be, or to withdraw our election as, a business development company unless approved by a majority of our outstanding voting securities as defined in the 1940 Act. A majority of the outstanding voting securities of a company is defined under the 1940 Act as the lesser of: (i) 67% or more of such company’s shares present at a meeting if more than 50% of the outstanding shares of such company are present or represented by proxy, or (ii) more than 50% of the outstanding shares of such company.
Qualifying Assets
Under the 1940 Act, a business development company may not acquire any asset other than assets of the type listed in Section 55(a) of the 1940 Act, which are referred to as qualifying assets, unless, at the time the acquisition is made, qualifying assets represent at least 70% of the company’s total assets. The principal categories of qualifying assets relevant to our proposed business are the following:
| (1) | Securities purchased in transactions not involving any public offering from the issuer of such securities, which issuer (subject to certain limited exceptions) is an eligible portfolio company, or from any person who is, or has been during the preceding 13 months, an affiliated person of an eligible portfolio company, or from any other person, subject to such rules as may be prescribed by the SEC. An eligible portfolio company is defined in the 1940 Act as any issuer which: |
| (a) | is organized under the laws of, and has its principal place of business in, the United States; |
| (b) | is not an investment company (other than a small business investment company wholly owned by the business development company) or a company that would be an investment company but for certain exclusions under the 1940 Act; and |
| (c) | does not have any class of securities listed on a national securities exchange; or if it has securities listed on a national securities exchange such company has a market capitalization of less than $250 million; is controlled by the business development company and has an affiliate of a business development company on its board of directors; or meets such other criteria as may be established by the SEC. |
| (2) | Securities of any portfolio company which we control. |
| (3) | Securities purchased in a private transaction from a U.S. issuer that is not an investment company or from an affiliated person of the issuer, or in transactions incident thereto, if the issuer is in bankruptcy and subject to reorganization or if the issuer, immediately prior to the purchase of its securities was unable to meet its obligations as they came due without material assistance other than conventional lending or financing arrangements. |
| (4) | Securities of an eligible portfolio company purchased from any person in a private transaction if there is no ready market for such securities and we already own 60% of the outstanding equity of the eligible portfolio company. |
| (5) | Securities received in exchange for or distributed on or with respect to securities described in (1) through (4) above, or pursuant to the exercise of warrants or rights relating to such securities. |
214
| (6) | Cash, cash equivalents, U.S. Government securities or high-quality debt securities maturing in one year or less from the time of investment. |
Control, as defined by the 1940 Act, is presumed to exist where a business development company beneficially owns more than 25% of the outstanding voting securities of the portfolio company.
We do not intend to acquire securities issued by any investment company, including other business development companies, that exceed the limits imposed by the 1940 Act. Under these limits, we generally cannot acquire more than 3% of the voting stock of any investment company (as defined in the 1940 Act), invest more than 5% of the value of our total assets in the securities of one such investment company or invest more than 10% of the value of our total assets in the securities of such other investment companies in the aggregate. With regard to that portion of our portfolio invested in securities issued by investment companies, it should be noted that such investments might subject our stockholders to additional expenses.
Significant Managerial Assistance
Business development companies generally must offer to make available to the issuer of the securities significant managerial assistance, except in circumstances where either (i) the business development company controls such issuer of securities or (ii) the business development company purchases such securities in conjunction with one or more other persons acting together and one of the other persons in the group makes available such managerial assistance. Making available significant managerial assistance means, among other things, any arrangement whereby the business development company, through its directors, officers or employees, offers to provide and, if accepted, does so provide, significant guidance and counsel concerning the management, operations or business objectives and policies of a portfolio company through monitoring of portfolio company operations, selective participation in board and management meetings, consulting with and advising a portfolio company’s officers or other organizational or financial guidance.
Temporary Investments
Pending investment in other types of qualifying assets, as described above, our investments may consist of cash, cash equivalents, U.S. government securities or high quality debt securities maturing in one year or less from the time of investment, which we refer to, collectively, as temporary investments, so that 70% of our assets are qualifying assets. We may invest in U.S. Treasury bills or in repurchase agreements, provided that such agreements are fully collateralized by cash or securities issued by the U.S. government or its agencies. A repurchase agreement involves the purchase by an investor, such as us, of a specified security and the simultaneous agreement by the seller to repurchase it at an agreed upon future date and at a price which is greater than the purchase price by an amount that reflects an agreed-upon interest rate. There is no percentage restriction on the proportion of our assets that may be invested in such repurchase agreements. However, if more than 25% of our total assets constitute repurchase agreements from a single counterparty, we generally would not meet the diversification tests imposed on us by the Code in order to qualify as a RIC for U.S. federal income tax purposes. Thus, we do not intend to enter into repurchase agreements with a single counterparty in excess of this limit. We will monitor the creditworthiness of the counterparties with which we enter into repurchase agreement transactions.
Warrants and Options
Under the 1940 Act, a business development company is subject to restrictions on the amount of warrants, options, restricted stock or rights to purchase shares of capital stock that it may have outstanding at any time. In particular, the amount of capital stock that would result from the conversion or exercise of all outstanding warrants, options or other rights to purchase capital stock cannot exceed 25% of the business development company’s total outstanding shares of capital stock. This amount is reduced to 20% of the business development company’s total outstanding shares of capital stock if the amount of warrants, options or rights issued pursuant to
215
an executive compensation plan would exceed 15% of the business development company’s total outstanding shares of capital stock. We have received exemptive relief from the SEC permitting us to issue stock options and restricted stock to our employees and directors subject to the above conditions, among others. For a discussion regarding the conditions of this exemptive relief, see “—Exemptive Relief” below and Note 7 to our consolidated financial statements.
Senior Securities; Coverage Ratio
We will be permitted, under specified conditions, to issue multiple classes of indebtedness and one class of stock senior to our common stock if our asset coverage, as defined in the 1940 Act, is at least equal to 200% immediately after each such issuance. In addition, we may not be permitted to declare any cash dividend distribution on our outstanding common shares, or purchase any such shares, unless, at the time of such declaration or purchase, we have asset coverage of at least 200% after deducting the amount of such distribution or purchase price. We may also borrow amounts up to 5% of the value of our total assets for temporary or emergency purposes. For a discussion of the risks associated with the resulting leverage, see “Risk Factors—Risks Related to Our Business Structure—Because we have substantial indebtedness, there could be increased risk in investing in our company.” On April 5, 2007, we received approval from the SEC on our request for exemptive relief that permits us to exclude the indebtedness of our wholly-owned subsidiaries that are small business investment companies from the 200% asset coverage requirement applicable to us.
Capital Structure
We are not generally able to issue and sell our common stock at a price below NAV per share. We may, however, sell our common stock, at a price below the current NAV of the common stock, or sell warrants, options or other rights to acquire such common stock, at a price below the current NAV of the common stock if our Board of Directors determines that such sale is in the best interests of the Company and our stockholders have approved the practice of making such sales. In connection with the receipt of such stockholder approval, we will limit the number of shares that we issue at a price below NAV pursuant to this authorization so that the aggregate dilutive effect on our then outstanding shares will not exceed 20%. Our Board of Directors, subject to its fiduciary duties and regulatory requirements, has the discretion to determine the amount of the discount, and as a result, the discount could be up to 100% of NAV per share.
Code of Ethics
We have adopted and will maintain a code of ethics that establishes procedures for personal investments and restricts certain personal securities transactions. Personnel subject to the code may invest in securities for their personal investment accounts, including securities that may be purchased or held by us, so long as such investments are made in accordance with the code’s requirements. Our code of ethics will generally not permit investments by our employees in securities that may be purchased or held by us. We may be prohibited under the 1940 Act from conducting certain transactions with our affiliates without the prior approval of our directors who are not interested persons and, in some cases, the prior approval of the SEC.
Our current code of ethics is posted on our website at www.htgc.com and was filed with the SEC as an exhibit to a Form 8-K filing on July 13, 2015. You may read and copy the code of ethics at the SEC’s Public Reference Room in Washington, D.C. You may obtain information on the operation of the Public Reference Room by calling the SEC at (202) 551-8090. In addition, the code of ethics is available on the EDGAR Database on the SEC’s Internet site at http://www.sec.gov. You may also obtain copies of the code of ethics, after paying a duplicating fee, by electronic request at the following e-mail address: publicinfo@sec.gov, or by writing the SEC’s Public Reference Section, 100 F Street, N.E., Washington, D.C. 20549.
Privacy Principles
We are committed to maintaining the privacy of our stockholders and safeguarding their non-public personal information. The following information is provided to help you understand what personal information
216
we collect, how we protect that information and why, in certain cases, we may share information with select other parties.
Generally, we do not receive any non-public personal information relating to our stockholders, although certain non-public personal information of our stockholders may become available to us. We do not disclose any non-public personal information about our stockholders or former stockholders, except as permitted by law or as is necessary in order to service stockholder accounts (for example, to a transfer agent).
We restrict access to non-public personal information about our stockholders to our employees with a legitimate business need for the information. We maintain physical, electronic and procedural safeguards designed to protect the non-public personal information of our stockholders.
Proxy Voting Policies and Procedures
We vote proxies relating to our portfolio securities in the best interest of our stockholders. We review on a case-by-case basis each proposal submitted to a stockholder vote to determine its impact on the portfolio securities held by us. Although we generally vote against proposals that may have a negative impact on our portfolio securities, we may vote for such a proposal if there exists compelling long-term reasons to do so.
Our proxy voting decisions are made by our investment committee, which is responsible for monitoring each of our investments. To ensure that our vote is not the product of a conflict of interest, we require that: (i) anyone involved in the decision making process disclose to our Chief Compliance Officer any potential conflict that he or she is aware of and any contact that he or she has had with any interested party regarding a proxy vote; and (ii) employees involved in the decision making process or vote administration are prohibited from revealing how we intend to vote on a proposal in order to reduce any attempted influence from interested parties.
Exemptive Relief
On June 21, 2005, we filed a request with the SEC for exemptive relief to allow us to take certain actions that would otherwise be prohibited by the 1940 Act, as applicable to business development companies. Specifically, we requested that the SEC permit us to issue stock options to our non-employee directors as contemplated by Section 61(a)(3)(B)(i)(II) of the 1940 Act. On February 15, 2007, we received approval from the SEC on this exemptive request. In addition, in June 2007, we filed an amendment to the February 2007 order to adjust the number of shares issued to the non-employee directors. On October 10, 2007, we received approval from the SEC on this amended exemptive request.
On April 5, 2007, we received approval from the SEC on our request for exemptive relief that permits us to exclude the indebtedness of our wholly-owned subsidiaries that are small business investment companies from the 200% asset coverage requirement applicable to us.
On May 23, 2007, we received approval from the SEC on our request for exemptive relief that permits us to issue restricted stock to our employees, officers and directors. On June 21, 2007, our shareholders approved amendments to the 2004 Equity Incentive Plan and 2006 Non-Employee Incentive Plan (collectively, the “Plans”) permitting such restricted grants. The maximum amount of shares that may be issued under the Plans will be 10% of the outstanding shares of our common stock on the effective date of the Plans plus 10% of the outstanding number of shares of our common stock issued or delivered by us (other than pursuant to compensation plans) during the term of the Plans. The amount of voting securities that would result from the exercise of all of our outstanding warrants, options, and rights, if any, together with any restricted stock issued pursuant to the Plans, at the time of issuance shall not exceed 25% of our outstanding voting securities, except that if such amount would exceed 15% of our outstanding voting securities, then the total amount of voting securities that would result from the exercise of all outstanding warrants, options, and rights, if any, together with any restricted stock issued pursuant to the Plans, at the time of issuance shall not exceed 20% of our outstanding voting securities.
217
On June 22, 2010 we received approval from the SEC on our request for exemptive relief that permits our employees to exercise their stock options and restricted stock and pay any related income taxes using a cashless exercise program.
Other
We will be periodically examined by the SEC for compliance with the Exchange Act and the 1940 Act.
We are required to provide and maintain a bond issued by a reputable fidelity insurance company to protect us against larceny and embezzlement. Furthermore, as a business development company, we are prohibited from protecting any director or officer against any liability to our stockholders arising from willful misfeasance, bad faith, gross negligence or reckless disregard of the duties involved in the conduct of such person’s office.
We are required to adopt and implement written policies and procedures reasonably designed to prevent violation of the federal securities laws, review these policies and procedures annually for their adequacy and the effectiveness of their implementation. Our Chief Compliance Officer is responsible for administering these policies and procedures.
Small Business Administration Regulations
We make investments in qualifying small businesses through our two wholly-owned SBIC subsidiaries, HT II and HT III. With our net investments of $44.0 million and $74.5 million in HT II and HT III, respectively, we have the combined capacity to issue a total of $190.2 million of SBA guaranteed debentures, subject to SBA approval. At June 30, 2017, we have issued $190.2 million in SBA guaranteed debentures in our SBIC subsidiaries.
We intend to seek an additional SBIC license to ensure continued access to the maximum statutory limit of SBA guaranteed debentures under the SBIC program. We have formed Hercules Technology IV, L.P. for that purpose. There can be no assurance of when or if we will receive SBA approval for another SBIC license.
SBICs are designed to stimulate the flow of private equity capital to eligible small businesses. Under present SBA regulations, eligible small businesses include businesses that have a tangible net worth not exceeding $19.5 million and have average annual fully taxed net income not exceeding $6.5 million for the two most recent fiscal years. In addition, SBICs must devote 25.0% of its investment activity to “smaller” enterprises as defined by the SBA. A smaller enterprise is one that has a tangible net worth not exceeding $6.0 million and has average annual fully taxed net income not exceeding $2.0 million for the two most recent fiscal years. SBA regulations also provide alternative size standard criteria to determine eligibility, which depend on the industry in which the business is engaged and are based on such factors as the number of employees and gross sales. According to SBA regulations, SBICs may make long-term loans to small businesses, invest in the equity securities of such businesses and provide them with consulting and advisory services. Through our wholly-owned subsidiaries HT II and HT III, we plan to provide long-term loans to qualifying small businesses, and in connection therewith, make equity investments.
HT II and HT III are periodically examined and audited by the SBA’s staff to determine their compliance with SBA regulations. If HT II or HT III fails to comply with applicable SBA regulations, the SBA could, depending on the severity of the violation, limit or prohibit HT II’s or HT III’s use of debentures, declare outstanding debentures immediately due and payable, and/or limit HT II or HT III from making new investments. In addition, HT II or HT III may also be limited in their ability to make distributions to the Company if they do not have sufficient capital in accordance with SBA regulations. Such actions by the SBA would, in turn, negatively affect the Company because HT II and III are our wholly owned subsidiaries. HT II and HT III were in compliance with the terms of the SBIC’s leverage as of June 30, 2017 as a result of having sufficient capital as defined under the SBA regulations.
218
HT II and HT III hold approximately $104.8 million and $271.5 million in assets, respectively, and accounted for approximately 5.8% and 14.9% of our total assets prior to consolidation at June 30, 2017.
The SBA restricts the ability of SBICs to repurchase their capital stock. SBA regulations also include restrictions on a “change of control” or transfer of an SBIC and require that SBICs invest idle funds in accordance with SBA regulations. In addition, HT II and HT III may also be limited in their ability to make distributions to us if they do not have sufficient capital and/or distributed earnings, in accordance with SBA regulations.
Our SBIC subsidiaries are subject to regulation and oversight by the SBA, including requirements with respect to maintaining certain minimum financial ratios and other covenants. Receipt of an SBIC license does not assure that our SBIC subsidiaries will receive SBA guaranteed debenture funding, which is dependent upon our SBIC subsidiaries continuing to be in compliance with SBA regulations and policies. The SBA, as a creditor, will have a superior claim to our SBIC subsidiaries’ assets over our stockholders in the event we liquidate our SBIC subsidiaries or the SBA exercises its remedies under the SBA-guaranteed debentures issued by our SBIC subsidiaries upon an event of default.
219
DETERMINATION OF NET ASSET VALUE
We determine the NAV per share of our common stock quarterly. The NAV per share is equal to the value of our total assets minus liabilities and any preferred stock outstanding divided by the total number of shares of common stock outstanding. As of the date of this prospectus, we do not have any preferred stock outstanding.
At June 30, 2017, approximately 87.8% of our total assets represented investments in portfolio companies whose fair value is determined in good faith by the Board of Directors. Value, as defined in Section 2(a)(41) of the 1940 Act, is (i) the market price for those securities for which a market quotation is readily available and (ii) for all other securities and assets, fair value is as determined in good faith by the Board of Directors. Our investments are carried at fair value in accordance with the 1940 Act and Accounting Standards Codification Topic 946 Financial Services—Investment Companies (“ASC 946”) and measured in accordance with Accounting Standards Codification Topic 820 Fair Value Measurements and Disclosures (“ASC 820”). Our debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of our investments in these portfolio companies are considered Level 3 assets under ASC 820 because there is no known or accessible market or market indexes for these investment securities to be traded or exchanged. As such, we value substantially all of our investments at fair value as determined in good faith pursuant to a consistent valuation policy by our Board of Directors in accordance with the provisions of ASC 820 and the 1940 Act. Due to the inherent uncertainty in determining the fair value of investments that do not have a readily available market value, the fair value of our investments determined in good faith by our Board of Directors may differ significantly from the value that would have been used had a readily available market existed for such investments, and the differences could be material.
We may from time to time engage an independent valuation firm to provide us with valuation assistance with respect to certain portfolio investments. We engage independent valuation firms on a discretionary basis. Specifically, on a quarterly basis, we will identify portfolio investments with respect to which an independent valuation firm will assist in valuing. We select these portfolio investments based on a number of factors, including, but not limited to, the potential for material fluctuations in valuation results, credit quality and the time lapse since the last valuation of the portfolio investment by an independent valuation firm.
We intend to continue to engage an independent valuation firm to provide management with assistance regarding our determination of the fair value of selected portfolio investments each quarter unless directed by the Board of Directors to cancel such valuation services. The scope of services rendered by an independent valuation firm is at the discretion of the Board of Directors. Our Board of Directors is ultimately, and solely, responsible for determining the fair value of our investments in good faith.
With respect to investments for which market quotations are not readily available or when such market quotations are deemed not to represent fair value, our Board of Directors has approved a multi-step valuation process each quarter, as described below:
(1) our quarterly valuation process begins with each portfolio company being initially valued by the investment professionals responsible for the portfolio investment;
(2) preliminary valuation conclusions are then documented and business based assumptions are discussed with our investment committee;
(3) the Audit Committee of the Board of Directors reviews the preliminary valuation of the investments in the portfolio as provided by the investment committee, which incorporates the results of the independent valuation firm as appropriate; and
220
(4) the Board of Directors, upon the recommendation of the Audit Committee, discusses valuations and determines the fair value of each investment in our portfolio in good faith based on the input of, where applicable, the respective independent valuation firm and the investment committee.
ASC 820 establishes a framework for measuring the fair value of assets and liabilities and outlines a fair value hierarchy which prioritizes the inputs used to measure fair value and the effect of fair value measures on earnings. ASC 820 also requires disclosures for fair value measurements based on the level within the hierarchy of the information used in the valuation. ASC 820 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value. ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
We have categorized all investments recorded at fair value in accordance with ASC 820 based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels, defined by ASC 820 and directly related to the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities, are as follows:
Level 1—Inputs are unadjusted, quoted prices in active markets for identical assets at the measurement date. The types of assets carried at Level 1 fair value generally are equities listed in active markets.
Level 2—Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset in connection with market data at the measurement date and for the extent of the instrument’s anticipated life. Fair valued assets that are generally included in this category are publicly held debt investments and warrants held in a public company.
Level 3—Inputs reflect management’s best estimate of what market participants would use in pricing the asset at the measurement date. It includes prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. Generally, assets carried at fair value and included in this category are the debt investments and warrants and equities held in a private company.
Debt Investments
We follow the guidance set forth in ASC 820 which establishes a framework for measuring the fair value of assets and liabilities and outlines a fair value hierarchy which prioritizes the inputs used to measure fair value and the effect of fair value measures on earnings. Our debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of our investments in these portfolio companies are considered Level 3 assets under ASC 820 because there is no known or accessible market or market indexes for debt instruments for these investment securities to be traded or exchanged. In addition, we may, from time to time, invest in public debt of companies that meet our investment objectives. These investments are considered Level 2 assets.
In making a good faith determination of the value of our investments, we generally start with the cost basis of the investment, which includes the value attributed to the OID, if any, and PIK interest or other receivables which have been accrued as earned. We then apply the valuation methods as set forth below.
We apply a procedure for debt investments that assumes the sale of each investment in a hypothetical market to a hypothetical market participant where buyers and sellers are willing participants. The hypothetical market does not include scenarios where the underlying security was simply repaid or extinguished, but includes an exit concept. We determine the yield at inception for each debt investment.
We then use senior secured, leveraged loan yields provided by third party providers to determine the change in market yields between inception of the debt security and the measurement date. Industry specific indices and other relevant market data are used to benchmark/assess market based movements.
221
Under this process, we also evaluate the collateral for recoverability of the debt investments. We consider each portfolio company’s credit rating, security liens and other characteristics of the investment to adjust the baseline yield to derive a credit adjusted hypothetical yield for each investment as of the measurement date. The anticipated future cash flows from each investment are then discounted at the hypothetical yield to estimate each investment’s fair value as of the measurement date.
Our process includes an analysis of, among other things, the underlying investment performance, the current portfolio company’s financial condition and market changing events that impact valuation, estimated remaining life, current market yield and interest rate spreads of similar securities as of the measurement date. We value its syndicated debt investments using broker quotes and bond indices amongst other factors. If there is a significant deterioration of the credit quality of a debt investment, we may consider other factors to estimate fair value, including the proceeds that would be received in a liquidation analysis.
We record unrealized depreciation on investments when it believes that an investment has decreased in value, including where collection of a debt investment is doubtful or, if under the in-exchange premise, when the value of a debt security is less than amortized cost of the investment. Conversely, where appropriate, we record unrealized appreciation if it believes that the underlying portfolio company has appreciated in value and, therefore, that its investment has also appreciated in value or, if under the in-exchange premise, the value of a debt security is greater than amortized cost.
When originating a debt instrument, we generally receive warrants or other equity-related securities from the borrower. We determine the cost basis of the warrants or other equity-related securities received based upon their respective fair values on the date of receipt in proportion to the total fair value of the debt and warrants or other equity-related securities received. Any resulting discount on the debt investments from recordation of the warrant or other equity instruments is accreted into interest income over the life of the debt investment.
Debt investments that are traded on a public exchange are valued at the prevailing market price as of the valuation date.
Equity-Related Securities and Warrants
Securities that are traded in the over-the-counter markets or on a stock exchange will be valued at the prevailing bid price at period end. We have a limited amount of equity securities in public companies. In accordance with the 1940 Act, unrestricted publicly traded securities for which market quotations are readily available are valued at the closing market quote on the measurement date.
We estimate the fair value of warrants using a Black Scholes option pricing model. At each reporting date, privately held warrant and equity-related securities are valued based on an analysis of various factors including, but not limited to, the portfolio company’s operating performance and financial condition and general market conditions, price to enterprise value or price to equity ratios, discounted cash flow, valuation comparisons to comparable public companies or other industry benchmarks. When an external event occurs, such as a purchase transaction, public offering, or subsequent equity sale, the pricing indicated by that external event is utilized to corroborate our valuation of the warrant and equity-related securities. We periodically review the valuation of our portfolio companies that have not been involved in a qualifying external event to determine if the enterprise value of the portfolio company may have increased or decreased since the last valuation measurement date.
Escrow Receivables
Escrow receivables are collected in accordance with the terms and conditions of the escrow agreement. Escrow balances are typically distributed over a period greater than one year and may accrue interest during the escrow period. Escrow balances are measured for collectability on at least a quarterly basis and fair value is determined based on the amount of the estimated recoverable balances and the contractual maturity date. As of June 30, 2017, there were no material past due escrow receivables.
222
Determinations In Connection With Offerings
In connection with each offering of shares of our common stock, the Board of Directors or a committee thereof is required to make the determination that we are not selling shares of our common stock at a price below our then current NAV at the time at which the sale is made, unless it is determined by the Board of Directors that such sale is in the best interests of our stockholders and such sale is otherwise approved by our stockholders. The Board of Directors considers the following factors, among others, in making such determination:
| • | the NAV of our common stock disclosed in the most recent periodic report we filed with the SEC; |
| • | our management’s assessment of whether any material change in the NAV has occurred (including through the realization of net gains on the sale of our portfolio investments) from the period beginning on the date of the most recently disclosed NAV to the period ending two days prior to the date of the sale of our common stock; and |
| • | the magnitude of the difference between (i) a value that our Board of Directors or an authorized committee thereof has determined reflects the current NAV of our common stock, which is generally based upon the NAV of our common stock disclosed in the most recent periodic report that we filed with the SEC, as adjusted to reflect our management’s assessment of any material change in the NAV of our common stock since the date of the most recently disclosed NAV of our common stock, and (ii) the offering price of the shares of our common stock in the proposed offering. |
Importantly, this determination does not require that we calculate NAV in connection with each offering of shares of our common stock, but instead it involves the determination by the Board of Directors or a committee thereof that we are not selling shares of our common stock at a price below the then current NAV at the time at which the sale is made.
Moreover, to the extent that there is a possibility that we may (i) issue shares of our common stock at a price below the then current NAV of our common stock at the time at which the sale is made or (ii) trigger the undertaking (which we will provide to the SEC in a registration statement to which a prospectus will be a part) to suspend the offering of shares of our common stock pursuant to a prospectus if the NAV fluctuates by certain amounts in certain circumstances until such prospectus is amended, the Board of Directors or a committee thereof will elect, in the case of clause (i) above, either to postpone the offering until such time that there is no longer the possibility of the occurrence of such, events or to undertake to determine NAV within two days prior to any such sale to ensure that such sale will not be below our then current NAV, and, in the case of clause (ii) above, to comply with such undertaking or to undertake to determine NAV to ensure that such undertaking has not been triggered.
These processes and procedures are part of our compliance policies and procedures. Records will be made contemporaneously with all determinations described in this section and these records will be maintained with other records we are required to maintain under the 1940 Act.
223
SALES OF COMMON STOCK BELOW NET ASSET VALUE
We are not generally able to issue and sell our common stock at a price below NAV per share. We may, however, sell our common stock, at a price below the current NAV of the common stock, or sell warrants, options or other rights to acquire such common stock, at a price below the current NAV of the common stock if our Board of Directors determines that such sale is in our best interests and the best interests of our stockholders and our stockholders have approved the practice of making such sales. In connection with the receipt of such stockholder approval, we will agree to limit the number of shares that we issue at a price below NAV pursuant to this authorization so that the aggregate dilutive effect on our then outstanding shares will not exceed 20%. Our Board of Directors, subject to its fiduciary duties and regulatory requirements, has the discretion to determine the amount of the discount, and as a result, the discount could be up to 100% of NAV per share.
In order to sell shares pursuant to this authorization:
| • | a majority of our independent directors who have no financial interest in the sale must have approved the sale; and |
| • | a majority of such directors, who are not interested persons of the Company, in consultation with the underwriter or underwriters of the offering if it is to be underwritten, must have determined in good faith, and as of a time immediately prior to the first solicitation by us or on our behalf of firm commitments to purchase such shares or immediately prior to the issuance of such shares, that the price at which such shares are to be sold is not less than a price which closely approximates the market value of those shares, less any underwriting commission or discount; and |
Any offering of common stock below NAV per share will be designed to raise capital for investment in accordance with our investment objectives and business strategies.
In making a determination that an offering below NAV per share is in our and our stockholders’ best interests, our Board of Directors would consider a variety of factors including:
| • | The effect that an offering below NAV per share would have on our stockholders, including the potential dilution they would experience as a result of the offering; |
| • | The amount per share by which the offering price per share and the net proceeds per share are less than the most recently determined NAV per share; |
| • | The relationship of recent market prices of our common stock to NAV per share and the potential impact of the offering on the market price per share of our common stock; |
| • | Whether the proposed offering price would closely approximate the market value of our shares; |
| • | The potential market impact of being able to raise capital during the current financial market difficulties; |
| • | The nature of any new investors anticipated to acquire shares in the offering; |
| • | The anticipated rate of return on and quality, type and availability of investments to be funded with the proceeds from the offering, if any; and |
| • | The leverage available to us, both before and after any offering, and the terms thereof. |
Sales by us of our common stock at a discount from NAV pose potential risks for our existing stockholders whether or not they participate in the offering, as well as for new investors who participate in the offering.
The following three headings and accompanying tables will explain and provide hypothetical examples on the impact of an offering at a price less than NAV per share on three different sets of investors:
| • | existing stockholders who do not purchase any shares in the offering; |
224
| • | existing stockholders who purchase a relatively small amount of shares in the offering or a relatively large amount of shares in the offering; and |
| • | new investors who become stockholders by purchasing shares in the offering. |
Impact on Existing Stockholders not Participating in the Offering
Our existing stockholders who do not participate in an offering below NAV per share or who do not buy additional shares in the secondary market at the same or lower price we obtain in the offering (after expenses and commissions) face the greatest potential risks. All stockholders will experience an immediate decrease (often called dilution) in the NAV of the shares they hold. Stockholders who do not participate in the offering will also experience a disproportionately greater decrease in their participation in our earnings and assets and their voting power than stockholders who do participate in the offering. All stockholders may also experience a decline in the market price of their shares, which often reflects to some degree announced or potential decreases in NAV per share. This decrease could be more pronounced as the size of the offering and level of discount to NAV increases.
The following table illustrates the level of NAV dilution that would be experienced by a nonparticipating stockholder in different hypothetical offerings of different sizes and levels of discount from NAV per share. Actual sales prices and discounts may differ from the presentation below.
225
The examples assume that Company XYZ has 3,000,000 common shares outstanding, $40,000,000 in total assets and $10,000,000 in total liabilities. The current NAV and NAV are thus $30,000,000 and $10.00, respectively. The table illustrates the dilutive effect on nonparticipating Stockholder A of (1) an offering of 300,000 shares (10% of the outstanding shares) with proceeds to the Company XYZ at $9.00 per share after offering expenses and commissions, and (2) an offering of 600,000 shares (20% of the outstanding shares) with proceeds to the Company at $0.001 per share after offering expenses and commissions (a 100% discount from NAV).
| Prior to Sale Below NAV |
Example 1 10% Offering at 10% Discount |
Example 2 20% Offering at 100% Discount |
||||||||||||||||||
| Following Sale |
% Change |
Following Sale |
% Change |
|||||||||||||||||
| Offering Price |
||||||||||||||||||||
| Price per Share to Public(1) |
— | $ | 9.47 | — | $ | 0.001 | — | |||||||||||||
| Net Proceeds per Share to Issuer |
— | $ | 9.00 | — | $ | 0.001 | — | |||||||||||||
| Decrease to NAV |
||||||||||||||||||||
| Total Shares Outstanding |
3,000,000 | 3,300,000 | 10.00 | % | 3,600,000 | 20.00 | % | |||||||||||||
| NAV per Share |
$ | 10.00 | $ | 9.91 | (0.90 | )% | $ | 8.33 | (16.67 | )% | ||||||||||
| Share Dilution to Stockholder |
||||||||||||||||||||
| Shares Held by Stockholder A |
30,000 | 30,000 | — | 30,000 | — | |||||||||||||||
| Percentage of Shares Held by Stockholder A |
1.00 | % | 0.91 | % | (9.09 | )% | 0.83 | % | (16.67 | )% | ||||||||||
| Total Asset Values |
||||||||||||||||||||
| Total NAV Held by Stockholder A |
$ | 300,000 | $ | 297,273 | (0.90 | )% | $ | 250,005 | (16.67 | )% | ||||||||||
| Total Investment by Stockholder A (Assumed to Be $10.00 per Share) |
$ | 300,000 | $ | 300,000 | — | $ | 300,000 | — | ||||||||||||
| Total Dilution to Stockholder A (Change in Total NAV Held By Stockholder) |
— | $ | (2,727 | ) | — | $ | (49,995 | ) | — | |||||||||||
| Per Share Amounts |
||||||||||||||||||||
| NAV per Share Held by Stockholder A |
— | $ | 9.91 | — | $ | 8.33 | — | |||||||||||||
| Investment per Share Held by Stockholder A (Assumed to be $10.00 per Share on Shares Held Prior to Sale) |
$ | 10.00 | $ | 10.00 | — | $ | 10.00 | — | ||||||||||||
| Dilution per Share Held by Stockholder A |
— | $ | (0.09 | ) | — | $ | (1.67 | ) | — | |||||||||||
| Percentage Dilution per Share Held by Stockholder A |
— | — | (0.90 | )% | — | (16.67 | )% | |||||||||||||
| (1) | Assumes 5% in selling compensation and expenses paid by Company XYZ. |
Impact on Existing Stockholders who do Participate in the Offering
Our existing stockholders who participate in an offering below NAV per share or who buy additional shares in the secondary market at the same or lower price as we obtain in the offering (after expenses and commissions) will experience the same types of NAV dilution as the nonparticipating stockholders, albeit at a lower level, to the extent they purchase less than the same percentage of the discounted offering as their interest in our shares immediately prior to the offering. The level of NAV dilution on an aggregate basis will decrease as the number of shares such stockholders purchase increases. Existing stockholders who buy more than their proportionate percentage will experience NAV dilution but will, in contrast to existing stockholders who purchase less than their proportionate share of the offering, experience an increase (often called accretion) in NAV per share over their investment per share and will also experience a disproportionately greater increase in their participation in our earnings and assets and their voting power than our increase in assets, potential earning power and voting interests due to the offering. The level of accretion will increase as the excess number of shares purchased by such stockholder increases. Even a stockholder who over-participates will, however, be subject to the risk that we
226
may make additional discounted offerings in which such stockholder does not participate, in which case such a stockholder will experience NAV dilution as described above in such subsequent offerings. These stockholders may also experience a decline in the market price of their shares, which often reflects to some degree announced or potential decreases in NAV per share. This decrease could be more pronounced as the size of the offering and the level of discount to NAV increases.
The following chart illustrates the level of dilution and accretion in the hypothetical 20% discount offering from the prior chart (Example 3) for a stockholder that acquires shares equal to (1) 50% of its proportionate share of the offering (i.e., 3,000 shares, which is 0.5% of an offering of 600,000 shares rather than its 1.0% proportionate share) and (2) 150% of such percentage (i.e., 9,000 shares, which is 1.5% of an offering of 600,000 shares rather than its 1.0% proportionate share). The prospectus supplement pursuant to which any discounted offering is made will include a chart for this example based on the actual number of shares in such offering and the actual discount from the most recently determined NAV per share.
| Prior to Sale Below NAV |
50% Participation |
150% Participation |
||||||||||||||||||
| Following Sale |
% Change |
Following Sale |
% Change |
|||||||||||||||||
| Offering Price |
||||||||||||||||||||
| Price per Share to Public(1) |
— | $ | 8.42 | — | $ | 8.42 | — | |||||||||||||
| Net Proceeds per Share to Issuer |
— | $ | 8.00 | — | $ | 8.00 | — | |||||||||||||
| Increase in Shares and Decrease to NAV |
||||||||||||||||||||
| Total Shares Outstanding |
3,000,000 | 3,600,000 | 20.00 | % | 3,600,000 | 20.00 | % | |||||||||||||
| NAV per Share |
$ | 10.00 | $ | 9.67 | (3.33 | )% | $ | 9.67 | (3.33 | )% | ||||||||||
| Dilution/Accretion to Participating Stockholder A |
||||||||||||||||||||
| Share Dilution/Accretion |
||||||||||||||||||||
| Shares Held by Stockholder A |
30,000 | 33,000 | 10.00 | % | 39,000 | 30.00 | % | |||||||||||||
| Percentage Outstanding Held by Stockholder A |
1.00 | % | 0.92 | % | (8.33 | )% | 1.08 | % | 8.33 | % | ||||||||||
| NAV Dilution/Accretion |
||||||||||||||||||||
| Total NAV Held by Stockholder A |
$ | 300,000 | $ | 319,110 | 6.33 | % | $ | 377,130 | 25.67 | % | ||||||||||
| Total Investment by Stockholder A (Assumed to be $10.00 per Share on Shares Held Prior to Sale) |
— | $ | 325,260 | — | $ | 375,780 | — | |||||||||||||
| Total Dilution/Accretion to Stockholder A (Total NAV Less Total Investment) |
— | $ | (6,150 | ) | — | $ | 1,350 | — | ||||||||||||
| NAV Dilution/Accretion per Share |
||||||||||||||||||||
| NAV per Share Held by Stockholder A |
— | $ | 9.67 | — | $ | 9.67 | — | |||||||||||||
| Investment per Share Held by Stockholder A (Assumed to be $10.00 per Share on Shares Held Prior to Sale) |
$ | 10.00 | $ | 9.86 | (1.44 | )% | $ | 9.64 | (3.65 | )% | ||||||||||
| NAV Dilution/Accretion per Share Experienced by Stockholder A (NAV per Share Less Investment per Share) |
— | $ | (0.19 | ) | — | $ | 0.03 | — | ||||||||||||
| Percentage NAV Dilution/Accretion Experienced by Stockholder A (NAV Dilution/Accretion per Share Divided by Investment per Share) |
— | — | (1.93 | )% | — | 0.31 | % | |||||||||||||
| (1) | Assumes 5% in selling compensation and expenses paid by Company XYZ. |
Impact on New Investors
Investors who are not currently stockholders, but who participate in an offering below NAV and whose investment per share is greater than the resulting NAV per share (due to selling compensation and expenses paid by us) will experience an immediate decrease, albeit small, in the NAV of their shares and their NAV per share
227
compared to the price they pay for their shares. Investors who are not currently stockholders and who participate in an offering below NAV per share and whose investment per share is also less than the resulting NAV per share will experience an immediate increase in the NAV of their shares and their NAV per share compared to the price they pay for their shares. All these investors will experience a disproportionately greater participation in our earnings and assets and their voting power than our increase in assets, potential earning power and voting interests. These investors will, however, be subject to the risk that we may make additional discounted offerings in which such new stockholder does not participate, in which case such new stockholder will experience dilution as described above in such subsequent offerings. These investors may also experience a decline in the market price of their shares, which often reflects to some degree announced or potential decreases in NAV per share. This decrease could be more pronounced as the size of the offering and level of discount to NAV increases.
The following chart illustrates the level of dilution or accretion for new investors that would be experienced by a new investor in the same hypothetical 10% and 100% discounted offerings as described in the first chart above. The illustration is for a new investor who purchases the same percentage (1.00%) of the shares in the offering as Stockholder A in the prior examples held immediately prior to the offering. The prospectus supplement pursuant to which any discounted offering is made will include a chart for these examples based on the actual number of shares in such offering and the actual discount from the most recently determined NAV per share.
| Prior to Sale Below NAV |
Example 1 10% Offering at 10% Discount |
Example 2 20% Offering at 100% Discount |
||||||||||||||||||
| Following Sale |
% Change |
Following Sale |
% Change |
|||||||||||||||||
| Offering Price |
||||||||||||||||||||
| Price per Share to Public(1) |
— | $ | 9.47 | — | $ | 0.001 | — | |||||||||||||
| Net Proceeds per Share to Issuer |
— | $ | 9.00 | — | $ | 0.001 | — | |||||||||||||
| Increase in Shares and Decrease to NAV |
||||||||||||||||||||
| Total Shares Outstanding |
3,000,000 | 3,300,000 | 10.00 | % | 3,600,000 | 20.00 | % | |||||||||||||
| NAV per Share |
$ | 10.00 | $ | 9.91 | (0.90 | )% | $ | 8.33 | (16.67 | )% | ||||||||||
| Dilution/Accretion to New Investor A |
||||||||||||||||||||
| Share Dilution |
||||||||||||||||||||
| Shares Held by Investor A |
— | 3,000 | — | 6,000 | — | |||||||||||||||
| Percentage Outstanding Held by Investor A |
0.00 | % | 0.09 | % | — | 0.17 | % | — | ||||||||||||
| NAV Dilution |
||||||||||||||||||||
| Total NAV Held by Investor A |
— | $ | 29,730 | — | $ | 50,001 | — | |||||||||||||
| Total Investment by Investor A (At Price to Public) |
— | $ | 28,410 | — | $ | 6 | — | |||||||||||||
| Total Dilution/Accretion to Investor A (Total NAV Less Total Investment) |
— | $ | 1,320 | — | $ | 49,995 | — | |||||||||||||
| NAV Dilution per Share |
||||||||||||||||||||
| NAV per Share Held by Investor A |
$ | 9.91 | — | $ | 8.33 | — | ||||||||||||||
| Investment per Share Held by Investor A |
— | $ | 9.47 | — | $ | 0.001 | — | |||||||||||||
| NAV Dilution/Accretion per Share Experienced by Investor A (NAV per Share Less Investment per Share) |
— | $ | 0.44 | — | $ | 8.33 | — | |||||||||||||
| Percentage NAV Dilution/Accretion Experienced by Investor A (NAV Dilution/Accretion per Share Divided by Investment per Share) |
— | — | 4.65 | % | — | 99.99 | % | |||||||||||||
| (1) | Assumes 5% in selling compensation and expenses paid by Company XYZ. |
228
We have adopted a dividend reinvestment plan (the “DRP”), through which all distributions are paid to our stockholders in the form of additional shares of our common stock, unless a stockholder elects to receive cash as provided below. In this way, a stockholder can maintain an undiluted investment in our common stock and still allow us to pay out the required distributable income.
No action is required on the part of a registered stockholder to receive a distribution in shares of our common stock. A registered stockholder may elect to receive an entire distribution in cash by notifying American Stock Transfer & Trust Company, the plan administrator and our transfer agent and registrar, so that such notice is received by the plan administrator no later than three days prior to the payment date for distributions to stockholders. The plan administrator will set up an account for shares acquired through the DRP for each stockholder who has not elected to receive distributions in cash (each a “Participant”) and hold such shares in non-certificated form. Upon request by a Participant, received not less than three days prior to the payment date, the plan administrator will, instead of crediting shares to the Participant’s account, issue a certificate registered in the Participant’s name for the number of whole shares of our common stock and a check for any fractional share.
Those stockholders whose shares are held by a broker or other financial intermediary may receive distributions in cash by notifying their broker or other financial intermediary of their election.
We expect to use primarily newly-issued shares to implement the DRP, whether our shares are trading at a premium or at a discount to NAV, although we have the option under the DRP to purchase shares in the market to fulfill DRP requirements. The number of shares to be issued to a stockholder is determined by dividing the total dollar amount of the distribution payable to such stockholder by the market price per share of our common stock at the close of regular trading on the NYSE on the valuation date for such distribution. Market price per share on that date will be the closing price for such shares on the NYSE or, if no sale is reported for such day, at the average of their electronically-reported bid and asked prices. The number of shares of our common stock to be outstanding after giving effect to payment of the distribution cannot be established until the value per share at which additional shares will be issued has been determined and elections of our stockholders have been tabulated.
There is no charge to our stockholders for receiving their distributions in the form of additional shares of our common stock. The plan administrator’s fees for handling distributions in stock are paid by us. There are no brokerage charges with respect to shares we have issued directly as a result of distributions payable in stock. If a Participant elects by internet or by written or telephonic notice to the plan administrator to have the plan administrator sell part or all of the shares held by the plan administrator in the Participant’s account and remit the proceeds to the Participant, the plan administrator is authorized to deduct a $15.00 transaction fee plus brokerage commissions from the proceeds.
Any shares issued in connection with a stock split or stock dividend will be added to a Participant’s account with the Plan Administrator. The Plan Administrator may curtail or suspend transaction processing until the completion of such stock split or payment of such stock dividend.
Stockholders who receive distributions in the form of stock generally are subject to the same federal, state and local tax consequences as are stockholders who elect to receive their distributions in cash. A stockholder’s basis for determining gain or loss upon the sale of stock received in a distribution from us will be equal to the total dollar amount of the distribution payable to the stockholder.
The DRP may be terminated by us upon notice in writing mailed to each Participant at least 30 days prior to any record date for the payment of any distribution by us. All correspondence concerning the DRP, including requests for additional information, should be directed to the plan administrator by mail at American Stock Transfer & Trust Company, Attn: Dividend Reinvestment Department, P.O. Box 922, Wall Street Station, New York, NY 10269-0560 or by phone at 1-866-669-9888.
229
The following description is based on relevant portions of the Maryland General Corporation Law and on our charter and bylaws. This summary may not contain all of the information that is important to you, and we refer you to the Maryland General Corporation Law and our charter and bylaws for a more detailed description of the provisions summarized below.
Under the terms of our charter, our authorized capital stock consists of 200,000,000 shares of common stock, par value $0.001 per share, of which 82,864,802 shares are outstanding as of August 30, 2017. Under our charter, our Board of Directors is authorized to classify and reclassify any unissued shares of stock into other classes or series of stock, and to cause the issuance of such shares, without obtaining stockholder approval. In addition, as permitted by the Maryland General Corporation Law, but subject to the 1940 Act, our charter provides that the Board of Directors, without any action by our stockholders, may amend the charter from time to time to increase or decrease the aggregate number of shares of stock or the number of shares of stock of any class or series that we have authority to issue. Under Maryland law, our stockholders generally are not personally liable for our debts or obligations.
Common Stock
All shares of our common stock have equal rights as to earnings, assets, distributions and voting privileges, except as described below and, when they are issued, will be duly authorized, validly issued, fully paid and nonassessable.
Distributions may be paid to the holders of our common stock if, as and when authorized by our Board of Directors and declared by us out of assets legally available therefor. Shares of our common stock have no conversion, exchange, preemptive or redemption rights. In the event of a liquidation, dissolution or winding up of Hercules each share of our common stock would be entitled to share ratably in all of our assets that are legally available for distribution after we pay all debts and other liabilities and subject to any preferential rights of holders of our preferred stock, if any preferred stock is outstanding at such time. Each share of our common stock is entitled to one vote on all matters submitted to a vote of stockholders, including the election of directors. Except as provided with respect to any other class or series of stock, the holders of our common stock will possess exclusive voting power. There is no cumulative voting in the election of directors, which means that holders of a majority of the outstanding shares of common stock will elect all of our directors, and holders of less than a majority of such shares will be unable to elect any director.
| Title of Class |
Amount Authorized |
Amount Held by Company for its Account |
Amount Outstanding |
|||||||||
| Common Stock, $0.001 par value per share |
200,000,000 | — | 82,864,802 | |||||||||
Preferred Stock
Our charter authorizes our Board of Directors to classify and reclassify any unissued shares of stock into other classes or series of stock, including preferred stock. Prior to issuance of shares of each class or series, the Board of Directors is required by Maryland law and by our charter to set the terms, preferences, conversion or other rights, voting powers, restrictions, limitations as to dividends or other distributions, qualifications and terms or conditions of redemption for each class or series. Thus, the Board of Directors could authorize the issuance of shares of preferred stock with terms and conditions which could have the effect of delaying, deferring or preventing a transaction or a change in control that might involve a premium price for holders of our common stock or otherwise be in their best interest. You should note, however, that any issuance of preferred stock must comply with the requirements of the 1940 Act. The 1940 Act requires, among other things, that (1) immediately after issuance and before any dividend or other distribution is made with respect to our common stock and before
230
any purchase of common stock is made, such preferred stock together with all other senior securities must not exceed an amount equal to 50% of our total assets after deducting the amount of such dividend, distribution or purchase price, as the case may be, and (2) the holders of shares of preferred stock, if any are issued, must be entitled as a class to elect two directors at all times and to elect a majority of the directors if distributions on such preferred stock are in arrears by two years or more. Certain matters under the 1940 Act require the separate vote of the holders of any issued and outstanding preferred stock. We believe that the availability for issuance of preferred stock will provide us with increased flexibility in structuring future financings and acquisitions.
Limitation on Liability of Directors and Officers; Indemnification and Advance of Expenses
Maryland law permits a Maryland corporation to include in its charter a provision limiting the liability of its directors and officers to the corporation and its stockholders for money damages except for liability resulting from (a) actual receipt of an improper benefit or profit in money, property or services or (b) active and deliberate dishonesty established by a final judgment as being material to the cause of action. Our charter contains such a provision which eliminates directors’ and officers’ liability to the maximum extent permitted by Maryland law, subject to the requirements of the 1940 Act.
Our charter authorizes us, to the maximum extent permitted by Maryland law and subject to the requirements of the 1940 Act, to indemnify any present or former director or officer or any individual who, while a director or officer and at our request, serves or has served another corporation, real estate investment trust, partnership, joint venture, trust, employee benefit plan or other enterprise as a director, officer, partner or trustee, from and against any claim or liability to which such person may become subject or which such person may incur by reason of his or her service in any such capacity, except with respect to any matter as to which such person shall have been finally adjudicated in any proceeding not to have acted in good faith in the reasonable belief that their action was in our best interest or to be liable to us or our stockholders by reason of willful misfeasance, bad faith, gross negligence or reckless disregard of the duties involved in the conduct of such person’s office. Our charter also provides that, to the maximum extent permitted by Maryland law, with the approval of our Board of Directors and provided that certain conditions described in our charter are met, we may pay certain expenses incurred by any such indemnified person in advance of the final disposition of a proceeding upon receipt of an undertaking by or on behalf of such indemnified person to repay amounts we have so paid if it is ultimately determined that indemnification of such expenses is not authorized under our charter. Our bylaws obligate us, to the maximum extent permitted by Maryland law and subject to the requirements of the 1940 Act, to indemnify any present or former director or officer or any individual who, while a director or officer and at our request, serves or has served another corporation, real estate investment trust, partnership, joint venture, trust, employee benefit plan or other enterprise as a director, officer, partner or trustee and who is made, or threatened to be made, a party to the proceeding by reason of his or her service in any such capacity from and against any claim or liability to which that person may become subject or which that person may incur by reason of his or her service in any such capacity, except with respect to any matter as to which such person shall have been finally adjudicated in any proceeding not to have acted in good faith in the reasonable belief that their action was in our best interest or to be liable to us or our stockholders by reason of willful misfeasance, bad faith, gross negligence or reckless disregard of the duties involved in the conduct of such person’s office. Our bylaws also provide that, to the maximum extent permitted by Maryland law, with the approval of our Board of Directors and provided that certain conditions described in our bylaws are met, we may pay certain expenses incurred by any such indemnified person in advance of the final disposition of a proceeding upon receipt of an undertaking by or on behalf of such indemnified person to repay amounts we have so paid if it is ultimately determined that indemnification of such expenses is not authorized under our bylaws.
Maryland law requires a corporation (unless its charter provides otherwise, which our charter does not) to indemnify a director or officer who has been successful in the defense of any proceeding to which he or she is made, or threatened to be made, a party by reason of his or her service in that capacity. Maryland law permits a corporation to indemnify its present and former directors and officers, among others, against judgments,
231
penalties, fines, settlements and reasonable expenses actually incurred by them in connection with any proceeding to which they may be made, or threatened to be made, a party by reason of their service in those or other capacities unless it is established that (a) the act or omission of the director or officer was material to the matter giving rise to the proceeding and (1) was committed in bad faith or (2) was the result of active and deliberate dishonesty, (b) the director or officer actually received an improper personal benefit in money, property or services or (c) in the case of any criminal proceeding, the director or officer had reasonable cause to believe that the act or omission was unlawful. However, under Maryland law, a Maryland corporation may not indemnify for an adverse judgment in a suit by or in the right of the corporation or for a judgment of liability on the basis that a personal benefit was improperly received, unless in either case a court orders indemnification, and then only for expenses. In addition, Maryland law permits a corporation to advance reasonable expenses to a director or officer upon the corporation’s receipt of (a) a written affirmation by the director or officer of his or her good faith belief that he or she has met the standard of conduct necessary for indemnification by the corporation and (b) a written undertaking by him or her or on his or her behalf to repay the amount paid or reimbursed by the corporation if it is ultimately determined that the standard of conduct was not met.
We currently have in effect a directors’ and officers’ insurance policy covering our directors and officers and us for any acts and omissions committed, attempted or allegedly committed by any director or officer during the policy period. The policy is subject to customary exclusions.
Provisions of the Maryland General Corporation Law and Our Charter and Bylaws
The Maryland General Corporation Law and our charter and bylaws contain provisions that could make it more difficult for a potential acquiror to acquire us by means of a tender offer, proxy contest or otherwise. These provisions are expected to discourage certain coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to negotiate first with our Board of Directors. We believe that the benefits of these provisions outweigh the potential disadvantages of discouraging any such acquisition proposals because, among other things, the negotiation of such proposals may improve their terms.
Classified Board of Directors
Our Board of Directors is divided into three classes of directors serving staggered three-year terms. The terms of the first, second and third classes will expire in 2017, 2015 and 2016, respectively. Upon expiration of their current terms, directors of each class are eligible to serve for three-year terms or until their successors are duly elected and qualify. Each year one class of directors will be elected by the stockholders. A classified board may render a change in control or removal of our incumbent management more difficult. We believe, however, that the longer time required to elect a majority of a classified Board of Directors will help to ensure the continuity and stability of our management and policies.
Election of Directors
Our charter provides that, except as otherwise provided in the bylaws, the affirmative vote of the holders of a majority of the outstanding shares of stock entitled to vote in the election of directors will be required to elect each director. Our bylaws currently provide that directors are elected by a plurality of the votes cast in the election of directors. Pursuant to our charter and bylaws, our Board of Directors may amend the bylaws to alter the vote required to elect directors.
Number of Directors; Vacancies; Removal
Our charter provides that the number of directors will be set only by the Board of Directors in accordance with our bylaws. Our bylaws provide that a majority of our entire Board of Directors may at any time increase or decrease the number of directors. However, unless the bylaws are amended, the number of directors may never
232
be less than one nor more than 12. We have elected to be subject to the provision of Subtitle 8 of Title 3 of the Maryland General Corporation Law, as amended (the “Maryland General Corporation Law”), regarding the filling of vacancies on the Board of Directors. Accordingly, at such time, except as may be provided by the Board of Directors in setting the terms of any class or series of preferred stock, any and all vacancies on the Board of Directors may be filled only by the affirmative vote of a majority of the remaining directors in office, even if the remaining directors do not constitute a quorum, and any director elected to fill a vacancy shall serve for the remainder of the full term of the directorship in which the vacancy occurred and until a successor is elected and qualifies, subject to any applicable requirements of the 1940 Act.
Our charter provides that a director may be removed only for cause, as defined in the charter, and then only by the affirmative vote of at least two-thirds of the votes entitled to be cast in the election of directors.
Action by Stockholders
Under the Maryland General Corporation Law, stockholder action may be taken only at an annual or special meeting of stockholders or by unanimous consent in lieu of a meeting (unless the charter provides for stockholder action by less than unanimous written consent, which our charter does not). These provisions, combined with the requirements of our bylaws regarding the calling of a stockholder-requested special meeting of stockholders discussed below, may have the effect of delaying consideration of a stockholder proposal until the next annual meeting.
Advance Notice Provisions for Stockholder Nominations and Stockholder Proposals
Our bylaws provide that with respect to an annual meeting of stockholders, nominations of persons for election to the Board of Directors and the proposal of business to be considered by stockholders may be made only (1) pursuant to our notice of the meeting, (2) by the Board of Directors or (3) by a stockholder who is entitled to vote at the meeting and who has complied with the advance notice procedures of the bylaws. With respect to special meetings of stockholders, only the business specified in our notice of the meeting may be brought before the meeting. Nominations of persons for election to the Board of Directors at a special meeting may be made only (1) pursuant to our notice of the meeting, (2) by the Board of Directors or (3) provided that the Board of Directors has determined that directors will be elected at the meeting, by a stockholder who is entitled to vote at the meeting and who has complied with the advance notice provisions of the bylaws.
The purpose of requiring stockholders to give us advance notice of nominations and other business is to afford our Board of Directors a meaningful opportunity to consider the qualifications of the proposed nominees and the advisability of any other proposed business and, to the extent deemed necessary or desirable by our Board of Directors, to inform stockholders and make recommendations about such qualifications or business, as well as to provide a more orderly procedure for conducting meetings of stockholders. Although our bylaws do not give our Board of Directors any power to disapprove stockholder nominations for the election of directors or proposals recommending certain action, they may have the effect of precluding a contest for the election of directors or the consideration of stockholder proposals if proper procedures are not followed and of discouraging or deterring a third party from conducting a solicitation of proxies to elect its own slate of directors or to approve its own proposal without regard to whether consideration of such nominees or proposals might be harmful or beneficial to us and our stockholders.
Calling of Special Meeting of Stockholders
Our bylaws provide that special meetings of stockholders may be called by our Board of Directors and certain of our officers. Additionally, our bylaws provide that, subject to the satisfaction of certain procedural and informational requirements by the stockholders requesting the meeting, a special meeting of stockholders shall be called by our secretary upon the written request of stockholders entitled to cast not less than a majority of all of the votes entitled to be cast at such meeting.
233
Approval of Extraordinary Corporate Action; Amendment of Charter and Bylaws
Under Maryland law, a Maryland corporation generally cannot dissolve, amend its charter, merge, sell all or substantially all of its assets, engage in a share exchange or engage in similar transactions outside the ordinary course of business, unless approved by the affirmative vote of stockholders entitled to cast at least two-thirds of the votes entitled to be cast on the matter. However, a Maryland corporation may provide in its charter for approval of these matters by a lesser percentage, but not less than a majority of all of the votes entitled to be cast on the matter. Our charter generally provides for approval of charter amendments and extraordinary transactions by the stockholders entitled to cast at least a majority of the votes entitled to be cast on the matter. Our charter also provides that certain charter amendments and any proposal for our conversion, whether by merger or otherwise, from a closed-end company to an open-end company or any proposal for our liquidation or dissolution requires the approval of the stockholders entitled to cast at least 75% of the votes entitled to be cast on such matter. However, if such amendment or proposal is approved by at least 75% of our continuing directors (in addition to approval by our Board of Directors), such amendment or proposal may be approved by the stockholders entitled to cast a majority of the votes entitled to be cast on such a matter. The “continuing directors” are defined in our charter as our current directors, as well as those directors whose nomination for election by the stockholders or whose election by the directors to fill vacancies is approved by a majority of the continuing directors then on the Board of Directors.
Our charter and bylaws provide that the Board of Directors will have the exclusive power to make, alter, amend or repeal any provision of our bylaws.
No Appraisal Rights
Except with respect to appraisal rights arising in connection with the Control Share Act discussed below, as permitted by the Maryland General Corporation Law, our charter provides that stockholders will not be entitled to exercise appraisal rights.
Control Share Acquisitions
The Maryland Control Share Acquisition Act (the “Control Share Act”) provides that control shares of a Maryland corporation acquired in a control share acquisition have no voting rights except to the extent approved by a vote of two-thirds of the votes entitled to be cast on the matter. Shares owned by the acquiror, by officers or by directors who are employees of the corporation are excluded from shares entitled to vote on the matter. Control shares are voting shares of stock which, if aggregated with all other shares of stock owned by the acquiror or in respect of which the acquiror is able to exercise or direct the exercise of voting power (except solely by virtue of a revocable proxy), would entitle the acquiror to exercise voting power in electing directors within one of the following ranges of voting power:
| • | one-tenth or more but less than one-third; |
| • | one-third or more but less than a majority; or |
| • | a majority or more of all voting power. |
The requisite stockholder approval must be obtained each time an acquiror crosses one of the thresholds of voting power set forth above. Control shares do not include shares the acquiring person is then entitled to vote as a result of having previously obtained stockholder approval. A control share acquisition means the acquisition of control shares, subject to certain exceptions.
A person who has made or proposes to make a control share acquisition may compel the Board of Directors of the corporation to call a special meeting of stockholders to be held within 50 days of demand to consider the voting rights of the shares. The right to compel the calling of a special meeting is subject to the satisfaction of certain conditions, including an undertaking to pay the expenses of the meeting. If no request for a meeting is made, the corporation may itself present the question at any stockholders meeting.
234
If voting rights are not approved at the meeting or if the acquiring person does not deliver an acquiring person statement as required by the statute, then the corporation may repurchase for fair value any or all of the control shares, except those for which voting rights have previously been approved. The right of the corporation to repurchase control shares is subject to certain conditions and limitations. Fair value is determined, without regard to the absence of voting rights for the control shares, as of the date of the last control share acquisition by the acquiror or of any meeting of stockholders at which the voting rights of the shares are considered and not approved. If voting rights for control shares are approved at a stockholders meeting and the acquiror becomes entitled to vote a majority of the shares entitled to vote, all other stockholders may exercise appraisal rights. The fair value of the shares as determined for purposes of appraisal rights may not be less than the highest price per share paid by the acquiror in the control share acquisition.
The Control Share Act does not apply (a) to shares acquired in a merger, consolidation or share exchange if the corporation is a party to the transaction or (b) to acquisitions approved or exempted by the charter or bylaws of the corporation.
Our bylaws contain a provision exempting from the Control Share Act any and all acquisitions by any person of our shares of stock.
Business Combinations
Under the Maryland Business Combination Act (the “Business Combination Act”), “business combinations” between a Maryland corporation and an interested stockholder or an affiliate of an interested stockholder are prohibited for five years after the most recent date on which the interested stockholder becomes an interested stockholder. These business combinations include a merger, consolidation, share exchange or, in circumstances specified in the statute, an asset transfer or issuance or reclassification of equity securities. An interested stockholder is defined as:
| • | any person who beneficially owns 10% or more of the voting power of the corporation’s shares; or |
| • | an affiliate or associate of the corporation who, at any time within the two-year period prior to the date in question, was the beneficial owner of 10% or more of the voting power of the then outstanding voting stock of the corporation. |
A person is not an interested stockholder under this statute if the Board of Directors approved in advance the transaction by which such stockholder otherwise would have become an interested stockholder. However, in approving a transaction, the Board of Directors may provide that its approval is subject to compliance, at or after the time of approval, with any terms and conditions determined by the board.
After the 5-year prohibition, any business combination between the Maryland corporation and an interested stockholder generally must be recommended by the Board of Directors of the corporation and approved by the affirmative vote of at least:
| • | 80% of the votes entitled to be cast by holders of outstanding shares of voting stock of the corporation; and |
| • | two-thirds of the votes entitled to be cast by holders of voting stock of the corporation other than shares held by the interested stockholder with whom or with whose affiliate the business combination is to be effected or held by an affiliate or associate of the interested stockholder. |
These super-majority vote requirements do not apply if the corporation’s common stockholders receive a minimum price, as defined under Maryland law, for their shares in the form of cash or other consideration in the same form as previously paid by the interested stockholder for its shares.
The statute permits various exemptions from its provisions, including business combinations that are exempted by the Board of Directors before the time that the interested stockholder becomes an interested
235
stockholder. Our Board of Directors has adopted a resolution exempting any business combination between us and any other person from the provisions of the Business Combination Act, provided that the business combination is first approved by the Board of Directors, including a majority of the directors who are not interested persons as defined in the 1940 Act.
Conflict with 1940 Act
Our bylaws provide that, if and to the extent that any provision of the Maryland General Corporation Law, or any provision of our charter or bylaws conflicts with any provision of the 1940 Act, the applicable provision of the 1940 Act will control.
Regulatory Restrictions
Our wholly-owned subsidiaries, HT II and HT III, have obtained SBIC licenses. The SBA prohibits, without prior SBA approval, a “change of control” or transfers which would result in any person (or group of persons acting in concert) owning 10% or more of any class of capital stock of a SBIC. A “change of control” is any event which would result in a transfer of the power, direct or indirect, to direct the management and policies of a SBIC, whether through ownership, contractual arrangements or otherwise.
236
DESCRIPTION OF OUR PREFERRED STOCK
In addition to shares of common stock, our charter authorizes the issuance of preferred stock. We may issue preferred stock from time to time in one or more classes or series, without stockholder approval. If we offer preferred stock under this prospectus we will issue an appropriate prospectus supplement. Prior to issuance of shares of each class or series, our Board of Directors is required by Maryland law and by our charter to set the terms, preferences, conversion or other rights, voting powers, restrictions, limitations as to dividends or other distributions, qualifications and terms or conditions of redemption for each class or series. Thus, the Board of Directors could authorize the issuance of shares of preferred stock with terms and conditions that could have the effect of delaying, deferring or preventing a transaction or a change in control that might involve a premium price for holders of our common stock or otherwise be in their best interest. You should note, however, that any such an issuance must adhere to the requirements of the 1940 Act, Maryland law and any other limitations imposed by law.
The following is a general description of the terms of the preferred stock we may issue from time to time. Particular terms of any preferred stock we offer will be described in the prospectus supplement accompanying each preferred share offering.
The 1940 Act requires, among other things, that (i) immediately after issuance and before any dividend or other distribution is made with respect to our common stock and before any purchase of common stock is made, such preferred stock together with all other senior securities must not exceed an amount equal to 50% of our total assets after deducting the amount of such dividend, distribution or purchase price, as the case may be, (ii) the holders of shares of preferred stock, if any are issued, must be entitled as a class to elect two directors at all times and to elect a majority of the directors if dividends or other distribution on the preferred stock are in arrears by two years or more, and (iii) such shares be cumulative as to distributions and have a complete preference over our common stock to payment of their liquidation in event of dissolution. Some matters under the 1940 Act require the separate vote of the holders of any issued and outstanding preferred stock. For example, holders of preferred stock would vote separately from the holders of common stock on a proposal to cease operations as a business development company. We believe that the availability for issuance of preferred stock will provide us with increased flexibility in structuring future financings and acquisitions.
For any series of preferred stock that we may issue, our Board of Directors will determine and the articles supplementary and the prospectus supplement relating to such series will describe:
| • | the designation and number of shares of such series; |
| • | the rate and time at which, and the preferences and conditions under which, any dividends or other distributions will be paid on shares of such series, as well as whether such dividends or other distributions are participating or non-participating; |
| • | any provisions relating to convertibility or exchangeability of the shares of such series, including adjustments to the conversion price of such series; |
| • | the rights and preferences, if any, of holders of shares of such series upon our liquidation, dissolution or winding up of our affairs; |
| • | the voting powers, if any, of the holders of shares of such series; |
| • | any provisions relating to the redemption of the shares of such series; |
| • | any limitations on our ability to pay dividends or make distributions on, or acquire or redeem, other securities while shares of such series are outstanding; |
| • | any conditions or restrictions on our ability to issue additional shares of such series or other securities; |
| • | if applicable, a discussion of certain U.S. federal income tax considerations; and |
237
| • | any other relative powers, preferences and participating, optional or special rights of shares of such series, and the qualifications, limitations or restrictions thereof. |
All shares of preferred stock that we may issue will be identical and of equal rank except as to the particular terms thereof that may be fixed by our Board of Directors, and all shares of each series of preferred stock will be identical and of equal rank except as to the dates from which dividends or other distributions, if any, thereon will be cumulative. To the extent we issue preferred stock, the payment of distributions to holders of our preferred stock will take priority over payment of distributions to our common stockholders.
238
DESCRIPTION OF OUR SUBSCRIPTION RIGHTS
The following is a general description of the terms of the subscription rights we may issue from time to time. Particular terms of any subscription rights we offer will be described in the prospectus supplement relating to such subscription rights.
We may issue subscription rights to our stockholders to purchase common stock. Subscription rights may be issued independently or together with any other offered security and may or may not be transferable by the person purchasing or receiving the subscription rights. In connection with a subscription rights offering to our stockholders, we would distribute certificates evidencing the subscription rights and a prospectus supplement to our stockholders on the record date that we set for receiving subscription rights in such subscription rights offering.
Our stockholders will indirectly bear all of the expenses of the subscription rights offering, regardless of whether our stockholders exercise any subscription rights.
A prospectus supplement will describe the particular terms of any subscription rights we may issue, including the following:
| • | the period of time the offering would remain open (which shall be open a minimum number of days such that all record holders would be eligible to participate in the offering and shall not be open longer than 120 days); |
| • | the title and aggregate number of such subscription rights; |
| • | the exercise price for such subscription rights (or method of calculation thereof); |
| • | the currency or currencies, including composite currencies, in which the price of such subscription rights may be payable; |
| • | if applicable, the designation and terms of the securities with which the subscription rights are issued and the number of subscription rights issued with each such security or each principal amount of such security; |
| • | the ratio of the offering (which, in the case of transferable rights, will require a minimum of three shares to be held of record before a person is entitled to purchase an additional share); |
| • | the number of such subscription rights issued to each stockholder; |
| • | the extent to which such subscription rights are transferable and the market on which they may be traded if they are transferable; |
| • | the date on which the right to exercise such subscription rights shall commence, and the date on which such right shall expire (subject to any extension); |
| • | if applicable, the minimum or maximum number of subscription rights that may be exercised at one time; |
| • | the extent to which such subscription rights include an over-subscription privilege with respect to unsubscribed securities and the terms of such over-subscription privilege; |
| • | any termination right we may have in connection with such subscription rights offering; |
| • | the terms of any rights to redeem, or call such subscription rights; |
| • | information with respect to book-entry procedures, if any; |
| • | the terms of the securities issuable upon exercise of the subscription rights; |
| • | the material terms of any standby underwriting, backstop or other purchase arrangement that we may enter into in connection with the subscription rights offering; |
239
| • | if applicable, a discussion of certain U.S. federal income tax considerations applicable to the issuance or exercise of such subscription rights; and |
| • | any other terms of such subscription rights, including exercise, settlement and other procedures and limitations relating to the transfer and exercise of such subscription rights. |
Each subscription right will entitle the holder of the subscription right to purchase for cash or other consideration such amount of shares of common stock at such subscription price as shall in each case be set forth in, or be determinable as set forth in, the prospectus supplement relating to the subscription rights offered thereby. Subscription rights may be exercised as set forth in the prospectus supplement beginning on the date specified therein and continuing until the close of business on the expiration date for such subscription rights set forth in the prospectus supplement. After the close of business on the expiration date, all unexercised subscription rights will become void.
Upon receipt of payment and the subscription rights certificate properly completed and duly executed at the corporate trust office of the subscription rights agent or any other office indicated in the prospectus supplement we will forward, as soon as practicable, the shares of common stock purchasable upon such exercise. If less than all of the rights represented by such subscription rights certificate are exercised, a new subscription certificate will be issued for the remaining rights. Prior to exercising their subscription rights, holders of subscription rights will not have any of the rights of holders of the securities purchasable upon such exercise. To the extent permissible under applicable law, we may determine to offer any unsubscribed offered securities directly to persons other than stockholders, to or through agents, underwriters or dealers or through a combination of such methods, as set forth in the applicable prospectus supplement.
Under the 1940 Act, we may generally only offer subscription rights (other than rights to subscribe expiring not later than 120 days after their issuance and issued exclusively and ratably to a class or classes of our security holders) on the condition that (1) the subscription rights expire by their terms within ten years; (2) the exercise price is not less than the current market value at the date of issuance; (3) our stockholders authorize the proposal to issue such subscription rights, and a “required” majority of our Board of Directors approves of such issuance on the basis that the issuance is in the best interests of the Company and our stockholders; and (4) if the subscription rights are accompanied by other securities, the subscription rights are not separately transferable unless no class of such subscription rights and the securities accompanying them has been publicly distributed. A “required” majority of our Board of Directors is a vote of both a majority of our directors who have no financial interest in the transaction and a majority of the directors who are not interested persons of the company. The 1940 Act also provides that the amount of our voting securities that would result from the exercise of all outstanding warrants, options and subscription rights at the time of issuance may not exceed 25% of our outstanding voting securities.
For information regarding the dilutive impact of rights offerings, please see “Risk Factors—Risks Related to Our Securities” Your interest in us may be diluted if you do not fully exercise your subscription rights in any rights offering. In addition, if the subscription price is less than our NAV per share, then you will experience an immediate dilution of the aggregate NAV of your shares.”
240
The following is a general description of the terms of the warrants we may issue from time to time. Particular terms of any warrants we offer will be described in the prospectus supplement relating to such warrants and will be subject to compliance with the 1940 Act.
We may issue warrants to purchase shares of our common stock, preferred stock or debt securities. Such warrants may be issued independently or together with shares of common stock, preferred stock or debt securities and may be attached or separate from such securities. We will issue each series of warrants under a separate warrant agreement to be entered into between us and a warrant agent. The warrant agent will act solely as our agent and will not assume any obligation or relationship of agency for or with holders or beneficial owners of warrants.
A prospectus supplement will describe the particular terms of any series of warrants we may issue, including the following:
| • | the title and aggregate number of such warrants; |
| • | the price or prices at which such warrants will be issued; |
| • | the currency or currencies, including composite currencies, in which the price of such warrants may be payable; |
| • | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| • | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at which and the currency or currencies, including composite currencies, in which this principal amount of debt securities may be purchased upon such exercise; |
| • | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon exercise of one warrant and the price at which and the currency or currencies, including composite currencies, in which these shares may be purchased upon such exercise; |
| • | the date on which the right to exercise such warrants shall commence and the date on which such right will expire (subject to any extension); |
| • | whether such warrants will be issued in registered form or bearer form; |
| • | if applicable, the minimum or maximum amount of such warrants that may be exercised at any one time; |
| • | if applicable, the date on and after which such warrants and the related securities will be separately transferable; |
| • | the terms of any rights to redeem, or call such warrants; |
| • | information with respect to book-entry procedures, if any; |
| • | the terms of the securities issuable upon exercise of the warrants; |
| • | if applicable, a discussion of certain U.S. federal income tax considerations; and |
| • | any other terms of such warrants, including terms, procedures and limitations relating to the exchange and exercise of such warrants. |
We and the warrant agent may amend or supplement the warrant agreement for a series of warrants without the consent of the holders of the warrants issued thereunder to effect changes that are not inconsistent with the provisions of the warrants and that do not materially and adversely affect the interests of the holders of the warrants.
241
Each warrant will entitle the holder to purchase for cash such common stock or preferred stock at the exercise price or such principal amount of debt securities as shall in each case be set forth in, or be determinable as set forth in, the prospectus supplement relating to the warrants offered thereby. Warrants may be exercised as set forth in the prospectus supplement beginning on the date specified therein and continuing until the close of business on the expiration date set forth in the prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Upon receipt of payment and a warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the prospectus supplement, we will, as soon as practicable, forward the securities purchasable upon such exercise. If less than all of the warrants represented by such warrant certificate are exercised, a new warrant certificate will be issued for the remaining warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Prior to exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including, in the case of warrants to purchase debt securities, the right to receive principal, premium, if any, or interest payments, on the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture or, in the case of warrants to purchase common stock or preferred stock, the right to receive dividends or other distributions, if any, or payments upon our liquidation, dissolution or winding up or to exercise any voting rights.
Under the 1940 Act, we may generally only offer warrants provided that (i) the warrants expire by their terms within ten years, (ii) the exercise or conversion price is not less than the current market value at the date of issuance, (iii) our stockholders authorize the proposal to issue such warrants, and our Board of Directors approves such issuance on the basis that the issuance is in the best interests of the Company and its stockholders and (iv) if the warrants are accompanied by other securities, the warrants are not separately transferable unless no class of such warrants and the securities accompanying them has been publicly distributed. The 1940 Act also provides that the amount of our voting securities that would result from the exercise of all outstanding warrants, as well as options and rights, at the time of issuance may not exceed 25% of our outstanding voting securities.
242
DESCRIPTION OF OUR DEBT SECURITIES
We may issue debt securities in one or more series. The specific terms of each series of debt securities will be described in this prospectus and in the particular prospectus supplement relating to that series. The prospectus supplement may or may not modify the general terms found in this prospectus and will be filed with the SEC. For a complete description of the terms of a particular series of debt securities, including any supplemental indenture, you should read both this prospectus and the prospectus supplement relating to that particular series.
As required by federal law for all bonds and notes of companies that are publicly offered, the debt securities are governed by a document called an “indenture.” An indenture is a contract between us and U.S. Bank National Association, a financial institution acting as trustee on your behalf, and is subject to and governed by the Trust Indenture Act of 1939, as amended. The trustee has two main roles. First, the trustee can enforce your rights against us if we default. There are some limitations on the extent to which the trustee acts on your behalf, described in the second paragraph under “Events of Default—Remedies if an Event of Default Occurs.” Second, the trustee performs certain administrative duties for us.
Because this section is a summary, it does not describe every aspect of the debt securities and the indenture. The following description summarizes the material provisions of the indenture. We urge you to read the indenture because it, and not this description, defines your rights as a holder of debt securities. For example, in this section, we use capitalized words to signify terms that are specifically defined in the indenture. We have filed the form of the indenture with the SEC. See “Available Information” for information on how to obtain a copy of the indenture.
A prospectus supplement, which will accompany this prospectus, will describe the particular terms of any series of debt securities being offered, including the following:
| • | the designation or title of the series of debt securities; |
| • | the total principal amount of the series of debt securities; |
| • | the percentage of the principal amount at which the series of debt securities will be offered; |
| • | the date or dates on which principal will be payable; |
| • | the rate or rates (which may be either fixed or variable) and/or the method of determining such rate or rates of interest, if any; |
| • | the date or dates from which any interest will accrue, or the method of determining such date or dates, and the date or dates on which any interest will be payable; |
| • | the terms for redemption, extension or early repayment, if any; |
| • | the currencies in which the series of debt securities are issued and payable; |
| • | whether the amount of payments of principal, premium or interest, if any, on a series of debt securities will be determined with reference to an index, formula or other method (which could be based on one or more currencies, commodities, equity indices or other indices) and how these amounts will be determined; |
| • | the place or places, if any, other than or in addition to the City of New York, of payment, transfer, conversion and/or exchange of the debt securities; |
| • | the denominations in which the offered debt securities will be issued; |
| • | the provision for any sinking fund; |
| • | any restrictive covenants; |
| • | any Events of Default; |
243
| • | whether the series of debt securities are issuable in certificated form; |
| • | any provisions for defeasance or covenant defeasance; |
| • | if applicable, U.S. federal income tax considerations relating to OID; |
| • | whether and under what circumstances we will pay additional amounts in respect of any tax, assessment or governmental charge and, if so, whether we will have the option to redeem the debt securities rather than pay the additional amounts (and the terms of this option); |
| • | any provisions for convertibility or exchangeability of the debt securities into or for any other securities; |
| • | whether the debt securities are subject to subordination and the terms of such subordination; |
| • | the listing, if any, on a securities exchange; and |
| • | any other terms. |
The debt securities may be secured or unsecured obligations. Unless the prospectus supplement states otherwise, principal (and premium, if any) and interest, if any, will be paid by us in immediately available funds.
We are permitted, under specified conditions, to issue multiple classes of indebtedness if our asset coverage, as defined in the 1940 Act, is at least equal to 200% immediately after each such issuance. In addition, while any indebtedness and other senior securities remain outstanding, we must make provisions to prohibit any distribution to our stockholders or the repurchase of such securities or shares unless we meet the applicable asset coverage ratios at the time of the distribution or repurchase. We may also borrow amounts up to 5% of the value of our total assets for temporary or emergency purposes without regard to asset coverage. For a discussion of the risks associated with leverage, see “Risk Factors—Risks Related to Our Business Structure.”
General
The indenture provides that any debt securities proposed to be sold under this prospectus and the attached prospectus supplement (“offered debt securities”) and any debt securities issuable upon the exercise of warrants or upon conversion or exchange of other offered securities (“underlying debt securities”), may be issued under the indenture in one or more series.
For purposes of this prospectus, any reference to the payment of principal of or premium or interest, if any, on debt securities will include additional amounts if required by the terms of the debt securities.
The indenture does not limit the amount of debt securities that may be issued thereunder from time to time. Debt securities issued under the indenture, when a single trustee is acting for all debt securities issued under the indenture, are called the “indenture securities.” The indenture also provides that there may be more than one trustee thereunder, each with respect to one or more different series of indenture securities. See “Resignation of Trustee” section below. At a time when two or more trustees are acting under the indenture, each with respect to only certain series, the term “indenture securities” means the one or more series of debt securities with respect to which each respective trustee is acting. In the event that there is more than one trustee under the indenture, the powers and trust obligations of each trustee described in this prospectus will extend only to the one or more series of indenture securities for which it is trustee. If two or more trustees are acting under the indenture, then the indenture securities for which each trustee is acting would be treated as if issued under separate indentures.
We refer you to the prospectus supplement for information with respect to any deletions from, modifications of or additions to the Events of Default or our covenants that are described below, including any addition of a covenant or other provision providing event risk or similar protection.
244
We have the ability to issue indenture securities with terms different from those of indenture securities previously issued and, without the consent of the holders thereof, to reopen a previous issue of a series of indenture securities and issue additional indenture securities of that series unless the reopening was restricted when that series was created.
Conversion and Exchange
If any debt securities are convertible into or exchangeable for other securities, the prospectus supplement will explain the terms and conditions of the conversion or exchange, including the conversion price or exchange ratio (or the calculation method), the conversion or exchange period (or how the period will be determined), if conversion or exchange will be mandatory or at the option of the holder or us, provisions for adjusting the conversion price or the exchange ratio and provisions affecting conversion or exchange in the event of the redemption of the underlying debt securities. These terms may also include provisions under which the number or amount of other securities to be received by the holders of the debt securities upon conversion or exchange would be calculated according to the market price of the other securities as of a time stated in the prospectus supplement.
Issuance of Securities in Registered Form
We may issue the debt securities in registered form, in which case we may issue them either in book-entry form only or in “certificated” form. Debt securities issued in book-entry form will be represented by global securities. We expect that we will usually issue debt securities in book-entry only form represented by global securities.
Book-Entry Holders
We will issue registered debt securities in book-entry form only, unless we specify otherwise in the applicable prospectus supplement. This means debt securities will be represented by one or more global securities registered in the name of a depositary that will hold them on behalf of financial institutions that participate in the depositary’s book-entry system. These participating institutions, in turn, hold beneficial interests in the debt securities held by the depositary or its nominee. These institutions may hold these interests on behalf of themselves or customers.
Under the indenture, only the person in whose name a debt security is registered is recognized as the holder of that debt security. Consequently, for debt securities issued in book-entry form, we will recognize only the depositary as the holder of the debt securities and we will make all payments on the debt securities to the depositary. The depositary will then pass along the payments it receives to its participants, which in turn will pass the payments along to their customers who are the beneficial owners. The depositary and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so under the terms of the debt securities.
As a result, investors will not own debt securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other financial institution that participates in the depositary’s book-entry system or holds an interest through a participant. As long as the debt securities are represented by one or more global securities, investors will be indirect holders, and not holders, of the debt securities.
Street Name Holders
In the future, we may issue debt securities in certificated form or terminate a global security. In these cases, investors may choose to hold their debt securities in their own names or in “street name.” Debt securities held in street name are registered in the name of a bank, broker or other financial institution chosen by the investor, and the investor would hold a beneficial interest in those debt securities through the account he or she maintains at that institution.
245
For debt securities held in street name, we will recognize only the intermediary banks, brokers and other financial institutions in whose names the debt securities are registered as the holders of those debt securities and we will make all payments on those debt securities to them. These institutions will pass along the payments they receive to their customers who are the beneficial owners, but only because they agree to do so in their customer agreements or because they are legally required to do so. Investors who hold debt securities in street name will be indirect holders, and not holders, of the debt securities.
Legal Holders
Our obligations, as well as the obligations of the applicable trustee and those of any third parties employed by us or the applicable trustee, run only to the legal holders of the debt securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect means. This will be the case whether an investor chooses to be an indirect holder of a debt security or has no choice because we are issuing the debt securities only in book-entry form.
For example, once we make a payment or give a notice to the holder, we have no further responsibility for the payment or notice even if that holder is required, under agreements with depositary participants or customers or by law, to pass it along to the indirect holders but does not do so. Similarly, if we want to obtain the approval of the holders for any purpose (for example, to amend an indenture or to relieve us of the consequences of a default or of our obligation to comply with a particular provision of an indenture), we would seek the approval only from the holders, and not the indirect holders, of the debt securities. Whether and how the holders contact the indirect holders is up to the holders.
When we refer to you, we mean those who invest in the debt securities being offered by this prospectus, whether they are the holders or only indirect holders of those debt securities. When we refer to your debt securities, we mean the debt securities in which you hold a direct or indirect interest.
Special Considerations for Indirect Holders
If you hold debt securities through a bank, broker or other financial institution, either in book-entry form or in street name, we urge you to check with that institution to find out:
| • | how it handles securities payments and notices, |
| • | whether it imposes fees or charges, |
| • | how it would handle a request for the holders’ consent, if ever required, |
| • | whether and how you can instruct it to send you debt securities registered in your own name so you can be a holder, if that is permitted in the future for a particular series of debt securities, |
| • | how it would exercise rights under the debt securities if there were a default or other event triggering the need for holders to act to protect their interests, and |
| • | if the debt securities are in book-entry form, how the depositary’s rules and procedures will affect these matters. |
Global Securities
As noted above, we usually will issue debt securities as registered securities in book-entry form only. A global security represents one or any other number of individual debt securities. Generally, all debt securities represented by the same global securities will have the same terms.
Each debt security issued in book-entry form will be represented by a global security that we deposit with and register in the name of a financial institution or its nominee that we select. The financial institution that we
246
select for this purpose is called the depositary. Unless we specify otherwise in the applicable prospectus supplement, The Depository Trust Company, New York, New York, known as DTC, will be the depositary for all debt securities issued in book-entry form.
A global security may not be transferred to or registered in the name of anyone other than the depositary or its nominee, unless special termination situations arise. We describe those situations below under “Special Situations when a Global Security Will Be Terminated.” As a result of these arrangements, the depositary, or its nominee, will be the sole registered owner and holder of all debt securities represented by a global security, and investors will be permitted to own only beneficial interests in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial institution that in turn has an account with the depositary or with another institution that has an account with the depositary. Thus, an investor whose security is represented by a global security will not be a holder of the debt security, but only an indirect holder of a beneficial interest in the global security.
Special Considerations for Global Securities
As an indirect holder, an investor’s rights relating to a global security will be governed by the account rules of the investor’s financial institution and of the depositary, as well as general laws relating to securities transfers. The depositary that holds the global security will be considered the holder of the debt securities represented by the global security.
If debt securities are issued only in the form of a global security, an investor should be aware of the following:
| • | An investor cannot cause the debt securities to be registered in his or her name, and cannot obtain certificates for his or her interest in the debt securities, except in the special situations we describe below. |
| • | An investor will be an indirect holder and must look to his or her own bank or broker for payments on the debt securities and protection of his or her legal rights relating to the debt securities, as we describe under “Issuance of Securities in Registered Form” above. |
| • | An investor may not be able to sell interests in the debt securities to some insurance companies and other institutions that are required by law to own their securities in non-book-entry form. |
| • | An investor may not be able to pledge his or her interest in a global security in circumstances where certificates representing the debt securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective. |
| • | The depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in a global security. We and the trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in a global security. We and the trustee also do not supervise the depositary in any way. |
| • | If we redeem less than all the debt securities of a particular series being redeemed, DTC’s practice is to determine by lot the amount to be redeemed from each of its participants holding that series. |
| • | An investor is required to give notice of exercise of any option to elect repayment of its debt securities, through its participant, to the applicable trustee and to deliver the related debt securities by causing its participant to transfer its interest in those debt securities, on DTC’s records, to the applicable trustee. |
| • | DTC requires that those who purchase and sell interests in a global security deposited in its book-entry system use immediately available funds. Your broker or bank may also require you to use immediately available funds when purchasing or selling interests in a global security. |
| • | Financial institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in a global security, may also have their own policies affecting payments, |
247
| notices and other matters relating to the debt securities. There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries. |
Special Situations when a Global Security will be Terminated
In a few special situations described below, a global security will be terminated and interests in it will be exchanged for certificates in non-book-entry form (certificated securities). After that exchange, the choice of whether to hold the certificated debt securities directly or in street name will be up to the investor. Investors must consult their own banks or brokers to find out how to have their interests in a global security transferred on termination to their own names, so that they will be holders. We have described the rights of legal holders and street name investors under “Issuance of Securities in Registered Form” above.
The prospectus supplement may list situations for terminating a global security that would apply only to the particular series of debt securities covered by the prospectus supplement. If a global security is terminated, only the depositary, and not we or the applicable trustee, is responsible for deciding the names of the institutions in whose names the debt securities represented by the global security will be registered and, therefore, who will be the holders of those debt securities.
Payment and Paying Agents
We will pay interest to the person listed in the applicable trustee’s records as the owner of the debt security at the close of business on a particular day in advance of each due date for interest, even if that person no longer owns the debt security on the interest due date. That day, often approximately two weeks in advance of the interest due date, is called the “record date.” Because we will pay all the interest for an interest period to the holders on the record date, holders buying and selling debt securities must work out between themselves the appropriate purchase price. The most common manner is to adjust the sales price of the debt securities to prorate interest fairly between buyer and seller based on their respective ownership periods within the particular interest period. This prorated interest amount is called “accrued interest.”
Payments on Global Securities
We will make payments on a global security in accordance with the applicable policies of the depositary as in effect from time to time. Under those policies, we will make payments directly to the depositary, or its nominee, and not to any indirect holders who own beneficial interests in the global security. An indirect holder’s right to those payments will be governed by the rules and practices of the depositary and its participants.
Payments on Certificated Securities
We will make payments on a certificated debt security as follows. We will pay interest that is due on an interest payment date by check mailed on the interest payment date to the holder at his or her address shown on the trustee’s records as of the close of business on the regular record date. We will make all payments of principal and premium, if any, by check at the office of the applicable trustee in New York, New York and/or at other offices that may be specified in the prospectus supplement or in a notice to holders against surrender of the debt security.
Alternatively, if the holder asks us to do so, we will pay any amount that becomes due on the debt security by wire transfer of immediately available funds to an account at a bank in New York City, on the due date. To request payment by wire, the holder must give the applicable trustee or other paying agent appropriate transfer instructions at least 15 business days before the requested wire payment is due. In the case of any interest payment due on an interest payment date, the instructions must be given by the person who is the holder on the relevant regular record date. Any wire instructions, once properly given, will remain in effect unless and until new instructions are given in the manner described above.
248
Payment when Offices are Closed
If any payment is due on a debt security on a day that is not a business day, we will make the payment on the next day that is a business day. Payments made on the next business day in this situation will be treated under the indenture as if they were made on the original due date, except as otherwise indicated in the attached prospectus supplement. Such payment will not result in a default under any debt security or the indenture, and no interest will accrue on the payment amount from the original due date to the next day that is a business day.
Book-entry and other indirect holders should consult their banks or brokers for information on how they will receive payments on their debt securities.
Events of Default
You will have rights if an Event of Default occurs in respect of the debt securities of your series and is not cured, as described later in this subsection.
The term “Event of Default” in respect of the debt securities of your series means any of the following (unless the prospectus supplement relating to such debt securities states otherwise):
| • | we do not pay the principal of, or any premium on, a debt security of the series on its due date, and do not cure this default within five days; |
| • | we do not pay interest on a debt security of the series when due, and such default is not cured within 30 days; |
| • | we do not deposit any sinking fund payment in respect of debt securities of the series on its due date, and do not cure this default within five days; |
| • | we remain in breach of a covenant in respect of debt securities of the series for 60 days after we receive a written notice of default stating we are in breach. The notice must be sent by either the trustee or holders of at least 25% of the principal amount of debt securities of the series; |
| • | we file for bankruptcy or certain other events of bankruptcy, insolvency or reorganization occur and remain undischarged or unstayed for a period of 60 days; |
| • | on the last business day of each of 24 consecutive calendar months, we have an asset coverage of less than 100%; and |
| • | any other Event of Default in respect of debt securities of the series described in the applicable prospectus supplement occurs. |
An Event of Default for a particular series of debt securities does not necessarily constitute an Event of Default for any other series of debt securities issued under the same or any other indenture. The trustee may withhold notice to the holders of debt securities of any default, except in the payment of principal, premium or interest, if it considers the withholding of notice to be in the best interests of the holders.
Remedies if an Event of Default Occurs
If an Event of Default has occurred and has not been cured, the trustee or the holders of at least 25% in principal amount of the debt securities of the affected series may declare the entire principal amount of all the debt securities of that series to be due and immediately payable. This is called a declaration of acceleration of maturity. In certain circumstances, a declaration of acceleration of maturity may be canceled by the holders of a majority in principal amount of the debt securities of the affected series.
The trustee is not required to take any action under the indenture at the request of any holders unless the holders offer the trustee reasonable protection from expenses and liability (called an “indemnity”). If reasonable
249
indemnity is provided, the holders of a majority in principal amount of the outstanding debt securities of the relevant series may direct the time, method and place of conducting any lawsuit or other formal legal action seeking any remedy available to the trustee. The trustee may refuse to follow those directions in certain circumstances. No delay or omission in exercising any right or remedy will be treated as a waiver of that right, remedy or Event of Default.
Before you are allowed to bypass your trustee and bring your own lawsuit or other formal legal action or take other steps to enforce your rights or protect your interests relating to the debt securities, the following must occur:
| • | the holder must give your trustee written notice that an Event of Default has occurred and remains uncured; |
| • | the holders of at least 25% in principal amount of all outstanding debt securities of the relevant series must make a written request that the trustee take action because of the default and must offer reasonable indemnity to the trustee against the cost and other liabilities of taking that action; |
| • | the trustee must not have taken action for 60 days after receipt of the above notice and offer of indemnity; and |
| • | the holders of a majority in principal amount of the debt securities must not have given the trustee a direction inconsistent with the above notice during that 60 day period. |
However, you are entitled at any time to bring a lawsuit for the payment of money due on your debt securities on or after the due date.
Holders of a majority in principal amount of the debt securities of the affected series may waive any past defaults other than:
| • | the payment of principal, any premium or interest; or |
| • | in respect of a covenant that cannot be modified or amended without the consent of each holder. |
Book-entry and other indirect holders should consult their banks or brokers for information on how to give notice or direction to or make a request of the trustee and how to declare or cancel an acceleration of maturity.
Each year, we will furnish to each trustee a written statement of certain of our officers certifying that to their knowledge we are in compliance with the indenture and the debt securities, or else specifying any default.
Merger or Consolidation
Under the terms of the indenture, we are generally permitted to consolidate or merge with another entity. We may also be permitted to sell all or substantially all of our assets to another entity. However, unless the prospectus supplement relating to certain debt securities states otherwise, we may not take any of these actions unless all the following conditions are met:
| • | where we merge out of existence or sell our assets, the resulting entity must agree to be legally responsible for our obligations under the debt securities; |
| • | immediately after giving effect to such transaction, no Default or Event of Default shall have happened and be continuing; |
| • | under the indenture, no merger or sale of assets may be made if as a result any of our property or assets or any property or assets of one of our subsidiaries, if any, would become subject to any mortgage, lien or other encumbrance unless either (a) the mortgage, lien or other encumbrance could be created |
250
| • | pursuant to the limitation on liens covenant in the indenture without equally and ratably securing the indenture securities or (b) the indenture securities are secured equally and ratably with or prior to the debt secured by the mortgage, lien or other encumbrance; |
| • | we must deliver certain certificates and documents to the trustee; and |
| • | we must satisfy any other requirements specified in the prospectus supplement relating to a particular series of debt securities. |
Modification or Waiver
There are three types of changes we can make to the indenture and the debt securities issued thereunder.
Changes Requiring Approval
First, there are changes that we cannot make to debt securities without specific approval of all of the holders. The following is a list of those types of changes:
| • | change the stated maturity of the principal of or interest on a debt security; |
| • | reduce any amounts due on a debt security; |
| • | reduce the amount of principal payable upon acceleration of the maturity of a security following a default; |
| • | adversely affect any right of repayment at the holder’s option; |
| • | change the place (except as otherwise described in the prospectus or prospectus supplement) or currency of payment on a debt security; |
| • | impair your right to sue for payment; |
| • | adversely affect any right to convert or exchange a debt security in accordance with its terms; |
| • | modify the subordination provisions in the indenture in a manner that is adverse to holders of the debt securities; |
| • | reduce the percentage of holders of debt securities whose consent is needed to modify or amend the indenture; |
| • | reduce the percentage of holders of debt securities whose consent is needed to waive compliance with certain provisions of the indenture or to waive certain defaults; |
| • | modify any other aspect of the provisions of the indenture dealing with supplemental indentures, modification and waiver of past defaults, changes to the quorum or voting requirements or the waiver of certain covenants; and |
| • | change any obligation we have to pay additional amounts. |
Changes Not Requiring Approval
The second type of change does not require any vote by the holders of the debt securities. This type is limited to clarifications and certain other changes that would not adversely affect holders of the outstanding debt securities in any material respect. We also do not need any approval to make any change that affects only debt securities to be issued under the indenture after the change takes effect.
Changes Requiring Majority Approval
Any other change to the indenture and the debt securities would require the following approval:
| • | if the change affects only one series of debt securities, it must be approved by the holders of a majority in principal amount of that series; and |
251
| • | if the change affects more than one series of debt securities issued under the same indenture, it must be approved by the holders of a majority in principal amount of all of the series affected by the change, with all affected series voting together as one class for this purpose. |
The holders of a majority in principal amount of all of the series of debt securities issued under an indenture, voting together as one class for this purpose, may waive our compliance with some of our covenants in that indenture. However, we cannot obtain a waiver of a payment default or of any of the matters covered by the bullet points included above under “—Changes Requiring Approval.”
Further Details Concerning Voting
When taking a vote, we will use the following rules to decide how much principal to attribute to a debt security:
| • | for OID securities, we will use the principal amount that would be due and payable on the voting date if the maturity of these debt securities were accelerated to that date because of a default; |
| • | for debt securities whose principal amount is not known (for example, because it is based on an index), we will use a special rule for that debt security described in the prospectus supplement; and |
| • | for debt securities denominated in one or more foreign currencies, we will use the U.S. dollar equivalent. |
Debt securities will not be considered outstanding, and therefore not eligible to vote, if we have deposited or set aside in trust money for their payment or redemption. Debt securities will also not be eligible to vote if they have been fully defeased as described later under “Defeasance—Full Defeasance.”
We will generally be entitled to set any day as a record date for the purpose of determining the holders of outstanding indenture securities that are entitled to vote or take other action under the indenture. If we set a record date for a vote or other action to be taken by holders of one or more series, that vote or action may be taken only by persons who are holders of outstanding indenture securities of those series on the record date and must be taken within eleven months following the record date.
Book-entry and other indirect holders should consult their banks or brokers for information on how approval may be granted or denied if we seek to change the indenture or the debt securities or request a waiver.
Defeasance
The following provisions will be applicable to each series of debt securities unless we state in the applicable prospectus supplement that the provisions of covenant defeasance and full defeasance will not be applicable to that series.
Covenant Defeasance
Under current U.S. federal tax law, we can make the deposit described below and be released from some of the restrictive covenants in the indenture under which the particular series was issued. This is called “covenant defeasance.” In that event, you would lose the protection of those restrictive covenants but would gain the protection of having money and government securities set aside in trust to repay your debt securities. If applicable, you also would be released from the subordination provisions as described under the “Indenture Provisions—Subordination” section below. In order to achieve covenant defeasance, we must do the following:
| • | if the debt securities of the particular series are denominated in U.S. dollars, we must deposit in trust for the benefit of all holders of such debt securities a combination of money and U.S. government or U.S. government agency notes or bonds that will generate enough cash to make interest, principal and any other payments on the debt securities on their various due dates; |
252
| • | we must deliver to the trustee a legal opinion of our counsel confirming that, under current U.S. federal income tax law, we may make the above deposit without causing you to be taxed on the debt securities any differently than if we did not make the deposit and just repaid the debt securities ourselves at maturity; and |
| • | we must deliver to the trustee a legal opinion of our counsel stating that the above deposit does not require registration by us under the 1940 Act, as amended, and a legal opinion and officers’ certificate stating that all conditions precedent to covenant defeasance have been complied with. |
If we accomplish covenant defeasance, you can still look to us for repayment of the debt securities if there were a shortfall in the trust deposit or the trustee is prevented from making payment. For example, if one of the remaining Events of Default occurred (such as our bankruptcy) and the debt securities became immediately due and payable, there might be a shortfall. Depending on the event causing the default, you may not be able to obtain payment of the shortfall.
Full Defeasance
If there is a change in U.S. federal tax law, as described below, we can legally release ourselves from all payment and other obligations on the debt securities of a particular series (called “full defeasance”) if we put in place the following other arrangements for you to be repaid:
| • | if the debt securities of the particular series are denominated in U.S. dollars, we must deposit in trust for the benefit of all holders of such debt securities a combination of money and United States government or United States government agency notes or bonds that will generate enough cash to make interest, principal and any other payments on the debt securities on their various due dates. |
| • | we must deliver to the trustee a legal opinion confirming that there has been a change in current U.S. federal tax law or an IRS ruling that allows us to make the above deposit without causing you to be taxed on the debt securities any differently than if we did not make the deposit and just repaid the debt securities ourselves at maturity. Under current U.S. federal tax law, the deposit and our legal release from the debt securities would be treated as though we paid you your share of the cash and notes or bonds at the time the cash and notes or bonds were deposited in trust in exchange for your debt securities and you would recognize gain or loss on the debt securities at the time of the deposit; |
| • | we must deliver to the trustee a legal opinion of our counsel stating that the above deposit does not require registration by us under the 1940 Act, as amended, and a legal opinion and officers’ certificate stating that all conditions precedent to defeasance have been complied with; |
| • | Defeasance must not result in a breach of the indenture or any other material agreements; and |
| • | Satisfy the conditions for covenant defeasance contained in any supplemental indentures. |
If we ever did accomplish full defeasance, as described above, you would have to rely solely on the trust deposit for repayment of the debt securities. You could not look to us for repayment in the unlikely event of any shortfall. Conversely, the trust deposit would most likely be protected from claims of our lenders and other creditors if we ever became bankrupt or insolvent. If applicable, you would also be released from the subordination provisions described later under “Indenture Provisions—Subordination.”
Form, Exchange and Transfer of Certificated Registered Securities
Holders may exchange their certificated securities, if any, for debt securities of smaller denominations or combined into fewer debt securities of larger denominations, as long as the total principal amount is not changed.
Holders may exchange or transfer their certificated securities, if any, at the office of their trustee. We have appointed the trustee to act as our agent for registering debt securities in the names of holders transferring debt securities. We may appoint another entity to perform these functions or perform them ourselves.
253
Holders will not be required to pay a service charge to transfer or exchange their certificated securities, if any, but they may be required to pay any tax or other governmental charge associated with the transfer or exchange. The transfer or exchange will be made only if our transfer agent is satisfied with the holder’s proof of legal ownership.
If we have designated additional transfer agents for your debt security, they will be named in your prospectus supplement. We may appoint additional transfer agents or cancel the appointment of any particular transfer agent. We may also approve a change in the office through which any transfer agent acts.
If any certificated securities of a particular series are redeemable and we redeem less than all the debt securities of that series, we may block the transfer or exchange of those debt securities during the period beginning 15 days before the day we mail the notice of redemption and ending on the day of that mailing, in order to freeze the list of holders to prepare the mailing. We may also refuse to register transfers or exchanges of any certificated securities selected for redemption, except that we will continue to permit transfers and exchanges of the unredeemed portion of any debt security that will be partially redeemed.
Resignation of Trustee
Each trustee may resign or be removed with respect to one or more series of indenture securities provided that a successor trustee is appointed to act with respect to these series. In the event that two or more persons are acting as trustee with respect to different series of indenture securities under the indenture, each of the trustees will be a trustee of a trust separate and apart from the trust administered by any other trustee.
Indenture Provisions—Subordination
Upon any distribution of our assets upon our dissolution, winding up, liquidation or reorganization, the payment of the principal of (and premium, if any) and interest, if any, on any indenture securities denominated as subordinated debt securities is to be subordinated to the extent provided in the indenture in right of payment to the prior payment in full of all senior indebtedness (as defined below), but our obligation to you to make payment of the principal of (and premium, if any) and interest, if any, on such subordinated debt securities will not otherwise be affected. In addition, no payment on account of principal (or premium, if any), sinking fund or interest, if any, may be made on such subordinated debt securities at any time unless full payment of all amounts due in respect of the principal (and premium, if any), sinking fund and interest on senior indebtedness has been made or duly provided for in money or money’s worth.
In the event that, notwithstanding the foregoing, any payment by us is received by the trustee in respect of subordinated debt securities or by the holders of any of such subordinated debt securities before all senior indebtedness is paid in full, the payment or distribution must be paid over to the holders of the senior indebtedness or on their behalf for application to the payment of all the senior indebtedness remaining unpaid until all the senior indebtedness has been paid in full, after giving effect to any concurrent payment or distribution to the holders of the senior indebtedness. Subject to the payment in full of all senior indebtedness upon this distribution by us, the holders of such subordinated debt securities will be subrogated to the rights of the holders of the senior indebtedness to the extent of payments made to the holders of the senior indebtedness out of the distributive share of such subordinated debt securities.
By reason of this subordination, in the event of a distribution of our assets upon our insolvency, certain of our senior creditors may recover more, ratably, than holders of any subordinated debt securities. The indenture provides that these subordination provisions will not apply to money and securities held in trust under the defeasance provisions of the indenture.
254
Senior indebtedness is defined in the indenture as the principal of (and premium, if any) and unpaid interest on:
| • | our indebtedness (including indebtedness of others guaranteed by us), whenever created, incurred, assumed or guaranteed, for money borrowed (other than indenture securities issued under the indenture and denominated as subordinated debt securities), unless in the instrument creating or evidencing the same or under which the same is outstanding it is provided that this indebtedness is not senior or prior in right of payment to the subordinated debt securities; and |
| • | renewals, extensions, modifications and refinancings of any of this indebtedness. |
If this prospectus is being delivered in connection with the offering of a series of indenture securities denominated as subordinated debt securities, the accompanying prospectus supplement to this prospectus will set forth the approximate amount of our senior indebtedness outstanding as of a recent date.
Secured Indebtedness
Certain of our indebtedness, including certain series of indenture securities, may be secured. The prospectus supplement for each series of indenture securities will describe the terms of any security interest for such series and will indicate the approximate amount of our secured indebtedness as of a recent date. In the event of a distribution of our assets upon our insolvency, the holders of unsecured indenture securities may recover less, ratably, than holders of any of our secured indebtedness.
The Trustee under the Indenture
U.S. Bank National Association will serve as the trustee under the indenture.
Certain Considerations Relating to Foreign Currencies
Debt securities denominated or payable in foreign currencies may entail significant risks. These risks include the possibility of significant fluctuations in the foreign currency markets, the imposition or modification of foreign exchange controls and potential illiquidity in the secondary market. These risks will vary depending upon the currency or currencies involved and will be more fully described in the applicable prospectus supplement.
255
We may offer, from time to time, in one or more offerings or series, up to $600,000,000 of our common stock, preferred stock, debt securities, subscription rights to purchase shares of our common stock or warrants representing rights to purchase shares of our common stock, preferred stock or debt securities, in one or more underwritten public offerings, at-the-market offerings to or through a market maker or into an existing trading market for the securities, on an exchange, or otherwise, negotiated transactions, block trades, best efforts, auctions or a combination of these methods. The holders of our common stock will indirectly bear any fees and expenses in connection with any such offerings. We may sell the securities through underwriters or dealers, directly to one or more purchasers, including existing stockholders in a rights offering, through agents or through a combination of any such methods of sale. Any underwriter or agent involved in the offer and sale of the securities will be named in the applicable prospectus supplement. A prospectus supplement or supplements will also describe the terms of the offering of the securities, including: the purchase price of the securities and the proceeds we will receive from the sale; any over-allotment options under which underwriters may purchase additional securities from us; any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; any expenses we incur in connection with the sale of such securities; the public offering price; any discounts or concessions allowed or re-allowed or paid to dealers; and any securities exchange or market on which the securities may be listed. Only underwriters named in the applicable prospectus supplement will be underwriters of the securities offered by the applicable prospectus supplement.
The distribution of the securities may be effected from time to time in one or more transactions at a fixed price or prices, which may be changed, at prevailing market prices at the time of sale, at prices related to such prevailing market prices, at negotiated prices, or at prices determined by an auction process, provided, however, that the offering price per share of our common stock, less any underwriting commissions or discounts, must equal or exceed the NAV per share of our common stock at the time of the offering except (1) in connection with a rights offering to our existing stockholders, (2) with the consent of the majority of our voting securities or (3) under such circumstances as the SEC may permit. The price at which securities may be distributed may represent a discount from prevailing market prices. Although we are not currently authorized to issue shares of our common stock at a price below our NAV per share, we may seek stockholder approval of this proposal again at a special meeting of stockholders or our next annual meeting of stockholders. Our Board of Directors, subject to its fiduciary duties and regulatory requirements, has the discretion to determine the amount of the discount, and as a result, the discount could be up to 100% of NAV per share.
In connection with the sale of our securities, underwriters or agents may receive compensation from us or from purchasers of our securities, for whom they may act as agents, in the form of discounts, concessions or commissions. Underwriters may sell our securities to or through dealers and such dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters and/or commissions from the purchasers for whom they may act as agents. Underwriters, dealers and agents that participate in the distribution of our securities may be deemed to be underwriters under the Securities Act, and any discounts and commissions they receive from us and any profit realized by them on the resale of our securities may be deemed to be underwriting discounts and commissions under the Securities Act. Any such underwriter or agent will be identified and any such compensation received from us will be described in the applicable prospectus supplement.
Any underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of
256
the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any underwriters that are qualified market makers on the NYSE may engage in passive market making transactions in our common stock on the NYSE in accordance with Regulation M under the Exchange Act, during the business day prior to the pricing of the offering, before the commencement of offers or sales of our common stock. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the applicable prospectus supplement. Unless the applicable prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
Unless otherwise specified in the applicable prospectus supplement, each class or series of securities will be a new issue with no trading market, other than our common stock, which is traded on the NYSE. We may elect to list any other class or series of securities on any exchanges, but we are not obligated to do so. We cannot guarantee the liquidity of the trading markets for any securities.
Under agreements that we may enter, underwriters, dealers and agents who participate in the distribution of our securities may be entitled to indemnification by us against certain liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Underwriters, dealers and agents may engage in transactions with, or perform services for, us in the ordinary course of business.
If so indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by certain institutions to purchase our securities from us pursuant to contracts providing for payment and delivery on a future date. Institutions with which such contracts may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and others, but in all cases such institutions must be approved by us. The obligations of any purchaser under any such contract will be subject to the condition that the purchase of our securities shall not at the time of delivery be prohibited under the laws of the jurisdiction to which such purchaser is subject. The underwriters and such other agents will not have any responsibility in respect of the validity or performance of such contracts. Such contracts will be subject only to those conditions set forth in the applicable prospectus supplement, and the applicable prospectus supplement will set forth the commission payable for solicitation of such contracts.
We may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third parties in such sale transactions will be underwriters and, if not identified in this prospectus, will be identified in the applicable prospectus supplement.
In compliance with the guidelines of the Financial Industry Regulatory Authority, the maximum compensation to the underwriters or dealers in connection with the sale of our securities pursuant to this
257
prospectus and the applicable prospectus supplement may not exceed 8% of the aggregate offering price of the securities as set forth on the cover page of the applicable prospectus supplement.
In order to comply with the securities laws of certain states, if applicable, our securities offered hereby will be sold in such jurisdictions only through registered or licensed brokers or dealers.
BROKERAGE ALLOCATION AND OTHER PRACTICES
Because we generally acquire and dispose of our investments in privately negotiated transactions, we typically do not use brokers in the normal course of business. However, from time to time, we may work with brokers to sell positions we have acquired in the securities of publicly listed companies or to acquire positions (principally equity) in companies where we see a market opportunity to acquire such securities at attractive valuations. In cases where we do use a broker, we do not execute transactions through any particular broker or dealer, but will seek to obtain the best net results for the Company, taking into account such factors as price (including the applicable brokerage commission or dealer spread), size of order, difficulty of execution, and operational facilities of the firm and the firm’s risk and skill in positioning blocks of securities. While we generally seek reasonably competitive execution costs, we may not necessarily pay the lowest spread or commission available. Subject to applicable legal requirements, we may select a broker based partly upon brokerage or research services provided to us. In return for such services, we may pay a higher commission than other brokers would charge if we determine in good faith that such commission is reasonable in relation to the services provided.
CUSTODIAN, TRANSFER AND DIVIDEND PAYING AGENT AND REGISTRAR
Securities we hold in connection with our investments are held under a custody agreement with Union Bank of California. The address of the custodian is 475 Sansome Street, 15th Floor, San Francisco, California 94111. We have also entered into a custody agreement with U.S. Bank National Association, which is located at One Federal Street, Third Floor, Boston, Massachusetts 02110. The transfer agent and registrar for our common stock, American Stock Transfer & Trust Company, will act as our transfer agent, dividend paying and reinvestment agent and registrar. The principal business address of the transfer agent is 6201 15th Avenue, Brooklyn, New York 11219.
Certain legal matters regarding the securities offered by this prospectus will be passed upon for us by Dechert LLP, Washington, D.C. Certain legal matters will be passed upon for underwriters, if any, by the counsel named in the prospectus supplement.
The consolidated financial statements as of December 31, 2016 and December 31, 2015 and for each of the three years in the period ended December 31, 2016 and management’s assessment of the effectiveness of internal control over financial reporting (which is included in Management’s Report on Internal Control over Financial Reporting) as of December 31, 2016 included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
258
We have filed with the SEC a registration statement on Form N-2, together with all amendments and related exhibits, under the Securities Act, with respect to our securities offered by this prospectus. The registration statement contains additional information about us and our securities being offered by this prospectus.
We file annual, quarterly and current periodic reports, proxy statements and other information with the SEC under the Exchange Act. You may inspect and copy these reports, proxy statements and other information, as well as the registration statement of which this prospectus forms a part and the related exhibits and schedules, at the Public Reference Room of the SEC at 100 F Street, N.E., Washington, D.C. 20549-0102. You may obtain information on the operation of the Public Reference Room by calling the SEC at 202-551-8090. The SEC maintains an Internet website that contains reports, proxy and information statements and other information filed electronically by us with the SEC which are available on the SEC’s Internet website at http://www.sec.gov. Copies of these reports, proxy and information statements and other information may be obtained, after paying a duplicating fee, by electronic request at the following E-mail address: publicinfo@sec.gov, or by writing the SEC’s Public Reference Section, Washington, D.C. 20549-0102.
259
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To Board of Directors and Shareholders of
Hercules Capital, Inc.
In our opinion, the accompanying consolidated statements of assets and liabilities, including the consolidated schedules of investments, and the related consolidated statements of operations, of changes in net assets, and of cash flows present fairly, in all material respects, the financial position of Hercules Capital, Inc. and its subsidiaries at December 31, 2016 and 2015, and the results of their operations, their changes in net assets and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework 2013 issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits, which included confirmation of securities as of December 31, 2016 by correspondence with the custodian, borrowers and brokers, and the application of alternative auditing procedures where replies have not been received provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
San Francisco, California
February 23, 2017
F-2
CONSOLIDATED STATEMENT OF ASSETS AND LIABILITIES
(in thousands, except per share data)
| December 31, 2016 | December 31, 2015 | |||||||
| Assets |
||||||||
| Investments: |
||||||||
| Non-control/Non-affiliate investments (cost of $1,475,918 and $1,238,539, respectively) |
$ | 1,414,210 | $ | 1,192,652 | ||||
| Control investments (cost of $22,598 and $0, respectively) |
4,700 | — | ||||||
| Affiliate investments (cost of $13,010 and $13,742, respectively) |
5,032 | 7,986 | ||||||
|
|
|
|
|
|||||
| Total investments, at value (cost of $1,511,526 and $1,252,281, respectively) |
1,423,942 | 1,200,638 | ||||||
| Cash and cash equivalents |
13,044 | 95,196 | ||||||
| Restricted cash |
8,322 | 9,191 | ||||||
| Interest receivable |
11,614 | 9,239 | ||||||
| Other assets |
7,282 | 9,720 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 1,464,204 | $ | 1,323,984 | ||||
|
|
|
|
|
|||||
| Liabilities |
||||||||
| Accounts payable and accrued liabilities |
$ | 21,463 | $ | 17,241 | ||||
| Convertible Notes, net (principal of $0 and $17,604)(1) |
— | 17,478 | ||||||
| Credit Facilities |
5,016 | 50,000 | ||||||
| 2021 Asset-Backed Notes, net (principal of $109,205 and $129,300, respectively)(1) |
107,972 | 126,995 | ||||||
| 2019 Notes, net (principal of $110,364 and $110,364, respectively)(1) |
108,818 | 108,179 | ||||||
| 2024 Notes, net (principal of $252,873 and $103,000, respectively)(1) |
245,490 | 100,128 | ||||||
| Long-Term SBA Debentures, net (principal of $190,200 and $190,200, respectively)(1) |
187,501 | 186,829 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
$ | 676,260 | $ | 606,850 | ||||
| Commitments and Contingencies (Note 10) |
||||||||
| Net assets consist of: |
||||||||
| Common stock, par value |
80 | 73 | ||||||
| Capital in excess of par value |
839,657 | 752,244 | ||||||
| Unrealized depreciation on investments(2) |
(89,025 | ) | (52,808 | ) | ||||
| Accumulated undistributed realized gains on investments |
37,603 | 27,993 | ||||||
| Distributions in excess of net investment income |
(371 | ) | (10,368 | ) | ||||
|
|
|
|
|
|||||
| Total net assets |
$ | 787,944 | $ | 717,134 | ||||
|
|
|
|
|
|||||
| Total liabilities and net assets |
$ | 1,464,204 | $ | 1,323,984 | ||||
|
|
|
|
|
|||||
| Shares of common stock outstanding ($0.001 par value, 200,000,000 and 100,000,000 authorized, respectively) |
79,555 | 72,118 | ||||||
| Net asset value per share |
$ | 9.90 | $ | 9.94 | ||||
| (1) | The Company’s SBA debentures, 2019 Notes, 2024 Notes, 2021 Asset-Backed Notes, and Convertible Notes, as each term is defined herein, are presented net of the associated debt issuance costs for each instrument. See “Note 2—Summary of Significant Accounting Policies” and “Note 4—Borrowings”. |
| (2) | Amounts includes $1.4 million and $1.2 million in net unrealized depreciation on other assets and accrued liabilities, including escrow receivables, estimated taxes payable and Citigroup warrant participation agreement liabilities as of December 31, 2016 and 2015, respectively. |
See notes to consolidated financial statements.
F-3
The following table presents the assets and liabilities of our consolidated securitization trust for the 2021 Asset-Backed Notes (see Note 4), which is a variable interest entity (“VIE”). The assets of our securitization VIE can only be used to settle obligations of our consolidated securitization VIE, these liabilities are only the obligations of our consolidated securitization VIE, and the creditors (or beneficial interest holders) do not have recourse to our general credit. These assets and liabilities are included in the Consolidated Statements of Assets and Liabilities above.
| (Dollars in thousands) |
December 31, 2016 | December 31, 2015 | ||||||
| Assets |
||||||||
| Restricted Cash |
$ | 8,322 | $ | 9,191 | ||||
| Total investments, at value (cost of $244,695 and $258,748, respectively) |
242,349 | 257,657 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 250,671 | $ | 266,848 | ||||
|
|
|
|
|
|||||
| Liabilities |
||||||||
| 2021 Asset-Backed Notes, net (principal of $109,205 and $129,300, respectively)(1) |
$ | 107,972 | $ | 126,995 | ||||
|
|
|
|
|
|||||
| Total liabilities |
$ | 107,972 | $ | 126,995 | ||||
|
|
|
|
|
|||||
| (1) | The Company’s 2021 Asset-Backed Notes are presented net of the associated debt issuance costs for each instrument. See “Note 2—Summary of Significant Accounting Policies” and “Note 4—Borrowings”. |
See notes to consolidated financial statements.
F-4
CONSOLIDATED STATEMENT OF OPERATIONS
(in thousands, except per share data)
| For the Year Ended December 31, |
||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Investment income: |
||||||||||||
| Interest income |
||||||||||||
| Non-control/Non-affiliate investments |
$ | 158,489 | $ | 139,919 | $ | 124,776 | ||||||
| Control investments |
78 | — | — | |||||||||
| Affiliate investments |
160 | 347 | 1,842 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total interest income |
158,727 | 140,266 | 126,618 | |||||||||
|
|
|
|
|
|
|
|||||||
| Fee income |
||||||||||||
| Non-control/Non-affiliate investments |
16,318 | 16,865 | 17,013 | |||||||||
| Control investments |
6 | — | — | |||||||||
| Affiliate investments |
— | 1 | 34 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total fees |
16,324 | 16,866 | 17,047 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total investment income |
175,051 | 157,132 | 143,665 | |||||||||
| Operating expenses: |
||||||||||||
| Interest |
32,016 | 30,834 | 28,041 | |||||||||
| Loan fees |
5,042 | 6,055 | 5,919 | |||||||||
| General and administrative: |
||||||||||||
| Legal expenses |
4,823 | 3,079 | 1,366 | |||||||||
| Other expenses |
11,283 | 13,579 | 8,843 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total general and administrative |
16,106 | 16,658 | 10,209 | |||||||||
| Employee compensation: |
||||||||||||
| Compensation and benefits |
22,500 | 20,713 | 16,604 | |||||||||
| Stock-based compensation |
7,043 | 9,370 | 9,561 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total employee compensation |
29,543 | 30,083 | 26,165 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
82,707 | 83,630 | 70,334 | |||||||||
| Other income (loss) |
8,000 | (1 | ) | (1,581 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net investment income |
100,344 | 73,501 | 71,750 | |||||||||
| Net realized gain on investments |
||||||||||||
| Non-control/Non-affiliate investments |
4,576 | 5,147 | 20,112 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total net realized gain on investments |
4,576 | 5,147 | 20,112 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net change in unrealized appreciation (depreciation) on investments |
||||||||||||
| Non-control/Non-affiliate investments |
(29,970 | ) | (36,839 | ) | (17,392 | ) | ||||||
| Control investments |
(4,025 | ) | — | — | ||||||||
| Affiliate investments |
(2,222 | ) | 1,107 | (3,282 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total net unrealized depreciation on investments |
(36,217 | ) | (35,732 | ) | (20,674 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total net realized and unrealized loss |
(31,641 | ) | (30,585 | ) | (562 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net increase in net assets resulting from operations |
$ | 68,703 | $ | 42,916 | $ | 71,188 | ||||||
|
|
|
|
|
|
|
|||||||
| Net investment income before investment gains and losses per common share: |
||||||||||||
| Basic |
$ | 1.34 | $ | 1.04 | $ | 1.13 | ||||||
|
|
|
|
|
|
|
|||||||
| Change in net assets resulting from operations per common share: |
||||||||||||
| Basic |
$ | 0.91 | $ | 0.60 | $ | 1.12 | ||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
$ | 0.91 | $ | 0.59 | $ | 1.10 | ||||||
|
|
|
|
|
|
|
|||||||
| Weighted average shares outstanding |
||||||||||||
| Basic |
73,753 | 69,479 | 61,862 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
73,775 | 69,663 | 63,225 | |||||||||
|
|
|
|
|
|
|
|||||||
| Distributions declared per common share: |
||||||||||||
| Basic |
$ | 1.24 | $ | 1.24 | $ | 1.24 | ||||||
See notes to consolidated financial statements.
F-5
CONSOLIDATED STATEMENTS OF CHANGES IN NET ASSETS
(dollars and shares in thousands)
|
Common Stock |
Capital in excess of par value |
Unrealized Appreciation (Depreciation) on Investments |
Accumulated Undistributed Realized Gains (Losses) on Investments |
Undistributed Net Investment Income/ (Distributions in Excess of Investment Income) |
Provision for Income Taxes on Investment Gains |
Net Assets |
||||||||||||||||||||||||||
| Shares | Par Value | |||||||||||||||||||||||||||||||
| Balance at December 31, 2013 |
61,837 | $ | 62 | $ | 656,594 | $ | 3,598 | $ | (15,240 | ) | $ | 5,335 | $ | (342 | ) | $ | 650,007 | |||||||||||||||
| Net increase (decrease) in net assets resulting from operations |
— | — | — | (20,674 | ) | 20,112 | 71,750 | — | 71,188 | |||||||||||||||||||||||
| Public offering, net of offering expenses |
2,111 | 2 | 9,007 | — | — | — | — | 9,009 | ||||||||||||||||||||||||
| Issuance of common stock due to stock option exercises |
354 | — | 3,955 | — | — | — | — | 3,955 | ||||||||||||||||||||||||
| Retired shares from net issuance |
(277 | ) | — | (4,564 | ) | — | — | — | — | (4,564 | ) | |||||||||||||||||||||
| Issuance of common stock under restricted stock plan |
990 | 1 | (1 | ) | — | — | — | — | — | |||||||||||||||||||||||
| Retired shares for restricted stock vesting |
(397 | ) | — | (3,292 | ) | — | — | — | — | (3,292 | ) | |||||||||||||||||||||
| Distributions reinvested in common stock |
97 | — | 1,485 | — | — | — | — | 1,485 | ||||||||||||||||||||||||
| Distributions |
— | — | — | — | — | (78,562 | ) | — | (78,562 | ) | ||||||||||||||||||||||
| Stock-based compensation(1) |
— | — | 9,638 | — | — | — | — | 9,638 | ||||||||||||||||||||||||
| Tax reclassification of stockholders’ equity in accordance with generally accepted accounting principles |
— | — | (15,589 | ) | — | 9,207 | 6,382 | — | — | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2014 |
64,715 | $ | 65 | $ | 657,233 | $ | (17,076 | ) | $ | 14,079 | $ | 4,905 | $ | (342 | ) | $ | 658,864 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net increase (decrease) in net assets resulting from operations |
— | $ | — | $ | — | $ | (35,732 | ) | $ | 5,147 | $ | 73,501 | $ | — | $ | 42,916 | ||||||||||||||||
| Public offering, net of offering expenses |
7,591 | 8 | 100,084 | — | — | — | — | 100,092 | ||||||||||||||||||||||||
| Acquisition of common stock under repurchase plan |
(437 | ) | — | (4,644 | ) | (4,644 | ) | |||||||||||||||||||||||||
| Issuance of common stock due to stock option exercises |
64 | — | 427 | — | — | — | — | 427 | ||||||||||||||||||||||||
| Retired shares from net issuance |
(29 | ) | — | (423 | ) | — | — | — | — | (423 | ) | |||||||||||||||||||||
| Issuance of common stock under restricted stock plan |
676 | 1 | (1 | ) | — | — | — | — | — | |||||||||||||||||||||||
| Retired shares for restricted stock vesting |
(662 | ) | (1 | ) | (4,566 | ) | — | — | — | — | (4,567 | ) | ||||||||||||||||||||
| Distributions reinvested in common stock |
200 | — | 2,446 | — | — | — | — | 2,446 | ||||||||||||||||||||||||
| Distributions |
— | — | — | — | — | (87,438 | ) | — | (87,438 | ) | ||||||||||||||||||||||
| Stock-based compensation(1) |
— | — | 9,461 | — | — | — | — | 9,461 | ||||||||||||||||||||||||
| Tax reclassification of stockholders’ equity in accordance with generally accepted accounting principles |
— | — | (7,773 | ) | — | 8,767 | (994 | ) | — | — | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2015 |
72,118 | $ | 73 | $ | 752,244 | $ | (52,808 | ) | $ | 27,993 | $ | (10,026 | ) | $ | (342 | ) | $ | 717,134 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net increase (decrease) in net assets resulting from operations |
$ | — | $ | — | $ | — | $ | (36,217 | ) | $ | 4,576 | $ | 100,344 | $ | — | $ | 68,703 | |||||||||||||||
| Public offering, net of offering expenses |
7,428 | 7 | 92,820 | — | — | — | — | 92,827 | ||||||||||||||||||||||||
| Acquisition of common stock under repurchase plan |
(450 | ) | (1 | ) | (4,789 | ) | — | — | — | — | (4,790 | ) | ||||||||||||||||||||
| Issuance of common stock due to stock option exercises |
55 | — | 654 | — | — | — | — | 654 | ||||||||||||||||||||||||
| Retired shares from net issuance |
(17 | ) | — | (235 | ) | — | — | — | — | (235 | ) | |||||||||||||||||||||
| Issuance of common stock under restricted stock plan |
556 | 1 | (1 | ) | — | — | — | — | — | |||||||||||||||||||||||
| Retired shares for restricted stock vesting |
(279 | ) | — | (2,944 | ) | — | — | — | — | (2,944 | ) | |||||||||||||||||||||
| Distributions reinvested in common stock |
144 | — | 1,799 | — | — | — | — | 1,799 | ||||||||||||||||||||||||
| Distributions |
— | — | — | — | — | (92,333 | ) | — | (92,333 | ) | ||||||||||||||||||||||
| Stock-based compensation(1) |
— | — | 7,129 | — | — | — | — | 7,129 | ||||||||||||||||||||||||
| Tax reclassification of stockholders’ equity in accordance with generally accepted accounting principles |
— | — | (7,020 | ) | — | 5,034 | 1,644 | 342 | — | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2016 |
79,555 | $ | 80 | $ | 839,657 | $ | (89,025 | ) | $ | 37,603 | $ | (371 | ) | $ | — | $ | 787,944 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Stock-based compensation includes $87, $90 and $77 of restricted stock and option expense related to director compensation for the years ended December 31, 2016, 2015 and 2014, respectively. |
See notes to consolidated financial statements.
F-6
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net increase in net assets resulting from operations |
$ | 68,703 | $ | 42,916 | $ | 71,188 | ||||||
| Adjustments to reconcile net increase in net assets resulting from operations to net cash provided by (used in) operating activities: |
||||||||||||
| Purchase of investments |
(680,971 | ) | (712,701 | ) | (623,232 | ) | ||||||
| Principal and fee payments received on investments |
444,758 | 509,593 | 503,003 | |||||||||
| Proceeds from the sale of investments |
18,998 | 17,892 | 33,432 | |||||||||
| Net unrealized depreciation on investments |
36,217 | 35,732 | 20,674 | |||||||||
| Net realized gain on investments |
(4,576 | ) | (5,147 | ) | (20,112 | ) | ||||||
| Accretion of paid-in-kind principal |
(7,319 | ) | (4,037 | ) | (2,549 | ) | ||||||
| Accretion of loan discounts |
(7,163 | ) | (8,049 | ) | (9,792 | ) | ||||||
| Accretion of loan discount on convertible notes |
82 | 246 | 843 | |||||||||
| Loss on debt extinguishment (convertible notes) |
— | 1 | 1,581 | |||||||||
| Payment of loan discount on convertible notes |
— | (5 | ) | (4,195 | ) | |||||||
| Accretion of loan exit fees |
(22,614 | ) | (14,947 | ) | (11,541 | ) | ||||||
| Change in deferred loan origination revenue |
347 | 1,904 | (281 | ) | ||||||||
| Unearned fees related to unfunded commitments |
(758 | ) | (2,064 | ) | (259 | ) | ||||||
| Amortization of debt fees and issuance costs |
3,773 | 5,161 | 5,256 | |||||||||
| Depreciation |
202 | 193 | 266 | |||||||||
| Stock-based compensation and amortization of restricted stock grants(1) |
7,129 | 9,461 | 9,638 | |||||||||
| Change in operating assets and liabilities: |
||||||||||||
| Interest and fees receivable |
(2,375 | ) | 213 | (490 | ) | |||||||
| Prepaid expenses and other assets |
3,234 | 4,826 | 1,351 | |||||||||
| Accounts payable |
56 | (639 | ) | 271 | ||||||||
| Accrued liabilities |
3,892 | 5,090 | (1,583 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(138,385 | ) | (114,361 | ) | (26,531 | ) | ||||||
| Cash flows from investing activities: |
||||||||||||
| Purchases of capital equipment |
(252 | ) | (187 | ) | (190 | ) | ||||||
| Reduction of (investment in) restricted cash |
869 | 3,469 | (6,389 | ) | ||||||||
| Other long-term assets |
— | — | 25 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
617 | 3,282 | (6,554 | ) | ||||||||
| Cash flows from financing activities: |
||||||||||||
| Issuance of common stock, net |
92,827 | 100,092 | 9,837 | |||||||||
| Repurchase of common stock, net |
(4,790 | ) | (4,645 | ) | — | |||||||
| Retirement of employee shares |
(2,525 | ) | (4,562 | ) | (3,901 | ) | ||||||
| Distributions paid |
(90,534 | ) | (84,992 | ) | (77,076 | ) | ||||||
| Issuance of 2024 Notes |
149,873 | — | 103,000 | |||||||||
| Issuance of 2021 Asset-Backed Notes |
— | — | 129,300 | |||||||||
| Repayments of 2017 Asset-Backed Notes |
— | (16,049 | ) | (73,508 | ) | |||||||
| Repayments of 2021 Asset-Backed Notes |
(20,095 | ) | — | — | ||||||||
| Repayments of Long-Term SBA Debentures |
— | — | (34,800 | ) | ||||||||
| Repayments of 2019 Notes |
— | (60,000 | ) | — | ||||||||
| Borrowings of credit facilities |
285,891 | 138,689 | — | |||||||||
| Repayments of credit facilities |
(330,877 | ) | (88,689 | ) | — | |||||||
| Cash paid for debt issuance costs |
(5,289 | ) | — | (6,669 | ) | |||||||
| Cash paid for redemption of convertible notes |
(17,604 | ) | (65 | ) | (53,131 | ) | ||||||
| Fees paid for credit facilities and debentures |
(1,261 | ) | (620 | ) | (1,219 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
55,616 | (20,841 | ) | (8,167 | ) | |||||||
| Net decrease in cash and cash equivalents |
(82,152 | ) | (131,920 | ) | (41,252 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at beginning of period |
95,196 | 227,116 | 268,368 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 13,044 | $ | 95,196 | $ | 227,116 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental non-cash investing and financing activities: |
||||||||||||
| Interest paid |
$ | 31,011 | $ | 30,527 | $ | 25,738 | ||||||
| Income taxes paid |
$ | 184 | $ | 973 | $ | 133 | ||||||
| Distributions reinvested |
$ | 1,799 | $ | 2,446 | $ | 1,485 | ||||||
| (1) | Stock-based compensation includes $87, $90 and $77 of restricted stock and option expense related to director compensation for the years ended December 31, 2016, 2015 and 2014, respectively. |
See notes to consolidated financial statements.
F-7
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type
of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| Debt Investments |
||||||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Exicure, Inc.(11)(14A) |
Biotechnology Tools | Senior Secured | September 2019 | Interest rate PRIME + 6.45% or Floor rate of 9.95% |
$ | 6,000 | $ 5,971 | $ 6,035 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
5,971 | 6,035 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Biotechnology Tools (0.77%)* |
|
5,971 | 6,035 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Communications & Networking |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(13)(14B) |
Communications & Networking | Senior Secured | August 2017 | PIK Interest 10.50% | $ | 1,278 | 1,304 | 1,304 | ||||||||||||||
| OpenPeak, Inc.(7) |
Communications & Networking | Senior Secured | April 2017 |
Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 12,211 | 8,975 | — | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
10,279 | 1,304 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Avanti Communications Group(4)(9) |
Communications & Networking | Senior Secured | October 2019 | Interest rate FIXED 10.00% | $ | 8,025 | 7,212 | 4,825 | ||||||||||||||
| SkyCross, Inc.(6)(7)(13)(14B)(15) |
Communications & Networking | Senior Secured | January 2018 | Interest rate FIXED 10.95%, PIK Interest 5.00% |
$ | 16,758 | 16,900 | — | ||||||||||||||
| Spring Mobile
Solutions, |
Communications & Networking | Senior Secured | January 2019 | Interest rate PRIME + 6.70% or Floor rate of 9.95% |
$ | 3,000 | 3,038 | 3,044 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
27,150 | 7,869 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Communications & Networking (1.16%)* |
|
37,429 | 9,173 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(14A)(15) |
Consumer & Business Products | Senior Secured | December 2019 | Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 20,000 | 19,837 | 19,837 | ||||||||||||||
| Consumer & Business Products | Senior Secured | December 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.50% |
$ | 1,000 | 965 | 965 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Antenna79 (p.k.a. Pong Research Corporation) |
$ | 21,000 | 20,802 | 20,802 | ||||||||||||||||||
| Nasty Gal(14B)(15) |
Consumer & Business Products | Senior Secured | May 2019 |
Interest rate PRIME + 5.45% or Floor rate of 8.95% |
$ | 13,241 | 13,148 | 13,148 | ||||||||||||||
| Second Time Around (Simplify Holdings, LLC)(14A)(15) |
Consumer & Business Products | Senior Secured | February 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ | 2,280 | 2,302 | 2,283 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
36,252 | 36,233 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Consumer & Business Products (4.60%)* |
|
36,252 | 36,233 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Drug Delivery |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(9)(10)(14A)(15) |
Drug Delivery |
Senior Secured | October 2017 | Interest rate PRIME + 3.85% or Floor rate of 9.10% |
$ | 20,466 | 21,151 | 21,151 | ||||||||||||||
| Celsion Corporation(10)(14A) |
Drug Delivery |
Senior Secured | June 2017 |
Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 2,246 | 2,575 | 2,575 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
23,726 | 23,726 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||||
| Agile Therapeutics, Inc.(10)(14A) |
Drug Delivery | Senior Secured | December 2018 | Interest rate PRIME + 4.75% or Floor rate of 9.00% |
$ | 16,500 | 16,524 | 16,434 | ||||||||||||||
| Aprecia
Pharmaceuticals |
Drug Delivery | Senior Secured | January 2020 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 20,000 | 19,700 | 19,706 | ||||||||||||||
See notes to consolidated financial statements.
F-8
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| BioQ Pharma Incorporated(10)(14A)(14B) |
Drug Delivery | Senior Secured | May 2018 |
Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 8,231 | $ 8,636 | $ 8,577 | ||||||||||||||
| Drug Delivery | Senior Secured | May 2018 |
Interest rate PRIME + 7.00% or Floor rate of 10.25% |
$ | 2,464 | 2,511 | 2,509 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total BioQ Pharma Incorporated |
$ | 10,695 | 11,147 | 11,086 | ||||||||||||||||||
| Edge Therapeutics, Inc.(11)(14A)(17) |
Drug Delivery | Senior Secured | February 2020 | Interest rate PRIME + 4.65% or Floor rate of 9.15% |
$ | 15,000 | 15,004 | 15,045 | ||||||||||||||
| Pulmatrix Inc.(8)(10)(14A) |
Drug Delivery | Senior Secured | July 2018 | Interest rate PRIME + 6.25% or Floor rate of 9.50% |
$ | 5,954 | 6,022 | 6,013 | ||||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(10)(14A) |
Drug Delivery | Senior Secured | December 2018 | Interest rate PRIME + 2.70% or Floor rate of 7.95% |
$ | 12,123 | 12,325 | 12,238 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
80,722 | 80,522 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Drug Delivery (13.23%)* |
|
104,448 | 104,248 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| Cerecor, Inc.(11)(14A) |
Drug Discovery & Development | Senior Secured | August 2017 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 2,374 | 2,499 | 2,499 | ||||||||||||||
| Neuralstem, Inc.(14A)(15) |
Drug Discovery & Development | Senior Secured | April 2017 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 3,766 | 3,996 | 3,996 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
6,495 | 6,495 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Auris Medical
Holding, |
Drug Discovery & Development | Senior Secured | January 2020 | Interest rate PRIME + 6.05% or Floor rate of 9.55% |
$ | 12,500 | 12,317 | 12,326 | ||||||||||||||
| Aveo
Pharmaceuticals, |
Drug Discovery & Development | Senior Secured | December 2019 | Interest rate PRIME + 6.90% or Floor rate of 11.90% |
$ | 10,000 | 10,269 | 10,218 | ||||||||||||||
| Drug Discovery & Development | Senior Secured | December 2019 | Interest rate PRIME + 6.90% or Floor rate of 11.90% |
$ | 5,000 | 4,926 | 4,918 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Aveo Pharmaceuticals, Inc. |
$ | 15,000 | 15,195 | 15,136 | ||||||||||||||||||
| Bellicum Pharmaceuticals, Inc.(14A)(14B)(15) |
Drug Discovery & Development | Senior Secured | March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 15,000 | 15,212 | 15,387 | ||||||||||||||
| Drug Discovery & Development | Senior Secured | March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 5,000 | 4,981 | 5,049 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Bellicum Pharmaceuticals, Inc. |
$ | 20,000 | 20,193 | 20,436 | ||||||||||||||||||
| Brickell Biotech, Inc.(11)(14B) |
Drug Discovery & Development | Senior Secured | September 2019 | Interest rate PRIME + 5.70% or Floor rate of 9.20% |
$ | 7,500 | 7,521 | 7,560 | ||||||||||||||
| Cerulean Pharma, Inc.(12)(14B) |
Drug Discovery & Development | Senior Secured | July 2018 | Interest rate PRIME + 1.55% or Floor rate of 7.30% |
$ | 13,078 | 13,994 | 13,908 | ||||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(10)(14A) |
Drug Discovery & Development | Senior Secured | December 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 19,548 | 19,276 | 19,372 | ||||||||||||||
| CytRx Corporation(10)(14B)(15) |
Drug Discovery & Development | Senior Secured | February 2020 | Interest rate PRIME + 6.00% or Floor rate of 9.50% |
$ | 25,000 | 25,086 | 25,166 | ||||||||||||||
| Epirus Biopharmaceuticals, Inc.(7)(14A) |
Drug Discovery & Development | Senior Secured | April 2018 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 3,066 | 3,349 | — | ||||||||||||||
| Genocea Biosciences, Inc.(10)(14A) |
Drug Discovery & Development | Senior Secured | January 2019 | Interest rate PRIME + 2.25% or Floor rate of 7.25% |
$ | 17,000 | 17,313 | 17,376 | ||||||||||||||
| Immune Pharmaceuticals(10)(14B) |
Drug Discovery & Development | Senior Secured | September 2018 | Interest rate PRIME + 4.75% or Floor rate of 10.00% |
$ | 3,271 | 3,350 | 2,693 | ||||||||||||||
| Insmed, Incorporated(10)(14A) |
Drug Discovery & Development | Senior Secured | October 2020 | Interest rate PRIME + 4.75% or Floor rate of 9.25% |
$ | 55,000 | 54,695 | 54,559 | ||||||||||||||
| Mast Therapeutics, Inc.(14A)(15) |
Drug Discovery & Development | Senior Secured | January 2019 | Interest rate PRIME + 5.70% or Floor rate of 8.95% |
$ | 3,347 | 3,921 | 3,923 | ||||||||||||||
| Melinta Therapeutics(12)(14A) |
Drug Discovery & Development | Senior Secured | June 2018 | Interest rate PRIME + 3.75% or Floor rate of 8.25% |
$ | 24,502 | 25,001 | 24,945 | ||||||||||||||
| Merrimack Pharmaceuticals, Inc.(9) |
Drug Discovery & Development | Senior Secured | December 2022 | Interest rate FIXED 11.50% | $ | 25,000 | 25,000 | 25,000 | ||||||||||||||
| Metuchen Pharmaceuticals LLC(13)(14A) |
Drug Discovery & Development | Senior Secured | October 2020 | Interest rate PRIME + 7.25% or Floor rate of 10.75%, PIK Interest 1.35% |
$ | 35,081 | 34,541 | 34,541 | ||||||||||||||
See notes to consolidated financial statements.
F-9
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(14A)(15) |
Drug Discovery & Development | |
Senior Secured |
|
September 2020 | Interest rate PRIME + 2.75% or Floor rate of 8.50% |
$ | 40,000 | $ 39,388 | $ 39,504 | ||||||||||||
| PhaseRx,Inc.(14B)(15) |
Drug Discovery & Development | |
Senior Secured |
|
December 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 6,000 | 5,921 | 5,945 | ||||||||||||
| Sorrento Therapeutics, Inc.(9)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
December 2020 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 50,000 | 48,069 | 48,069 | ||||||||||||
| uniQure B.V.(4)(9)(10)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
May 2020 | Interest rate PRIME + 3.00% or Floor rate of 8.25% |
$ | 20,000 | 20,133 | 20,081 | ||||||||||||
| XOMA Corporation(9)(14B)(15) |
Drug Discovery & Development | |
Senior Secured |
|
September 2018 | Interest rate PRIME + 2.15% or Floor rate of 9.40% |
$ | 16,380 | 16,970 | 16,901 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
411,233 | 407,441 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Drug Discovery & Development (52.53%)* |
|
417,728 | 413,936 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Persimmon |
Electronics & Computer Hardware | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 7.50% or Floor rate of 11.00%, PIK Interest 1.50% |
$ | 7,012 | 7,096 | 7,134 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
7,096 | 7,134 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.91%)* |
|
7,096 | 7,134 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| InstaMed Communications, LLC(14B)(15) |
Healthcare Services, Other | |
Senior Secured |
|
February 2019 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 10,000 | 10,125 | 10,261 | ||||||||||||
| PH Group Holdings |
Healthcare Services, Other | |
Senior Secured |
|
September 2020 | Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 20,000 | 19,802 | 19,802 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
29,927 | 30,063 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Healthcare Services, Other (3.82%)* |
|
29,927 | 30,063 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Aria Systems, Inc.(10)(13) |
Internet Consumer & Business Services | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 3.20% or Floor rate of 6.95%, PIK Interest 1.95% |
$ | 2,061 | 2,045 | 1,728 | ||||||||||||
| Internet Consumer & Business Services | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 5.20% or Floor rate of 8.95%, PIK Interest 1.95% |
$ | 18,463 | 18,307 | 15,467 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Aria Systems, Inc. |
$ | 20,524 | 20,352 | 17,195 | ||||||||||||||||||
| CloudOne, Inc.(10)(14B) |
Internet Consumer & Business Services | |
Senior Secured |
|
April 2019 | Interest rate PRIME + 6.35% or Floor rate of 9.85% |
$ | 5,000 | 5,091 | 5,138 | ||||||||||||
| Intent Media, Inc.(13)(14A)(15) |
Internet Consumer & Business Services | |
Senior Secured |
|
December 2018 | Interest rate PRIME + 5.25% or Floor rate of 8.75%, PIK Interest 1.00% |
$ | 5,000 | 4,851 | 4,851 | ||||||||||||
| LogicSource(14B)(15) |
Internet Consumer & Business Services | |
Senior Secured |
|
October 2019 | Interest rate PRIME + 6.25% or Floor rate of 9.75% |
$ | 8,500 | 8,533 | 8,649 | ||||||||||||
| Snagajob.com, Inc.(12)(13)(14A) |
Internet Consumer & Business Services | |
Senior Secured |
|
July 2020 | Interest rate PRIME + 5.15% or Floor rate of 9.15%, PIK Interest 1.95% |
$ | 35,293 | 34,517 | 35,067 | ||||||||||||
| Tectura Corporation(7)(8)(13) |
Internet Consumer & Business Services | |
Senior Secured |
|
June 2021 | Interest rate FIXED 6.00%, PIK Interest 3.00% |
$ | 19,691 | 19,691 | 19,691 | ||||||||||||
| Internet Consumer & Business Services | |
Senior Secured |
|
June 2021 | PIK Interest 8.00% | $ | 11,015 | 240 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Tectura Corporation |
$ | 30,706 | 19,931 | 19,691 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
93,275 | 90,591 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Internet Consumer & Business Services (11.50%)* |
|
93,275 | 90,591 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
See notes to consolidated financial statements.
F-10
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| Media/Content/Info |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| FanDuel, Inc.(14B) |
Media/Content/Info | |
Senior Secured |
|
November 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ | 20,000 | $ 19,352 | $ 19,352 | ||||||||||||
| Machine Zone, Inc.(13)(16) |
Media/Content/Info | |
Senior Secured |
|
May 2018 | Interest rate PRIME + 2.50% or Floor rate of 6.75%, PIK Interest 3.00% |
$ | 103,785 | 102,444 | 103,083 | ||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9)(11)(14B)(17) |
Media/Content/Info | |
Senior Secured |
|
April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ | 5,000 | 5,029 | 5,099 | ||||||||||||
| Media/Content/Info | |
Senior Secured |
|
April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ | 2,500 | 2,471 | 2,510 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total WP Technology, Inc. (Wattpad, Inc.) |
$ | 7,500 | 7,500 | 7,609 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
129,296 | 130,044 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Media/Content/Info (16.50%)* |
|
129,296 | 130,044 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| InspireMD, Inc.(4)(9)(14B) |
Medical Devices & Equipment | |
Senior Secured |
|
June 2017 | Interest rate PRIME + 5.00% or Floor rate of 10.50% |
$ | 2,237 | 2,743 | 2,743 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
2,743 | 2,743 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Amedica Corporation(8)(14B)(15) |
Medical Devices & Equipment | |
Senior Secured |
|
January 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 7,417 | 8,816 | 8,715 | ||||||||||||
| Aspire Bariatrics, Inc.(14B)(15) |
Medical Devices & Equipment | |
Senior Secured |
|
October 2018 | Interest rate PRIME + 4.00% or Floor rate of 9.25% |
$ | 5,295 | 5,400 | 5,368 | ||||||||||||
| Avedro, Inc.(14A)(15) |
Medical Devices & Equipment | |
Senior Secured |
|
June 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.25% |
$ | 9,777 | 9,975 | 9,982 | ||||||||||||
| Flowonix Medical Incorporated(12)(14B) |
Medical Devices & Equipment | |
Senior Secured |
|
May 2018 | Interest rate PRIME + 4.75% or Floor rate of 10.00% |
$ | 10,905 | 11,340 | 11,275 | ||||||||||||
| Medical Devices & Equipment | |
Senior Secured |
|
March 2019 | Interest rate PRIME + 6.50% or Floor rate of 10.00% |
$ | 4,255 | 4,243 | 4,214 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Flowonix Medical Incorporated |
$ | 15,160 | 15,583 | 15,489 | ||||||||||||||||||
| Gamma Medica, Inc.(10)(14B) |
Medical Devices & Equipment | |
Senior Secured |
|
January 2018 | Interest rate PRIME + 6.50% or Floor rate of 9.75% |
$ | 2,500 | 2,650 | 2,645 | ||||||||||||
| IntegenX, Inc.(14B)(15) |
Medical Devices & Equipment | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 15,000 | 15,068 | 15,168 | ||||||||||||
| Medical Devices & Equipment | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 1,750 | 1,694 | 1,730 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total IntegenX, Inc. |
$ | 16,750 | 16,762 | 16,898 | ||||||||||||||||||
| Micell Technologies, Inc.(11)(14B) |
Medical Devices & Equipment | |
Senior Secured |
|
August 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.50% |
$ | 8,277 | 8,255 | 8,321 | ||||||||||||
| Quanta Fluid Solutions(4)(9)(10)(14B) |
Medical Devices & Equipment | |
Senior Secured |
|
April 2020 | Interest rate PRIME + 8.05% or Floor rate of 11.55% |
$ | 12,500 | 12,547 | 12,500 | ||||||||||||
| Quanterix Corporation(10)(14A) |
Medical Devices & Equipment | |
Senior Secured |
|
February 2018 | Interest rate PRIME + 2.75% or Floor rate of 8.00% |
$ | 9,964 | 10,276 | 10,316 | ||||||||||||
| SynergEyes, Inc.(14B)(15) |
Medical Devices & Equipment | |
Senior Secured |
|
January 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 2,347 | 2,762 | 2,719 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
93,026 | 92,953 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Medical Devices & Equipment (12.15%)* |
|
95,769 | 95,696 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Semiconductors |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| Achronix Semiconductor Corporation(14B)(15)(17) |
Semiconductors | |
Senior Secured |
|
November 2017 | Interest rate PRIME + 7.00% or Floor rate of 10.50% |
$ | 1,682 | 1,682 | 1,682 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
1,682 | 1,682 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
See notes to consolidated financial statements.
F-11
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Achronix Semiconductor Corporation(14B)(15)(17) |
Semiconductors | |
Senior Secured |
|
July 2018 | Interest rate PRIME + 8.25% or Floor rate of 11.50% |
$ | 3,341 | $ 3,546 | $ 3,530 | ||||||||||||
| Avnera Corporation(10)(14A) |
Semiconductors | |
Senior Secured |
|
April 2018 | Interest rate PRIME + 5.25% or Floor rate of 8.50% |
$ | 5,577 | 5,699 | 5,816 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
9,245 | 9,346 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Semiconductors (1.40%)* |
|
10,927 | 11,028 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Software |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(7)(13)(14C)(15)(18) |
Software | Senior Secured | October 2016 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 1,566 | 1,698 | 730 | ||||||||||||||
| RedSeal Inc.(15)(17) |
Software | Senior Secured | June 2017 | Interest rate PRIME + 3.25% or Floor rate of 6.50% |
$ | 2,635 | 2,635 | 2,635 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
4,333 | 3,365 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Actifio, Inc.(13)(14A) |
Software | Senior Secured | January 2019 | Interest rate PRIME + 4.25% or Floor rate of 8.25%, PIK Interest 2.25% |
$ | 30,961 | 30,830 | 30,918 | ||||||||||||||
| Software | Senior Secured | January 2019 | Interest rate PRIME + 4.75% or Floor rate of 8.75%, PIK Interest 2.50% |
$ | 10,171 | 9,929 | 10,036 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Actifio, Inc. |
$ | 41,132 | 40,759 | 40,954 | ||||||||||||||||||
| Clickfox, Inc.(12)(14C) |
Software | Senior Secured | May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.50% |
$ | 12,000 | 12,261 | 12,273 | ||||||||||||||
| Cloud Technology Partners, Inc.(14A) |
Software | Senior Secured | June 2018 | Interest rate PRIME + 3.05% or Floor rate of 7.05% |
$ | 3,000 | 2,966 | 2,966 | ||||||||||||||
| Software | Senior Secured | December 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.75% |
$ | 10,000 | 9,863 | 9,863 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Cloud Technology Partners, Inc. |
$ | 13,000 | 12,829 | 12,829 | ||||||||||||||||||
| Druva, Inc.(10)(12)(14B)(17) |
Software | Senior Secured | March 2018 | Interest rate PRIME + 4.60% or Floor rate of 7.85% |
$ | 9,157 | 9,604 | 9,613 | ||||||||||||||
| Software | Senior Secured | May 2018 | Interest rate PRIME + 4.60% or Floor rate of 7.85% |
$ | 10,000 | 10,066 | 10,141 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Druva, Inc. |
$ | 19,157 | 19,670 | 19,754 | ||||||||||||||||||
| Evernote Corporation(15)(17) |
Software | Senior Secured | October 2020 | Interest rate PRIME + 5.45% or Floor rate of 8.95% |
$ | 6,000 | 5,961 | 5,961 | ||||||||||||||
| Lithium Technologies, Inc.(13)(14A)(15)(19) |
Software | Senior Secured | June 2020 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 1.80% |
$ | 25,019 | 24,999 | 24,999 | ||||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(7)(13)(14A)(15) |
Software | Senior Secured | March 2018 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 13,000 | 12,747 | 5,477 | ||||||||||||||
| Mattersight Corporation(11)(13) |
Software | Senior Secured | February 2020 | Interest rate PRIME + 6.25% or Floor rate of 9.75%, PIK Interest 2.15% |
$ | 22,664 | 22,023 | 22,280 | ||||||||||||||
| OneLogin, Inc.(13)(15) |
Software | Senior Secured | August 2019 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 3.25% |
$ | 15,369 | 15,249 | 15,488 | ||||||||||||||
| Quid, Inc.(13)(14A)(15) |
Software | Senior Secured | October 2019 | Interest rate PRIME + 4.75% or Floor rate of 8.25%, PIK Interest 2.25% |
$ | 8,116 | 8,126 | 8,220 | ||||||||||||||
| RedSeal Inc.(14A)(15)(17) |
Software | Senior Secured | June 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 5,000 | 5,120 | 5,107 | ||||||||||||||
| Software | Senior Secured | January 2020 | Interest rate PRIME + 7.75% or Floor rate of 11.25% |
$ | 5,000 | 4,880 | 4,880 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total RedSeal Inc. |
$ | 10,000 | 10,000 | 9,987 | ||||||||||||||||||
See notes to consolidated financial statements.
F-12
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| Signpost, Inc.(13)(14A)(15) |
Software | Senior Secured | February 2020 | Interest rate PRIME + 4.15% or Floor rate of 8.15%, PIK Interest 1.75% |
$ | 15,237 | $ 15,022 | $ 15,190 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
199,646 | 193,412 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Software (24.97%)* |
|
203,979 | 196,777 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Alimera Sciences, Inc.(10)(13)(14A) |
Specialty Pharmaceuticals | Senior Secured | November 2020 | Interest rate PRIME + 7.50% or Floor rate of 11.00%, PIK Interest 1.00% |
$ | 35,041 | 34,606 | 34,798 | ||||||||||||||
| Jaguar Animal Health, Inc.(10)(14B) |
Specialty Pharmaceuticals | Senior Secured | August 2018 | Interest rate PRIME + 5.65% or Floor rate of 9.90% |
$ | 3,511 | 3,803 | 3,725 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
38,409 | 38,523 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (4.89%)* |
|
38,409 | 38,523 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Surgical Devices |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Transmedics, Inc.(12)(14B) |
Surgical Devices | Senior Secured | February 2020 | Interest rate PRIME + 5.30% or Floor rate of 9.55% |
$ | 8,500 | 8,497 | 8,529 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
8,497 | 8,529 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Surgical Devices (1.08%)* |
|
8,497 | 8,529 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| American Superconductor Corporation(10)(14B) |
Sustainable and Renewable Technology | Senior Secured | June 2017 | Interest rate PRIME + 7.25% or Floor rate of 11.00% |
$ | 1,500 | 1,550 | 1,550 | ||||||||||||||
| Modumetal, Inc.(11)(14C)(14D) |
Sustainable and Renewable Technology | Senior Secured | March 2017 | Interest rate PRIME + 8.70% or Floor rate of 11.95% |
$ | 376 | 882 | 882 | ||||||||||||||
| Sustainable and Renewable Technology | Senior Secured | October 2017 | Interest rate PRIME + 6.00% or Floor rate of 9.25% |
$ | 3,370 | 4,115 | 4,115 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Modumetal, Inc. |
$ | 3,746 | 4,997 | 4,997 | ||||||||||||||||||
| Stion Corporation(5)(14A) |
Sustainable and Renewable Technology | Senior Secured | February 2017 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 333 | 333 | 333 | ||||||||||||||
| Sungevity, Inc.(12)(14D) |
Sustainable and Renewable Technology | Senior Secured | October 2017 | Interest rate PRIME + 3.70% or Floor rate of 6.95% |
$ | 35,000 | 39,834 | 29,709 | ||||||||||||||
| Sustainable and Renewable Technology | Senior Secured | October 2017 | Interest rate PRIME + 3.70% or Floor rate of 6.95% |
$ | 20,000 | 20,000 | 14,917 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Sungevity, Inc. |
$ | 55,000 | 59,834 | 44,626 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
66,714 | 51,506 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| FuelCell Energy, Inc.(11)(14B) |
Sustainable and Renewable Technology | Senior Secured | October 2018 | Interest rate PRIME + 5.50% or Floor rate of 9.50% |
$ | 20,000 | 20,488 | 20,707 | ||||||||||||||
| Proterra, Inc.(10)(14A)(14B) |
Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate PRIME + 6.95% or Floor rate of 10.20% |
$ | 30,000 | 30,670 | 30,592 | ||||||||||||||
See notes to consolidated financial statements.
F-13
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 10,000 | $ 9,921 | $ 9,916 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Proterra, Inc. |
$ | 40,000 | 40,591 | 40,508 | ||||||||||||||||
| Rive Technology, Inc.(14A)(15) |
Sustainable and Renewable Technology | Senior Secured | January 2019 | Interest rate PRIME + 6.20% or Floor rate of 9.45% |
$ | 7,500 | 7,586 | 7,650 | ||||||||||||
| Tendril Networks(11)(14B) |
Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate FIXED 7.25% | $ | 15,000 | 15,405 | 15,324 | ||||||||||||
| Verdezyne, Inc.(14B)(15) |
Sustainable and Renewable Technology | Senior Secured | April 2019 | Interest rate PRIME + 8.25% or Floor rate of 11.75% |
$ | 15,000 | 15,084 | 15,098 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
99,154 | 99,287 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (19.14%)* |
|
165,868 | 150,793 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total: Debt Investments (168.64%)* |
|
1,384,871 | 1,328,803 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-14
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||||||||
| Equity Investments |
||||||||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||||||||
| NuGEN Technologies, Inc.(15) |
Biotechnology Tools | Equity | Preferred Series C | 189,394 | $ | 500 | $ | 575 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
500 | 575 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Communications & Networking |
||||||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(15) |
|
Communications & Networking |
|
Equity | Common Stock | 100 | 4,000 | 3,396 | ||||||||||||||||
| GlowPoint, Inc.(3) |
|
Communications & Networking |
|
Equity | Common Stock | 114,192 | 101 | 31 | ||||||||||||||||
| Peerless Network Holdings, Inc. |
|
Communications & Networking |
|
Equity | Preferred Series A | 1,000,000 | 1,000 | 4,990 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Communications & Networking (1.07%)* |
|
5,101 | 8,417 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||||||
| Market Force Information, Inc. |
|
Consumer & Business Products |
|
Equity | Common Stock | 480,261 | — | 279 | ||||||||||||||||
| |
Consumer & Business Products |
|
Equity | Preferred Series B-1 | 187,970 | 500 | 273 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total Market Force Information, Inc. |
|
668,231 | 500 | 552 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Consumer & Business Products (0.07%)* |
|
500 | 552 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Diagnostic |
||||||||||||||||||||||||
| Singulex, Inc. |
Diagnostic | Equity | Common Stock | 937,998 | 750 | 574 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Diagnostic (0.07%)* |
|
750 | 574 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Drug Delivery |
||||||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9) |
Drug Delivery | Equity | Common Stock | 54,240 | 108 | 141 | ||||||||||||||||||
| BioQ Pharma Incorporated(15) |
Drug Delivery | Equity | Preferred Series D | 165,000 | 500 | 542 | ||||||||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 161,856 | 1,000 | 2,023 | ||||||||||||||||||
| Merrion Pharmaceuticals, Plc(4)(9) |
Drug Delivery | Equity | Common Stock | 20,000 | 9 | — | ||||||||||||||||||
| Neos Therapeutics, Inc.(3)(15) |
Drug Delivery | Equity | Common Stock | 125,000 | 1,500 | 731 | ||||||||||||||||||
| Revance Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 22,765 | 557 | 472 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Drug Delivery (0.50%)* |
|
3,674 | 3,909 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9)(15) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 426,931 | 1,060 | 231 | ||||||||||||||||
| Cerecor, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 119,087 | 1,000 | 105 | ||||||||||||||||
| Cerulean Pharma, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 135,501 | 1,000 | 96 | ||||||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 142,858 | 1,000 | 411 | ||||||||||||||||
| Dynavax Technologies(3)(9) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 20,000 | 550 | 79 | ||||||||||||||||
| Epirus Biopharmaceuticals, Inc. |
|
Drug Discovery & Development |
|
Equity | Common Stock | 200,000 | 1,000 | — | ||||||||||||||||
| Genocea Biosciences, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 223,463 | 2,000 | 921 | ||||||||||||||||
| Inotek Pharmaceuticals Corporation(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 3,778 | 1,500 | 23 | ||||||||||||||||
| Insmed, Incorporated(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 70,771 | 1,000 | 936 | ||||||||||||||||
| Melinta Therapeutics |
|
Drug Discovery & Development |
|
Equity | Preferred Series 4 | 1,914,448 | 2,000 | 2,042 | ||||||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 76,362 | 2,743 | 1,175 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Drug Discovery & Development (0.76%)* |
|
14,853 | 6,019 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
See notes to consolidated financial statements.
F-15
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||||
| Identiv, Inc.(3) |
Electronics & Computer Hardware |
Equity | Common Stock | 6,700 | $ | 34 | $ | 21 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.00%)* |
|
34 | 21 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Information Services |
||||||||||||||||||||
| DocuSign, Inc.(15) |
Information Services | Equity | Common Stock | 385,000 | 6,081 | 6,081 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Information Services (0.77%)* |
|
6,081 | 6,081 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Internet Consumer & Business Services |
|
|||||||||||||||||||
| Blurb, Inc.(15) |
Internet Consumer & Business Services |
Equity | Preferred Series B | 220,653 | 175 | 197 | ||||||||||||||
| Brigade Group, Inc. (p.k.a. Philotic, Inc.) |
Internet Consumer & Business Services |
Equity | Common Stock | 9,023 | 93 | — | ||||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services |
Equity | Preferred Series C | 230,030 | 250 | 228 | ||||||||||||||
| Internet Consumer & Business Services |
Equity | Preferred Series D | 198,677 | 250 | 221 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Lightspeed POS, Inc. |
428,707 | 500 | 449 | |||||||||||||||||
| OfferUp, Inc.(15) |
Internet Consumer & Business Services |
Equity | Preferred Series A | 286,080 | 1,663 | 1,663 | ||||||||||||||
| Internet Consumer & Business Services |
Equity | Preferred Series A-1 | 108,710 | 632 | 632 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total OfferUp, Inc. |
394,790 | 2,295 | 2,295 | |||||||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services |
Equity | Preferred Series G | 218,351 | 250 | 431 | ||||||||||||||
| Internet Consumer & Business Services |
Equity | Preferred Series H | 87,802 | 250 | 249 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Oportun (p.k.a. Progress Financial) |
306,153 | 500 | 680 | |||||||||||||||||
| RazorGator Interactive Group, Inc. |
Internet Consumer & Business Services |
Equity | Preferred Series AA | 34,783 | 15 | 34 | ||||||||||||||
| Tectura Corporation |
Internet Consumer & Business Services |
Equity | Preferred Series BB | 1,000,000 | — | — | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.46%)* |
|
3,578 | 3,655 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Media/Content/Info |
|
|||||||||||||||||||
| Pinterest, Inc. |
Media/Content/Info | Equity | Preferred Series Seed | 620,000 | 4,085 | 4,085 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (0.52%)* |
|
4,085 | 4,085 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||
| AtriCure, Inc.(3)(15) |
Medical Devices & Equipment |
Equity | Common Stock | 7,536 | 266 | 147 | ||||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment |
Equity | Preferred Series AA | 221,893 | 1,500 | 359 | ||||||||||||||
| Gelesis, Inc.(15) |
Medical Devices & Equipment |
Equity | Common Stock | 198,202 | — | 634 | ||||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series A-1 | 191,210 | 425 | 687 | |||||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series A-2 | 191,626 | 500 | 650 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Gelesis, Inc. |
581,038 | 925 | 1,971 | |||||||||||||||||
| Medrobotics Corporation(15) |
Medical Devices & Equipment |
Equity | Preferred Series E | 136,798 | 250 | 216 | ||||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series F | 73,971 | 155 | 188 | |||||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series G | 163,934 | 500 | 514 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Medrobotics Corporation |
374,703 | 905 | 918 | |||||||||||||||||
See notes to consolidated financial statements.
F-16
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Optiscan Biomedical, Corp.(5)(15) |
Medical Devices & Equipment |
Equity | Preferred Series B | 6,185,567 | $ | 3,000 | $ | 292 | ||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series C | 1,927,309 | 655 | 85 | |||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series D | 55,103,923 | 5,257 | 3,014 | |||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series E | 13,573,546 | 1,136 | 1,138 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Optiscan Biomedical, Corp. |
76,790,345 | 10,048 | 4,529 | |||||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment |
Equity | Preferred Series B | 232,061 | 527 | 548 | ||||||||||||
| Quanterix Corporation |
Medical Devices & Equipment |
Equity | Preferred Series D | 272,479 | 1,000 | 1,086 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (1.21%)* |
|
15,171 | 9,558 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| Box, Inc.(3) |
Software | Equity | Common Stock | 611,442 | 4,709 | 8,475 | ||||||||||||
| CapLinked, Inc. |
Software | Equity | Preferred Series A-3 | 53,614 | 51 | 86 | ||||||||||||
| Druva, Inc. |
Software | Equity | Preferred Series 2 | 458,841 | 1,000 | 1,288 | ||||||||||||
| ForeScout Technologies, Inc. |
Software | Equity | Preferred Series D | 319,099 | 398 | 1,725 | ||||||||||||
| Software | Equity | Preferred Series E | 80,587 | 131 | 440 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total ForeScout Technologies, Inc. |
399,686 | 529 | 2,165 | |||||||||||||||
| HighRoads, Inc. |
Software | Equity | Common Stock | 190 | 307 | — | ||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Equity | Preferred Series E | 669,173 | 963 | 1,025 | ||||||||||||
| Palantir Technologies |
Software | Equity | Preferred Series E | 727,696 | 5,431 | 5,431 | ||||||||||||
| WildTangent, Inc.(15) |
Software | Equity | Preferred Series 3 | 100,000 | 402 | 148 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (2.36%)* |
|
13,392 | 18,618 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||
| QuatRx Pharmaceuticals Company |
Specialty Pharmaceuticals |
Equity | Preferred Series E | 241,829 | 750 | — | ||||||||||||
| Specialty Pharmaceuticals |
Equity | Preferred Series E-1 | 26,955 | — | — | |||||||||||||
| Specialty Pharmaceuticals |
Equity | Preferred Series G | 4,667,636 | — | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total QuatRx Pharmaceuticals Company |
4,936,420 | 750 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.00%)* |
|
750 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
||||||||||||||||||
| Gynesonics, Inc.(15) |
Surgical Devices | Equity | Preferred Series B | 219,298 | 250 | 37 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 656,538 | 282 | 52 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 1,991,157 | 712 | 671 | |||||||||||||
| Surgical Devices | Equity | Preferred Series E | 2,786,367 | 429 | 450 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
5,653,360 | 1,673 | 1,210 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Equity | Preferred Series B | 88,961 | 1,100 | 357 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 119,999 | 300 | 291 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 260,000 | 650 | 912 | |||||||||||||
| Surgical Devices | Equity | Preferred Series F | 100,200 | 500 | 523 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
569,160 | 2,550 | 2,083 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.42%)* |
|
4,223 | 3,293 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Sustainable and Renewable Technology |
|
|||||||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
Sustainable and Renewable Technology |
Equity | Common Stock | 19,250 | 761 | — | ||||||||||||
| Glori Energy, Inc.(3) |
Sustainable and Renewable Technology |
Equity | Common Stock | 18,208 | 165 | 1 | ||||||||||||
| Modumetal, Inc. |
Sustainable and Renewable Technology |
Equity | Preferred Series C | 3,107,520 | 500 | 533 | ||||||||||||
See notes to consolidated financial statements.
F-17
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Proterra, Inc. |
Sustainable and Renewable Technology |
Equity | Preferred Series 5 | 99,280 | $ | 500 | $ | 512 | ||||||||||
| Sungevity, Inc.(15) |
Sustainable and Renewable Technology |
Equity | Preferred Series D | 68,807,339 | 6,750 | — | ||||||||||||
| TPI Composites, Inc.(3) |
Sustainable and Renewable Technology |
Equity | Common Stock | 78,018 | 273 | 1,251 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.29%)* |
|
8,949 | 2,297 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Equity Investments (8.59%)* |
|
81,641 | 67,654 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Warrant Investments |
||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||
| Exicure, Inc. |
Biotechnology Tools | Warrant | Preferred Series C | 104,348 | 107 | 181 | ||||||||||||
| Labcyte, Inc.(15) |
Biotechnology Tools | Warrant | Preferred Series C | 1,127,624 | 323 | 409 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
430 | 590 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Communications & Networking |
||||||||||||||||||
| Intelepeer, Inc.(15) |
Communications & Networking |
Warrant | Common Stock | 117,958 | 102 | — | ||||||||||||
| OpenPeak, Inc. |
Communications & Networking |
Warrant | Common Stock | 108,982 | 149 | — | ||||||||||||
| PeerApp, Inc. |
Communications & Networking |
Warrant | Preferred Series B | 298,779 | 61 | 14 | ||||||||||||
| Peerless Network Holdings, Inc. |
Communications & Networking |
Warrant | Preferred Series A | 135,000 | 95 | 415 | ||||||||||||
| SkyCross, Inc.(6)(15) |
Communications & Networking |
Warrant | Preferred Series F | 9,762,777 | 394 | — | ||||||||||||
| Spring Mobile Solutions, Inc. |
Communications & Networking |
Warrant | Common Stock | 2,834,375 | 418 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Communications & Networking (0.05%)* |
|
1,219 | 429 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Consumer & Business Products |
||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(15) |
Consumer & Business Products |
Warrant | Common Stock | 1,662,441 | 228 | — | ||||||||||||
| Intelligent Beauty, Inc.(15) |
Consumer & Business Products |
Warrant | Preferred Series B | 190,234 | 230 | 354 | ||||||||||||
| IronPlanet, Inc. |
Consumer & Business Products |
Warrant | Preferred Series D | 1,155,821 | 1,076 | 5,574 | ||||||||||||
| Nasty Gal(15) |
Consumer & Business Products |
Warrant | Preferred Series C | 845,194 | 23 | — | ||||||||||||
| The Neat Company(15) |
Consumer & Business Products |
Warrant | Preferred Series C-1 | 540,540 | 365 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Consumer & Business Products (0.75%)* |
|
1,922 | 5,928 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Delivery |
||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9)(15) |
Drug Delivery | Warrant | Common Stock | 176,730 | 785 | 92 | ||||||||||||
| Agile Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 180,274 | 730 | 269 | ||||||||||||
| Aprecia Pharmaceuticals Company |
Drug Delivery | Warrant | Preferred Series A-1 | 735,981 | 366 | 242 | ||||||||||||
| BIND Therapeutics, Inc.(15) |
Drug Delivery | Warrant | Common Stock | 152,586 | 488 | — | ||||||||||||
| BioQ Pharma Incorporated |
Drug Delivery | Warrant | Common Stock | 459,183 | 1 | 264 | ||||||||||||
| Celsion Corporation(3) |
Drug Delivery | Warrant | Common Stock | 194,986 | 428 | — | ||||||||||||
| Dance Biopharm, Inc.(15) |
Drug Delivery | Warrant | Common Stock | 110,882 | 74 | — | ||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 78,595 | 390 | 402 | ||||||||||||
| Kaleo, Inc. (p.k.a. Intelliject, Inc.) |
Drug Delivery | Warrant | Preferred Series B | 82,500 | 594 | 391 | ||||||||||||
| Neos Therapeutics, Inc.(3)(15) |
Drug Delivery | Warrant | Common Stock | 70,833 | 285 | 17 | ||||||||||||
| Pulmatrix Inc.(3) |
Drug Delivery | Warrant | Common Stock | 25,150 | 116 | — | ||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(3) |
Drug Delivery | Warrant | Common Stock | 72,379 | 266 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Delivery (0.21%)* |
|
4,523 | 1,677 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-18
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Drug Discovery & Development |
||||||||||||||||||
| ADMA Biologics, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 89,750 | $ | 295 | $ | 43 | ||||||||||
| Anthera Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 40,178 | 984 | — | ||||||||||||
| Auris Medical Holding, AG(3)(4)(9) |
Drug Discovery & Development |
Warrant | Common Stock | 156,726 | 249 | 51 | ||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9) |
Drug Discovery & Development |
Warrant | Common Stock | 2,069,880 | 396 | 123 | ||||||||||||
| Brickell Biotech, Inc. |
Drug Discovery & Development |
Warrant | Preferred Series C | 26,086 | 119 | 139 | ||||||||||||
| Cerecor, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 22,328 | 70 | — | ||||||||||||
| Cerulean Pharma, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 171,901 | 369 | 14 | ||||||||||||
| Chroma Therapeutics, Ltd.(4)(9) |
Drug Discovery & Development |
Warrant | Preferred Series D | 325,261 | 490 | — | ||||||||||||
| Cleveland BioLabs, Inc.(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 7,813 | 105 | — | ||||||||||||
| Concert Pharmaceuticals, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 70,796 | 367 | 56 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(3) |
Drug Discovery & Development |
Warrant | Common Stock | 292,398 | 165 | 8 | ||||||||||||
| CytRx Corporation(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 634,146 | 416 | 78 | ||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 200 | 28 | — | ||||||||||||
| Epirus Biopharmaceuticals, Inc. |
Drug Discovery & Development |
Warrant | Common Stock | 64,194 | 276 | — | ||||||||||||
| Fortress Biotech, Inc. (p.k.a. Coronado Biosciences, Inc.)(3) |
Drug Discovery & Development |
Warrant | Common Stock | 73,009 | 142 | 13 | ||||||||||||
| Genocea Biosciences, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 73,725 | 266 | 75 | ||||||||||||
| Immune Pharmaceuticals(3) |
Drug Discovery & Development |
Warrant | Common Stock | 214,853 | 164 | — | ||||||||||||
| Mast Therapeutics, Inc.(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 2,272,724 | 203 | 85 | ||||||||||||
| Melinta Therapeutics |
Drug Discovery & Development |
Warrant | Preferred Series 3 | 1,382,323 | 626 | 295 | ||||||||||||
| Nanotherapeutics, Inc.(15) |
Drug Discovery & Development |
Warrant | Common Stock | 171,389 | 838 | 767 | ||||||||||||
| Neothetics, Inc. (p.k.a. Lithera, Inc)(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 46,838 | 266 | 29 | ||||||||||||
| Neuralstem, Inc.(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 75,187 | 77 | 1 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 69,840 | 152 | 157 | ||||||||||||
| PhaseRx,Inc.(3)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 63,000 | 125 | 15 | ||||||||||||
| Sorrento Therapeutics, Inc.(3)(9) |
Drug Discovery & Development |
Warrant | Common Stock | 306,748 | 890 | 632 | ||||||||||||
| uniQure B.V.(3)(4)(9) |
Drug Discovery & Development |
Warrant | Common Stock | 37,174 | 218 | 8 | ||||||||||||
| XOMA Corporation(3)(9)(15) |
Drug Discovery & Development |
Warrant | Common Stock | 9,063 | 279 | 6 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Discovery & Development (0.33%)* |
|
8,575 | 2,595 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-19
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| Clustrix, Inc. |
Electronics & Computer Hardware |
Warrant | Common Stock | 50,000 | $ | 12 | $ | — | ||||||||||
| Persimmon Technologies |
Electronics & Computer Hardware |
Warrant | Preferred Series D | 63,348 | 40 | 509 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.06%)* |
|
52 | 509 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||
| Chromadex Corporation(3)(15) |
Healthcare Services, Other |
Warrant | Common Stock | 139,673 | 157 | 137 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Healthcare Services, Other (0.02%)* |
|
157 | 137 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Information Services |
||||||||||||||||||
| INMOBI Inc.(4)(9) |
Information Services | Warrant | Common Stock | 46,874 | 82 | — | ||||||||||||
| InXpo, Inc.(15) |
Information Services | Warrant | Preferred Series C | 648,400 | 98 | 4 | ||||||||||||
| Information Services | Warrant | Preferred Series C-1 | 1,165,183 | 74 | 6 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total InXpo, Inc. |
1,813,583 | 172 | 10 | |||||||||||||||
| RichRelevance, Inc.(15) |
Information Services | Warrant | Preferred Series E | 112,612 | 98 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Information Services (0.00%)* |
|
352 | 10 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
|
|||||||||||||||||
| Aria Systems, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series E | 239,692 | 73 | — | ||||||||||||
| Blurb, Inc.(15) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 234,280 | 636 | 96 | ||||||||||||
| CashStar, Inc.(15) |
Internet Consumer & Business Services |
Warrant | Preferred Series C-2 | 727,272 | 130 | 24 | ||||||||||||
| CloudOne, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series E | 968,992 | 19 | 46 | ||||||||||||
| Intent Media, Inc.(15) |
Internet Consumer & Business Services |
Warrant | Common Stock | 140,077 | 168 | 167 | ||||||||||||
| Just Fabulous, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series B | 206,184 | 1,102 | 1,093 | ||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 245,610 | 20 | 31 | ||||||||||||
| LogicSource(15) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 79,625 | 30 | 59 | ||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services |
Warrant | Preferred Series G | 174,562 | 78 | 190 | ||||||||||||
| Prism Education Group, Inc.(15) |
Internet Consumer & Business Services |
Warrant | Preferred Series B | 200,000 | 43 | — | ||||||||||||
| ShareThis, Inc.(15) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 493,502 | 547 | 1 | ||||||||||||
| Snagajob.com, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series A | 1,575,000 | 640 | 1,075 | ||||||||||||
| Tapjoy, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series D | 748,670 | 316 | 19 | ||||||||||||
| Tectura Corporation |
Internet Consumer & Business Services |
Warrant | Preferred Series B-1 | 253,378 | 51 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.36%)* |
|
3,853 | 2,801 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Media/Content/Info |
||||||||||||||||||
| FanDuel, Inc. |
Media/Content/Info | Warrant | Preferred Series E-1 | 4,648 | 730 | 682 | ||||||||||||
| Machine Zone, Inc.(16) |
Media/Content/Info | Warrant | Common Stock | 1,552,710 | 1,958 | 2,729 | ||||||||||||
| Rhapsody International, Inc.(15) |
Media/Content/Info | Warrant | Common Stock | 715,755 | 385 | 7 | ||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9) |
Media/Content/Info | Warrant | Common Stock | 127,909 | 1 | 6 | ||||||||||||
| Zoom Media Group, Inc. |
Media/Content/Info | Warrant | Preferred Series A | 1,204 | 348 | 14 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Media/Content/Info (0.44%)* |
|
3,422 | 3,438 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-20
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||||
| Amedica Corporation(3)(15) |
|
Medical Devices & Equipment |
|
Warrant | Common Stock | 103,225 | $ | 459 | $ | 14 | ||||||||||||
| Aspire Bariatrics, Inc.(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series D | 395,000 | 455 | 217 | ||||||||||||||
| Avedro, Inc.(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series AA | 300,000 | 401 | 254 | ||||||||||||||
| Flowonix Medical Incorporated |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series AA | 155,325 | 362 | 21 | ||||||||||||||
| Gamma Medica, Inc. |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series A | 450,956 | 170 | 234 | ||||||||||||||
| Gelesis, Inc.(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series A-1 | 74,784 | 78 | 153 | ||||||||||||||
| InspireMD, Inc.(3)(4)(9) |
|
Medical Devices & Equipment |
|
Warrant | Common Stock | 39,364 | 242 | 20 | ||||||||||||||
| IntegenX, Inc.(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series C | 547,752 | 15 | 35 | ||||||||||||||
| Medrobotics Corporation(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series E | 455,539 | 370 | 292 | ||||||||||||||
| Micell Technologies, Inc. |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series D-2 | 84,955 | 262 | 347 | ||||||||||||||
| NetBio, Inc. |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series A | 7,841 | 408 | 158 | ||||||||||||||
| NinePoint Medical, Inc.(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series A-1 | 587,840 | 170 | 65 | ||||||||||||||
| Optiscan Biomedical, Corp.(5)(15) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series D | 10,535,275 | 1,252 | 170 | ||||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series A | 500,000 | 402 | 355 | ||||||||||||||
| Quanterix Corporation |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series C | 173,428 | 180 | 104 | ||||||||||||||
| SonaCare Medical, LLC (p.k.a. US |
|
Medical Devices & Equipment |
|
Warrant | Preferred Series A | 6,464 | 188 | — | ||||||||||||||
| Strata Skin Sciences, Inc. (p.k.a. MELA Sciences, Inc.)(3) |
|
Medical Devices & Equipment |
|
Warrant | Common Stock | 69,320 | 402 | — | ||||||||||||||
| ViewRay, Inc.(3)(15) |
|
Medical Devices & Equipment |
|
Warrant | Common Stock | 128,231 | 333 | 2 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Medical Devices & Equipment (0.31%)* |
|
6,149 | 2,441 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Semiconductors |
||||||||||||||||||||||
| Achronix Semiconductor Corporation(15) |
Semiconductors | Warrant | Preferred Series C | 360,000 | 160 | 71 | ||||||||||||||||
| Semiconductors | Warrant | Preferred Series D-1 | 500,000 | 7 | 25 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Achronix Semiconductor Corporation |
|
860,000 | 167 | 96 | ||||||||||||||||||
| Aquantia Corp. |
Semiconductors | Warrant | Preferred Series G | 196,831 | 4 | 88 | ||||||||||||||||
| Avnera Corporation |
Semiconductors | Warrant | Preferred Series E | 141,567 | 46 | 114 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Semiconductors (0.04%)* |
|
217 | 298 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Software |
||||||||||||||||||||||
| Actifio, Inc. |
Software | Warrant | Common Stock | 73,584 | 249 | 83 | ||||||||||||||||
| Software | Warrant | Preferred Series F | 31,673 | 343 | 54 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Actifio, Inc. |
|
105,257 | 592 | 137 | ||||||||||||||||||
| Braxton Technologies, LLC |
Software | Warrant | Preferred Series A | 168,750 | 188 | — | ||||||||||||||||
| CareCloud Corporation(15) |
Software | Warrant | Preferred Series B | 413,433 | 258 | 488 | ||||||||||||||||
| Clickfox, Inc.(15) |
Software | Warrant | Preferred Series B | 1,038,563 | 330 | 63 | ||||||||||||||||
| Software | Warrant | Preferred Series C | 592,019 | 730 | 76 | |||||||||||||||||
| Software | Warrant | Preferred Series C-A | 2,218,214 | 230 | 1,604 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Clickfox, Inc. |
|
3,848,796 | 1,290 | 1,743 | ||||||||||||||||||
See notes to consolidated financial statements.
F-21
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||||
| Cloud Technology Partners, Inc. |
Software | Warrant | Preferred Series C | 113,960 | $ | 34 | $ | 35 | ||||||||||||
| Evernote Corporation(15) |
Software | Warrant | Common Stock | 62,500 | 106 | 110 | ||||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(15) |
Software | Warrant | Preferred Series E | 614,333 | 16 | — | ||||||||||||||
| Mattersight Corporation(3) |
Software | Warrant | Common Stock | 357,143 | 538 | 386 | ||||||||||||||
| Message Systems, Inc.(15) |
Software | Warrant | Preferred Series C | 503,718 | 334 | 325 | ||||||||||||||
| Mobile Posse, Inc.(15) |
Software | Warrant | Preferred Series C | 396,430 | 130 | 102 | ||||||||||||||
| Neos, Inc.(15) |
Software | Warrant | Common Stock | 221,150 | 22 | 64 | ||||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Warrant | Preferred Series E | 225,586 | 33 | 45 | ||||||||||||||
| OneLogin, Inc.(15) |
Software | Warrant | Common Stock | 228,972 | 150 | 188 | ||||||||||||||
| Poplicus, Inc.(15) |
Software | Warrant | Preferred Series C | 2,595,230 | — | 6 | ||||||||||||||
| Quid, Inc.(15) |
Software | Warrant | Preferred Series D | 71,576 | 1 | 8 | ||||||||||||||
| RedSeal Inc.(15) |
Software | Warrant | Preferred Series C-Prime | 640,603 | 66 | 65 | ||||||||||||||
| Signpost, Inc.(15) |
Software | Warrant | Preferred Series C | 324,005 | 314 | 167 | ||||||||||||||
| Soasta, Inc.(15) |
Software | Warrant | Preferred Series E | 410,800 | 691 | 190 | ||||||||||||||
| Sonian, Inc.(15) |
Software | Warrant | Preferred Series C | 185,949 | 106 | 105 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (0.53%)* |
|
4,869 | 4,164 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||
| Alimera Sciences, Inc.(3) |
Specialty Pharmaceuticals |
Warrant | Common Stock | 1,717,709 | 860 | 421 | ||||||||||||||
| QuatRx Pharmaceuticals Company |
Specialty Pharmaceuticals |
Warrant | Preferred Series E | 155,324 | 308 | — | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.05%)* |
|
1,168 | 421 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Surgical Devices |
||||||||||||||||||||
| Gynesonics, Inc.(15) |
Surgical Devices | Warrant | Preferred Series C | 180,480 | 75 | 14 | ||||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 1,575,965 | 320 | 240 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Gynesonics, Inc. |
|
1,756,445 | 395 | 254 | ||||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Warrant | Preferred Series B | 40,436 | 225 | 16 | ||||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 175,000 | 100 | 405 | |||||||||||||||
| Surgical Devices | Warrant | Preferred Series F | 50,544 | 38 | 56 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Transmedics, Inc. |
|
265,980 | 363 | 477 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (0.09%)* |
|
758 | 731 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||||
| Agrivida, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series D | 471,327 | 120 | 99 | ||||||||||||||
| Alphabet Energy, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series A | 86,329 | 82 | — | ||||||||||||||
| American Superconductor Corporation(3) |
Sustainable and Renewable Technology |
Warrant | Common Stock | 58,823 | 39 | 85 | ||||||||||||||
| Beamreach Solar (p.k.a. Solexel, Inc.)(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 1,171,625 | 1,162 | — | ||||||||||||||
| Brightsource Energy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series 1 | 116,666 | 104 | — | ||||||||||||||
| Calera, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 44,529 | 513 | — | ||||||||||||||
| EcoMotors, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series B | 437,500 | 308 | 30 | ||||||||||||||
| Fluidic, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series D | 61,804 | 102 | 20 | ||||||||||||||
See notes to consolidated financial statements.
F-22
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
Sustainable and Renewable Technology |
Warrant | Common Stock | 530,811 | $ | 181 | $ | — | ||||||||||
| Sustainable and Renewable Technology |
Warrant | Preferred Series 2-A | 6,229 | 50 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Flywheel Building Intelligence, Inc. (p.k.a. |
537,040 | 231 | — | |||||||||||||||
| Fulcrum Bioenergy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series C-1 | 280,897 | 275 | 201 | ||||||||||||
| GreatPoint Energy, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series D-1 | 393,212 | 548 | — | ||||||||||||
| Polyera Corporation(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 311,609 | 338 | — | ||||||||||||
| Proterra, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series 4 | 477,517 | 41 | 457 | ||||||||||||
| Rive Technology, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series E | 234,477 | 12 | 3 | ||||||||||||
| Stion Corporation(5) |
Sustainable and Renewable Technology |
Warrant | Preferred Series Seed | 2,154 | 1,378 | — | ||||||||||||
| Sungevity, Inc. |
Sustainable and Renewable Technology |
Warrant | Common Stock | 20,000,000 | 543 | — | ||||||||||||
| Sustainable and Renewable Technology |
Warrant | Preferred Series C | 32,472,222 | 902 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Sungevity, Inc. |
52,472,222 | 1,445 | — | |||||||||||||||
| TAS Energy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series AA | 428,571 | 299 | — | ||||||||||||
| Tendril Networks |
Sustainable and Renewable Technology |
Warrant | Preferred Series 3-A | 1,019,793 | 189 | 219 | ||||||||||||
| Trilliant, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series A | 320,000 | 162 | 202 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.17%)* |
|
7,348 | 1,316 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Warrant Investments (3.49%)* |
|
45,014 | 27,485 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total Investments (180.72%)* |
|
$ | 1,511,526 | $ | 1,423,942 | |||||||||||||
|
|
|
|
|
|||||||||||||||
| * | Value as a percent of net assets |
| (1) | Preferred and common stock, warrants, and equity interests are generally non-income producing. |
| (2) | Gross unrealized appreciation, gross unrealized depreciation, and net depreciation for federal income tax purposes totaled $24.7 million, $114.5 million and $89.8 million respectively. The tax cost of investments is $1.5 billion. |
| (3) | Except for warrants in 37 publicly traded companies and common stock in 19 publicly traded companies, all investments are restricted at December 31, 2016 and were valued at fair value as determined in good faith by the Company’s board of directors (the “Board of Directors”). No unrestricted securities of the same issuer are outstanding. The Company uses the Standard Industrial Code for classifying the industry grouping of its portfolio companies. |
| (4) | Non-U.S. company or the company’s principal place of business is outside the United States. |
| (5) | Affiliate investment as defined under the Investment Company Act of 1940, as amended, (the “1940 Act”) in which Hercules owns at least 5% but generally less than 25% of the company’s voting securities. |
| (6) | Control investment as defined under the 1940 Act in which Hercules owns at least 25% of the company’s voting securities or has greater than 50% representation on its board. |
| (7) | Debt is on non-accrual status at December 31, 2016, and is therefore considered non-income producing. Note that at December 31, 2016, only the $11.0 million PIK, or payment-in-kind, loan is on non-accrual for the Company’s debt investment in Tectura Corporation. |
| (8) | Denotes that all or a portion of the debt investment is convertible debt. |
See notes to consolidated financial statements.
F-23
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(dollars in thousands)
| (9) | Indicates assets that the Company deems not “qualifying assets” under section 55(a) of 1940 Act. Qualifying assets must represent at least 70% of the Company’s total assets at the time of acquisition of any additional non-qualifying assets. |
| (10) | Denotes that all or a portion of the debt investment secures the notes offered in the Debt Securitization (as defined in Note 4). |
| (11) | Denotes that all or a portion of the debt investment is pledged as collateral under the Wells Facility (as defined in Note 4). |
| (12) | Denotes that all or a portion of the debt investment is pledged as collateral under the Union Bank Facility (as defined in Note 4). |
| (13) | Denotes that all or a portion of the debt investment principal includes accumulated PIK interest and is net of repayments. |
| (14) | Denotes that all or a portion of the debt investment includes an exit fee receivable. |
| A. | This fee ranges from 1.0% to 5.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| B. | This fee ranges from 5.0% to 10.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| C. | This fee ranges from 10.0% to 15.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| D. | This fee is greater than 15.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| (15) | Denotes that all or a portion of the investment in this portfolio company is held by Hercules Technology II, L.P., or HT II, or Hercules Technology III, L.P., or HT III, the Company’s wholly owned small business investment companies, or SBIC, subsidiaries. |
| (16) | Denotes that the fair value of the Company’s total investments in this portfolio company represent greater than 5% of the Company’s total assets at December 31, 2016. |
| (17) | Denotes that there is an unfunded contractual commitment available at the request of this portfolio company at December 31, 2016. Refer to Note 10. |
| (18) | Repayment of debt investment is delinquent of the contractual maturity date as of December 31, 2016. |
| (19) | The stated PIK interest rate may be reduced to 1.45% subject to achievement of a milestone by the portfolio company. |
See notes to consolidated financial statements.
F-24
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Debt Investments |
||||||||||||||||||||
| Communications & Networking |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Avanti Communications Group(4)(9) |
Communications & Networking | Senior Secured | October 2019 | Interest rate FIXED 10.00% | $ | 10,000 | $ | 8,900 | $ | 7,812 | ||||||||||
| OpenPeak, Inc.(7) |
Communications & Networking | Senior Secured | April 2017 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 12,370 | 9,134 | 2,444 | ||||||||||||
| SkyCross, Inc.(7)(12)(13)(14) |
Communications & Networking | Senior Secured | January 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95%, PIK Interest 5.00% |
$ | 19,649 | 20,080 | 14,859 | ||||||||||||
| Spring Mobile Solutions, Inc.(13) |
Communications & Networking | Senior Secured | January 2019 | Interest rate PRIME + 6.70% or Floor rate of 9.95% |
$ | 3,000 | 2,935 | 2,935 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
41,049 | 28,050 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Communications & Networking (3.91%)* |
|
41,049 | 28,050 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Antenna79 (p.k.a. Pong |
Consumer & Business Products | Senior Secured | June 2016 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 308 | 308 | 308 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
308 | 308 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Antenna79 (p.k.a. Pong
Research |
Consumer & Business Products | Senior Secured | December 2017 | Interest rate PRIME + 6.75% or Floor rate of 10.00%, PIK Interest 2.50% |
$ | 4,955 | 4,785 | 4,783 | ||||||||||||
| Miles, Inc. (p.k.a. Fluc, Inc.)(8) |
Consumer & Business Products | Convertible Debt | March 2017 | Interest rate FIXED 4.00% | $ | 100 | 100 | — | ||||||||||||
| Nasty Gal(13)(14) |
Consumer & Business Products | Senior Secured | May 2019 | Interest rate PRIME + 5.45% or Floor rate of 8.95% |
$ | 15,000 | 14,876 | 14,876 | ||||||||||||
| The Neat Company(7)(12)(13)(14) |
Consumer & Business Products | Senior Secured | September 2017 | Interest rate PRIME + 7.75% or Floor rate of 11.00%, PIK Interest 1.00% |
$ | 15,936 | 15,545 | 5,527 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
35,306 | 25,186 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Consumer & Business Products (3.55%)* |
|
35,614 | 25,494 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Delivery |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(9)(10)(13)(14) |
Drug Delivery | Senior Secured | October 2017 | Interest rate PRIME + 3.85% or Floor rate of 9.10% |
$ | 20,466 | 20,772 | 20,678 | ||||||||||||
| Agile Therapeutics, Inc.(10)(13) |
Drug Delivery | Senior Secured | December 2018 | Interest rate PRIME + 4.75% or Floor rate of 9.00% |
$ | 16,500 | 16,231 | 16,107 | ||||||||||||
| BIND Therapeutics, Inc.(13)(14) |
Drug Delivery | Senior Secured | July 2018 | Interest rate PRIME + 5.10% or Floor rate of 8.35% |
$ | 15,000 | 15,119 | 15,044 | ||||||||||||
| BioQ Pharma Incorporated(10)(13) |
Drug Delivery | Senior Secured | May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 10,000 | 10,180 | 10,066 | ||||||||||||
| Drug Delivery | Senior Secured | May 2018 | Interest rate PRIME + 7.00% or Floor rate of 10.50% |
$ | 3,000 | 2,962 | 2,962 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total BioQ Pharma Incorporated |
$ | 13,000 | 13,142 | 13,028 | ||||||||||||||||
| Celator Pharmaceuticals, Inc.(10)(13) |
Drug Delivery | Senior Secured | June 2018 | Interest rate PRIME + 6.50% or Floor rate of 9.75% |
$ | 14,573 | 14,594 | 14,609 | ||||||||||||
| Celsion Corporation(10)(13) |
Drug Delivery | Senior Secured | June 2017 | Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 6,346 | 6,501 | 6,544 | ||||||||||||
| Dance Biopharm, Inc.(13)(14) |
Drug Delivery | Senior Secured | November 2017 | Interest rate PRIME + 7.40% or Floor rate of 10.65% |
$ | 2,705 | 2,776 | 2,757 | ||||||||||||
| Edge Therapeutics, Inc.(10)(13) |
Drug Delivery | Senior Secured | March 2018 | Interest rate PRIME + 6.45% or Floor rate of 9.95% |
$ | 5,466 | 5,431 | 5,455 | ||||||||||||
| Egalet Corporation(11)(13) |
Drug Delivery | Senior Secured | July 2018 | Interest rate PRIME + 6.15% or Floor rate of 9.40% |
$ | 15,000 | 14,967 | 15,036 | ||||||||||||
See notes to consolidated financial statements.
F-25
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Neos Therapeutics, Inc.(10)(13)(14) |
Drug Delivery | Senior Secured | October 2017 | Interest rate PRIME + 5.75% or Floor rate of 9.00% |
$ | 10,000 | $ | 10,000 | $ | 10,007 | ||||||||||
| Drug Delivery | Senior Secured | October 2017 | Interest rate PRIME + 7.25% or Floor rate of 10.50% |
$ | 10,000 | 10,043 | 9,998 | |||||||||||||
| Drug Delivery | Senior Secured | October 2017 | Interest rate PRIME + 5.75% or Floor rate of 9.00% |
$ | 5,000 | 4,977 | 4,957 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Neos Therapeutics, Inc. |
$ | 25,000 | 25,020 | 24,962 | ||||||||||||||||
| Pulmatrix Inc.(8)(10)(13) |
Drug Delivery | Senior Secured | July 2018 | Interest rate PRIME + 6.25% or Floor rate of 9.50% |
$ | 7,000 | 6,877 | 6,856 | ||||||||||||
| ZP Opco, Inc. (p.k.a.
Zosano |
Drug Delivery | Senior Secured | December 2018 | Interest rate PRIME + 2.70% or Floor rate of 7.95% |
$ | 15,000 | 14,925 | 14,781 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
156,355 | 155,857 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Delivery (21.73%)* |
|
156,355 | 155,857 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Aveo Pharmaceuticals, Inc.(9)(13) |
Drug Discovery & Development | Senior Secured | January 2018 | Interest rate PRIME + 6.65% or Floor rate of 11.90% |
$ | 10,000 | 10,076 | 9,944 | ||||||||||||
| Cerecor, Inc.(13) |
Drug Discovery & Development | Senior Secured | August 2017 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 5,688 | 5,705 | 5,740 | ||||||||||||
| Cerulean Pharma, Inc.(11)(13) |
Drug Discovery & Development | Senior Secured | July 2018 | Interest rate PRIME + 1.55% or Floor rate of 7.30% |
$ | 21,000 | 21,132 | 21,109 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell
Therapeutics, |
Drug Discovery & Development | Senior Secured | December 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 25,000 | 25,507 | 25,550 | ||||||||||||
| Epirus Biopharmaceuticals, Inc.(11)(13) |
Drug Discovery & Development | Senior Secured | April 2018 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 15,000 | 14,852 | 14,924 | ||||||||||||
| Genocea Biosciences, Inc.(10)(13) |
Drug Discovery & Development | Senior Secured | January 2019 | Interest rate PRIME + 3.75% or Floor rate of 7.25% |
$ | 17,000 | 17,008 | 16,948 | ||||||||||||
| Immune Pharmaceuticals(10)(13) |
Drug Discovery & Development | Senior Secured | September 2018 | Interest rate PRIME + 6.50% or Floor rate of 10.00% |
$ | 4,500 | 4,374 | 4,374 | ||||||||||||
| Insmed, Incorporated(10)(13) |
Drug Discovery & Development | Senior Secured | January 2018 | Interest rate PRIME + 4.75% or Floor rate of 9.25% |
$ | 25,000 | 25,128 | 24,991 | ||||||||||||
| Mast Therapeutics, Inc.(13)(14) |
Drug Discovery & Development | Senior Secured | January 2019 | Interest rate PRIME + 5.70% or Floor rate of 8.95% |
$ | 15,000 | 14,808 | 14,808 | ||||||||||||
| Melinta Therapeutics(11)(13) |
Drug Discovery & Development | Senior Secured | June 2018 | Interest rate PRIME + 3.75% or Floor rate of 8.25% |
$ | 30,000 | 29,843 | 29,703 | ||||||||||||
| Merrimack Pharmaceuticals, Inc.(9) |
Drug Discovery & Development | Senior Secured | December 2022 | Interest rate FIXED 11.50% | $ | 25,000 | 25,000 | 25,000 | ||||||||||||
| Neothetics, Inc. (p.k.a. Lithera, Inc.)(13)(14) |
Drug Discovery & Development | Senior Secured | January 2018 | Interest rate PRIME + 5.75% or Floor rate of 9.00% |
$ | 10,000 | 9,966 | 9,940 | ||||||||||||
| Neuralstem, Inc.(13)(14) |
Drug Discovery & Development | Senior Secured | April 2017 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 8,335 | 8,418 | 8,397 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(13)(14) |
Drug Discovery & Development | Senior Secured | September 2020 | Interest rate PRIME + 2.75% or Floor rate of 8.50% |
$ | 20,000 | 19,828 | 19,828 | ||||||||||||
| uniQure B.V.(4)(9)(10)(13) |
Drug Discovery & Development | Senior Secured | June 2018 | Interest rate PRIME + 5.00% or Floor rate of 10.25% |
$ | 20,000 | 19,956 | 19,929 | ||||||||||||
| XOMA Corporation(9)(13)(14) |
Drug Discovery & Development | Senior Secured | September 2018 | Interest rate PRIME + 2.15% or Floor rate of 9.40% |
$ | 20,000 | 19,974 | 19,815 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
271,575 | 271,000 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Discovery & Development (37.79%)* |
|
271,575 | 271,000 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Electronics & Computer Hardware |
|
|||||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Persimmon Technologies(13) |
Electronics & Computer Hardware | Senior Secured | June 2019 | Interest rate PRIME + 7.50% or Floor rate of 11.00% |
$ | 7,000 | 6,873 | 6,873 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
6,873 | 6,873 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.96%)* |
|
6,873 | 6,873 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-26
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| Agrivida, Inc.(13)(14) |
Sustainable and Renewable Technology | Senior Secured | December 2016 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 4,362 | $ | 4,587 | $ | 4,587 | ||||||||||
| American Superconductor Corporation(10)(13) |
Sustainable and Renewable Technology | Senior Secured | November 2016 | Interest rate PRIME + 7.25% or Floor rate of 11.00% |
$ | 3,667 | 4,106 | 4,106 | ||||||||||||
| Fluidic, Inc.(10)(13) |
Sustainable and Renewable Technology | Senior Secured | March 2016 | Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 784 | 931 | 931 | ||||||||||||
| Polyera Corporation(13)(14) |
Sustainable and Renewable Technology | Senior Secured | April 2016 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 637 | 890 | 890 | ||||||||||||
| Stion Corporation(5)(13) |
Sustainable and Renewable Technology | Senior Secured | March 2016 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 2,200 | 2,200 | 1,013 | ||||||||||||
| Sungevity, Inc.(11) |
Sustainable and Renewable Technology | Senior Secured | April 2016 | Interest rate PRIME + 3.70% or Floor rate of 6.95% |
$ | 20,000 | 20,000 | 20,000 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
32,714 | 31,527 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| American Superconductor Corporation(10)(13) |
Sustainable and Renewable Technology | Senior Secured | June 2017 | Interest rate PRIME + 7.25% or Floor rate of 11.00% |
$ | 1,500 | 1,496 | 1,484 | ||||||||||||
| Amyris, Inc.(9)(11)(13) |
Sustainable and Renewable Technology | Senior Secured | February 2017 | Interest rate PRIME + 6.25% or Floor rate of 9.50% |
$ | 17,543 | 17,543 | 17,499 | ||||||||||||
| Sustainable and Renewable Technology | Senior Secured | February 2017 | Interest rate PRIME + 5.25% or Floor rate of 8.50% |
$ | 3,497 | 3,497 | 3,488 | |||||||||||||
| Sustainable and Renewable Technology | Senior Secured | February 2017 | Interest rate PRIME + 6.25% or Floor rate of 9.50% |
$ | 10,960 | 11,045 | 11,045 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Amyris, Inc. |
$ | 32,000 | 32,085 | 32,032 | ||||||||||||||||
| Modumetal, Inc.(13) |
Sustainable and Renewable Technology | Senior Secured | March 2017 | Interest rate PRIME + 8.70% or Floor rate of 11.95% |
$ | 1,759 | 2,062 | 2,032 | ||||||||||||
| Sustainable and Renewable Technology | Senior Secured | October 2017 | Interest rate PRIME + 6.00% or Floor rate of 9.25% |
$ | 7,061 | 7,101 | 7,080 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Modumetal, Inc. |
$ | 8,820 | 9,163 | 9,112 | ||||||||||||||||
| Polyera Corporation(13) |
Sustainable and Renewable Technology | Senior Secured | January 2017 | Interest rate PRIME + 6.70% or Floor rate of 9.95% |
$ | 1,254 | 1,455 | 1,455 | ||||||||||||
| Proterra, Inc.(10)(13) |
Sustainable and Renewable Technology | Senior Secured | December 2018 | Interest rate PRIME + 6.95% or Floor rate of 10.20% |
$ | 25,000 | 24,995 | 24,550 | ||||||||||||
| Sungevity, Inc.(11)(13) |
Sustainable and Renewable Technology | Senior Secured | October 2017 | Interest rate PRIME + 3.70% or Floor rate of 6.95% |
$ | 35,000 | 34,733 | 34,773 | ||||||||||||
| Tendril Networks(13) |
Sustainable and Renewable Technology | Senior Secured | June 2019 | Interest rate FIXED 7.25% | $ | 15,000 | 14,735 | 14,477 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
118,662 | 117,883 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (20.83%)* |
|
151,376 | 149,410 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Healthcare Services, Other |
|
|||||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Chromadex Corporation(13)(14) |
Healthcare Services, Other | Senior Secured | April 2018 | Interest rate PRIME + 6.10% or Floor rate of 9.35% |
$ | 5,000 | 4,907 | 4,918 | ||||||||||||
| InstaMed
Communications, |
Healthcare Services, Other | Senior Secured | February 2019 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 10,000 | 10,048 | 10,049 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
14,955 | 14,967 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Healthcare Services, Other (2.09%)* |
|
14,955 | 14,967 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Information Services |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| Eccentex Corporation(13)(16) |
Information Services | Senior Secured | May 2015 | Interest rate PRIME + 7.00% or Floor rate of 10.25% |
$ | 13 | 28 | 28 | ||||||||||||
| InXpo, Inc.(13)(14) |
Information Services | Senior Secured | October 2016 | Interest rate PRIME + 7.50% or Floor rate of 10.75% |
$ | 1,589 | 1,624 | 1,624 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
1,652 | 1,652 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Information Services (0.23%)* |
|
1,652 | 1,652 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-27
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Internet Consumer & Business Services |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| NetPlenish(7)(8)(14) |
Internet Consumer & Business Services |
Convertible Debt | September 2016 | Interest rate FIXED 10.00% | $ | 381 | $ | 373 | $ | — | ||||||||||
| Internet Consumer & Business Services |
Senior Secured | April 2016 | Interest rate FIXED 10.00% | $ | 45 | 45 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total NetPlenish |
$ | 426 | 418 | — | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
418 | — | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Aria Systems, Inc.(10)(12) |
Internet Consumer & Business Services |
Senior Secured | June 2019 | Interest rate PRIME + 5.20% or Floor rate of 8.95%, PIK Interest 1.95% |
$ | 18,101 | 17,850 | 17,673 | ||||||||||||
| Internet Consumer & Business Services |
Senior Secured | June 2019 | Interest rate PRIME + 3.20% or Floor rate of 6.95%, PIK Interest 1.95% |
$ | 2,021 | 1,995 | 1,972 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Aria Systems, Inc. |
$ | 20,122 | 19,845 | 19,645 | ||||||||||||||||
| One Planet Ops Inc. (p.k.a. Reply! Inc.)(7)(12) |
Internet Consumer & Business Services |
Senior Secured | March 2019 | Interest rate PRIME + 4.25% or Floor rate of 7.50% |
$ | 6,321 | 5,811 | 5,811 | ||||||||||||
| Internet Consumer & Business Services |
Senior Secured | March 2019 | PIK Interest 2.00% | $ | 2,129 | 2,129 | 55 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total One Planet Ops Inc. (p.k.a. Reply! Inc.) |
$ | 8,450 | 7,940 | 5,866 | ||||||||||||||||
| ReachLocal(13) |
Internet Consumer & Business Services |
Senior Secured | April 2018 | Interest rate PRIME + 8.50% or Floor rate of 11.75% |
$ | 25,000 | 24,868 | 24,769 | ||||||||||||
| Tapjoy, Inc.(11)(13) |
Internet Consumer & Business Services |
Senior Secured | July 2018 | Interest rate PRIME + 6.50% or Floor rate of 9.75% |
$ | 20,000 | 19,598 | 19,514 | ||||||||||||
| Tectura Corporation(7)(12)(15) |
Internet Consumer & Business Services |
Senior Secured | May 2014 | Interest rate LIBOR + 10.00% or Floor rate of 13.00% |
$ | 6,468 | 6,468 | 4,851 | ||||||||||||
| Internet Consumer & Business Services |
Senior Secured | May 2014 | Interest rate LIBOR + 8.00% or Floor rate of 11.00%, PIK Interest 1.00% |
$ | 8,170 | 8,170 | 6,128 | |||||||||||||
| Internet Consumer & Business Services |
Senior Secured | May 2014 | Interest rate LIBOR + 10.00% or Floor rate of 13.00% |
$ | 563 | 563 | 422 | |||||||||||||
| Internet Consumer & Business Services |
Senior Secured | May 2014 | Interest rate LIBOR + 10.00% or Floor rate of 13.00% |
$ | 5,000 | 5,000 | 3,750 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Tectura Corporation |
$ | 20,201 | 20,201 | 15,151 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
92,452 | 84,945 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (11.85%)* |
|
92,870 | 84,945 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Media/Content/Info |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| Zoom Media Group, Inc. |
Media/Content/Info | Senior Secured | January 2016 | Interest rate PRIME + 5.25% or Floor rate of 8.50% |
$ | 5,060 | 5,060 | 5,060 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
5,060 | 5,060 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Machine Zone, Inc.(12) |
Media/Content/Info | Senior Secured | May 2018 | Interest rate PRIME + 2.50% or Floor rate of 6.75%, PIK Interest 3.00% |
$ | 90,729 | 88,730 | 88,101 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
88,730 | 88,101 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (12.99%)* |
|
93,790 | 93,161 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Medrobotics Corporation(13)(14) |
Medical Devices & Equipment | Senior Secured | March 2016 | Interest rate PRIME + 7.85% or Floor rate of 11.10% |
$ | 576 | 735 | 735 | ||||||||||||
| SonaCare Medical, LLC (p.k.a. US HIFU, LLC)(13) |
Medical Devices & Equipment | Senior Secured | April 2016 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 292 | 700 | 700 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
1,435 | 1,435 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-28
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Amedica Corporation(8)(13)(14) |
Medical Devices & Equipment | Senior Secured | January 2018 | Interest rate PRIME + 9.20% or Floor rate of 12.45% |
$ | 17,051 | $ | 17,642 | $ | 17,350 | ||||||||||
| Aspire Bariatrics, Inc.(13)(14) |
Medical Devices & Equipment | Senior Secured | October 2018 | Interest rate PRIME + 4.00% or Floor rate of 9.25% |
$ | 7,000 | 6,771 | 6,739 | ||||||||||||
| Avedro, Inc.(13)(14) |
Medical Devices & Equipment | Senior Secured | June 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.25% |
$ | 12,500 | 12,391 | 12,201 | ||||||||||||
| Flowonix Medical Incorporated(11)(13) |
Medical Devices & Equipment | Senior Secured | May 2018 | Interest rate PRIME + 6.50% or Floor rate of 10.00% |
$ | 15,000 | 15,071 | 14,974 | ||||||||||||
| Gamma Medica, Inc.(10)(13) |
Medical Devices & Equipment | Senior Secured | January 2018 | Interest rate PRIME + 6.50% or Floor rate of 9.75% |
$ | 4,000 | 4,009 | 3,989 | ||||||||||||
| InspireMD, Inc.(4)(9)(13) |
Medical Devices & Equipment | Senior Secured | February 2017 | Interest rate PRIME + 5.00% or Floor rate of 10.50% |
$ | 5,009 | 5,380 | 3,764 | ||||||||||||
| Quanterix Corporation(10)(13) |
Medical Devices & Equipment | Senior Secured | February 2018 | Interest rate PRIME + 2.75% or Floor rate of 8.00% |
$ | 9,661 | 9,718 | 9,659 | ||||||||||||
| SynergEyes, Inc.(13)(14) |
Medical Devices & Equipment | Senior Secured | January 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 4,263 | 4,516 | 4,464 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
75,498 | 73,140 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Medical Devices & Equipment (10.40%)* |
|
76,933 | 74,575 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Semiconductors |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(14) |
Semiconductors | Senior Secured | July 2016 | Interest rate PRIME + 4.75% or Floor rate of 8.00% |
$ | 5,000 | 5,000 | 5,000 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
5,000 | 5,000 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(13)(14) |
Semiconductors |
Senior Secured |
July 2018 |
Interest rate PRIME + 8.25% or Floor rate of 11.50% |
$ | 5,000 | 5,027 | 4,999 | ||||||||||||
| Aquantia Corp. |
Semiconductors |
Senior Secured |
February 2017 |
Interest rate PRIME + 2.95% or Floor rate of 6.20% |
$ | 5,001 | 5,001 | 5,001 | ||||||||||||
| Avnera Corporation(10)(13) |
Semiconductors |
Senior Secured |
April 2018 |
Interest rate PRIME + 5.25% or Floor rate of 8.50% |
$ | 7,500 | 7,498 | 7,568 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
17,526 | 17,568 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Semiconductors (3.15%)* |
|
22,526 | 22,568 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Software |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Clickfox, Inc.(13)(14)(16) |
Software | Senior Secured | December 2015 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 3,300 | 3,465 | 3,465 | ||||||||||||
| JumpStart Games, Inc. (p.k.a. Knowledge Adventure, Inc.)(12)(13)(14) |
Software | Senior Secured | October 2016 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 1,335 | 1,350 | 875 | ||||||||||||
| Neos, Inc.(13)(14) |
Software | Senior Secured | May 2016 | Interest rate PRIME + 6.75% or Floor rate of 10.50% |
$ | 729 | 895 | 895 | ||||||||||||
| Touchcommerce, Inc.(14) |
Software | Senior Secured | August 2016 | Interest rate PRIME + 2.25% or Floor rate of 6.50% |
$ | 5,511 | 5,511 | 5,511 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
11,221 | 10,746 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Actifio, Inc.(12) |
Software | Senior Secured | January 2019 | Interest rate PRIME + 4.25% or Floor rate of 8.25%, PIK Interest 2.25% |
$ | 30,263 | 30,019 | 29,712 | ||||||||||||
| Clickfox, Inc.(13)(14) |
Software | Senior Secured | March 2018 | Interest rate PRIME + 8.25% or Floor rate of 11.50% |
$ | 5,475 | 5,490 | 5,490 | ||||||||||||
| Druva, Inc.(10)(13) |
Software | Senior Secured | March 2018 | Interest rate PRIME + 4.60% or Floor rate of 7.85% |
$ | 12,000 | 12,080 | 12,034 | ||||||||||||
See notes to consolidated financial statements.
F-29
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| JumpStart Games, Inc. (p.k.a. Knowledge Adventure, Inc.)(12)(13)(14) |
Software | Senior Secured | March 2018 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 11,082 | $ | 11,174 | $ | 7,245 | ||||||||||
| Message Systems, Inc.(14) |
Software | Senior Secured | February 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.50% |
$ | 17,500 | 17,103 | 17,013 | ||||||||||||
| Software | Senior Secured | February 2017 | Interest rate PRIME + 2.75% or Floor rate of 6.00% |
$ | 1,618 | 1,618 | 1,616 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Message Systems, Inc. |
$ | 19,118 | 18,721 | 18,629 | ||||||||||||||||
| RedSeal Inc.(13)(14) |
Software | Senior Secured | June 2017 | Interest rate PRIME + 3.25% or Floor rate of 6.50% |
$ | 3,000 | 3,000 | 2,987 | ||||||||||||
| Software | Senior Secured | June 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 5,000 | 5,006 | 4,979 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total RedSeal Inc. |
$ | 8,000 | 8,006 | 7,966 | ||||||||||||||||
| Soasta, Inc.(13)(14) |
Software | Senior Secured | February 2018 | Interest rate PRIME + 2.25% or Floor rate of 5.50% |
$ | 3,500 | 3,432 | 3,419 | ||||||||||||
| Software | Senior Secured | February 2018 | Interest rate PRIME + 4.75% or Floor rate of 8.00% |
$ | 15,000 | 14,699 | 14,646 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Soasta, Inc. |
$ | 18,500 | 18,131 | 18,065 | ||||||||||||||||
| Touchcommerce, Inc.(13)(14) |
Software | Senior Secured | February 2018 | Interest rate PRIME + 6.00% or Floor rate of 10.25% |
$ | 12,000 | 11,853 | 11,721 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
115,474 | 110,862 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (16.96%)* |
|
126,695 | 121,608 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Cranford Pharmaceuticals, LLC(10)(12) |
Specialty Pharmaceuticals | Senior Secured | August 2016 | Interest rate LIBOR + 8.25% or Floor rate of 9.50% |
$ | 1,100 | 1,100 | 1,100 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
1,100 | 1,100 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Alimera Sciences, Inc.(10)(13) |
Specialty Pharmaceuticals | Senior Secured | May 2018 | Interest rate PRIME + 7.65% or Floor rate of 10.90% |
$ | 35,000 | 34,296 | 34,309 | ||||||||||||
| Cranford Pharmaceuticals, LLC(10)(12)(13)(14) |
Specialty Pharmaceuticals | Senior Secured | August 2017 | Interest rate LIBOR + 9.55% or Floor rate of 10.80%, PIK Interest 1.35% |
$ | 10,041 | 10,164 | 10,235 | ||||||||||||
| Jaguar Animal Health, Inc.(10)(13) |
Specialty Pharmaceuticals | Senior Secured |
August 2018 |
Interest rate PRIME + 5.65% or Floor rate of 9.90% |
$ | 6,000 | 6,009 | 6,009 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
50,469 | 50,553 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (7.20%)* |
|
51,569 | 51,653 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Surgical Devices |
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Transmedics, Inc.(13) |
Surgical Devices | Senior Secured | March 2019 | Interest rate PRIME + 5.30% or Floor rate of 9.55% |
$ | 8,500 | 8,471 | 8,396 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
8,471 | 8,396 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (1.17%)* |
|
8,471 | 8,396 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total Debt Investments (154.81%)* |
|
1,152,303 | 1,110,209 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-30
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type
of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||||||||
| Equity Investments |
|
|||||||||||||||||||||||
| Biotechnology Tools |
|
|||||||||||||||||||||||
| NuGEN Technologies, Inc.(14) |
Biotechnology Tools | Equity | Preferred Series C | 189,394 | $ | 500 | $ | 532 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
500 | 532 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Communications & Networking |
|
|||||||||||||||||||||||
| GlowPoint, Inc.(3) |
|
Communications & Networking |
|
Equity | Common Stock | 114,192 | 102 | 57 | ||||||||||||||||
| Peerless Network, Inc. |
|
Communications & Networking |
|
Equity | Preferred Series A | 1,000,000 | 1,000 | 4,380 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Communications & Networking (0.62%)* |
|
1,102 | 4,437 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Consumer & Business Products |
|
|||||||||||||||||||||||
| Market Force Information, Inc. |
|
Consumer & Business Products |
|
Equity | Common Stock | 480,261 | — | 217 | ||||||||||||||||
| |
Consumer & Business Products |
|
Equity | Preferred Series B-1 | 187,970 | 500 | 3 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total Market Force Information, Inc. |
|
668,231 | 500 | 220 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Consumer & Business Products (0.03%)* |
|
500 | 220 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Diagnostic |
|
|||||||||||||||||||||||
| Singulex, Inc. |
Diagnostic | Equity | Common Stock | 937,998 | 750 | 304 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Diagnostic (0.04%)* |
|
750 | 304 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Drug Delivery |
||||||||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9) |
Drug Delivery | Equity | Common Stock | 54,240 | 108 | 209 | ||||||||||||||||||
| BioQ Pharma Incorporated(14) |
Drug Delivery | Equity | Preferred Series D | 165,000 | 500 | 660 | ||||||||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 157,190 | 1,000 | 1,965 | ||||||||||||||||||
| Merrion Pharmaceuticals, Plc(3)(4)(9) |
Drug Delivery | Equity | Common Stock | 20,000 | 9 | — | ||||||||||||||||||
| Neos Therapeutics, Inc.(3)(14) |
Drug Delivery | Equity | Common Stock | 125,000 | 1,500 | 1,790 | ||||||||||||||||||
| Revance Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 22,765 | 557 | 778 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Drug Delivery (0.75%)* |
|
3,674 | 5,402 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9)(14) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 167,864 | 842 | 212 | ||||||||||||||||
| Cerecor, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 119,087 | 1,000 | 399 | ||||||||||||||||
| Cerulean Pharma, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 135,501 | 1,000 | 379 | ||||||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(14) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 142,858 | 1,000 | 1,695 | ||||||||||||||||
| Dynavax Technologies(3)(9) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 20,000 | 550 | 483 | ||||||||||||||||
| Epirus Biopharmaceuticals, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 200,000 | 1,000 | 618 | ||||||||||||||||
| Genocea Biosciences, Inc.(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 223,463 | 2,000 | 1,178 | ||||||||||||||||
| Inotek Pharmaceuticals Corporation(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 3,778 | 1,500 | 43 | ||||||||||||||||
| Insmed, Incorporated(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 70,771 | 1,000 | 1,284 | ||||||||||||||||
| Melinta Therapeutics |
|
Drug Discovery & Development |
|
Equity | Preferred Series 4 | 1,914,448 | 2,000 | 2,026 | ||||||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3) |
|
Drug Discovery & Development |
|
Equity | Common Stock | 76,362 | 2,743 | 1,450 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Subtotal: Drug Discovery & Development (1.36%)* |
|
14,635 | 9,767 | |||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
See notes to consolidated financial statements.
F-31
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| Identiv, Inc.(3) |
Electronics & Computer Hardware |
Equity | Common Stock | 6,700 | $ | 34 | $ | 13 | ||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.00%)* |
|
34 | 13 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||
| Glori Energy, Inc.(3) |
Sustainable and Renewable Technology |
Equity | Common Stock | 18,208 | 165 | 6 | ||||||||||||
| Modumetal, Inc. |
Sustainable and Renewable Technology |
Equity | Preferred Series C | 3,107,520 | 500 | 455 | ||||||||||||
| SCIEnergy, Inc. |
Sustainable and Renewable Technology |
Equity | Preferred Series 1 | 385,000 | 761 | — | ||||||||||||
| Sungevity, Inc.(14) |
Sustainable and Renewable Technology |
Equity | Preferred Series D | 68,807,339 | 6,750 | 6,912 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (1.03%)* |
|
8,176 | 7,373 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||
| Blurb, Inc.(14) |
Internet Consumer & Business Services |
Equity | Preferred Series B | 220,653 | 175 | 244 | ||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services |
Equity | Preferred Series C | 230,030 | 250 | 264 | ||||||||||||
| Internet Consumer & Business Services |
Equity | Preferred Series D | 198,677 | 250 | 249 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Lightspeed POS, Inc. |
428,707 | 500 | 513 | |||||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services |
Equity | Preferred Series G | 218,351 | 250 | 349 | ||||||||||||
| Internet Consumer & Business Services |
Equity | Preferred Series H | 87,802 | 250 | 248 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Oportun (p.k.a. Progress Financial) |
306,153 | 500 | 597 | |||||||||||||||
| Philotic, Inc. |
Internet Consumer & Business Services |
Equity | Common Stock | 9,023 | 93 | — | ||||||||||||
| RazorGator Interactive Group, Inc. |
Internet Consumer & Business Services |
Equity | Preferred Series AA | 34,783 | 15 | 28 | ||||||||||||
| Taptera, Inc. |
Internet Consumer & Business Services |
Equity | Preferred Series B | 454,545 | 150 | 99 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.21%)* |
|
1,433 | 1,481 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||
| AtriCure, Inc.(3)(14) |
Medical Devices & Equipment |
Equity | Common Stock | 7,536 | 266 | 155 | ||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment |
Equity | Preferred Series E | 221,893 | 1,500 | 1,953 | ||||||||||||
| Gelesis, Inc.(14) |
Medical Devices & Equipment |
Equity | Common Stock | 198,202 | — | 1,005 | ||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series A-1 | 191,210 | 425 | 1,051 | |||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series A-2 | 191,626 | 500 | 1,012 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gelesis, Inc. |
581,038 | 925 | 3,068 | |||||||||||||||
| Medrobotics Corporation(14) |
Medical Devices & Equipment |
Equity | Preferred Series E | 136,798 | 250 | 208 | ||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series F | 73,971 | 155 | 189 | |||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series G | 163,934 | 500 | 500 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Medrobotics Corporation |
374,703 | 905 | 897 | |||||||||||||||
See notes to consolidated financial statements.
F-32
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Novasys Medical, Inc. |
Medical Devices & Equipment |
Equity | Preferred Series D-1 | 4,118,444 | $ | 1,000 | $ | — | ||||||||||
| Optiscan Biomedical, Corp.(5)(14) |
Medical Devices & Equipment |
Equity | Preferred Series B | 6,185,567 | 3,000 | 565 | ||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series C | 1,927,309 | 655 | 169 | |||||||||||||
| Medical Devices & Equipment |
Equity | Preferred Series D | 55,103,923 | 5,257 | 5,927 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Optiscan Biomedical, Corp. |
63,216,799 | 8,912 | 6,661 | |||||||||||||||
| Oraya Therapeutics, Inc. |
Medical Devices & Equipment |
Equity | Preferred Series 1 | 1,086,969 | 500 | 266 | ||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment |
Equity | Preferred Series B | 232,061 | 527 | 543 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (1.89%)* |
|
14,535 | 13,543 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| Box, Inc.(3)(14) |
Software | Equity | Common Stock | 1,287,347 | 5,653 | 17,957 | ||||||||||||
| CapLinked, Inc. |
Software | Equity | Preferred Series A-3 | 53,614 | 51 | 79 | ||||||||||||
| Druva, Inc. |
Software | Equity | Preferred Series 2 | 458,841 | 1,000 | 1,031 | ||||||||||||
| ForeScout Technologies, Inc. |
Software | Equity | Preferred Series D | 319,099 | 398 | 1,368 | ||||||||||||
| Software | Equity | Preferred Series E | 80,587 | 131 | 350 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total ForeScout Technologies, Inc. |
399,686 | 529 | 1,718 | |||||||||||||||
| HighRoads, Inc. |
Software | Equity | Preferred Series B | 190,170 | 307 | — | ||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Equity | Preferred Series E | 669,173 | 963 | 1,016 | ||||||||||||
| WildTangent, Inc.(14) |
Software | Equity | Preferred Series 3 | 100,000 | 402 | 190 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (3.07%)* |
|
8,905 | 21,991 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||
| QuatRx Pharmaceuticals Company |
Specialty Pharmaceuticals |
Equity | Preferred Series E | 241,829 | 750 | — | ||||||||||||
| Specialty Pharmaceuticals |
Equity | Preferred Series E-1 | 26,955 | — | — | |||||||||||||
| Specialty Pharmaceuticals |
Equity | Preferred Series G | 4,667,636 | — | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total QuatRx Pharmaceuticals Company |
4,936,420 | 750 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.00%)* |
|
750 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
||||||||||||||||||
| Gynesonics, Inc.(14) |
Surgical Devices | Equity | Preferred Series B | 219,298 | 250 | 32 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 656,538 | 282 | 46 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 1,991,157 | 712 | 637 | |||||||||||||
| Surgical Devices | Equity | Preferred Series E | 2,785,402 | 429 | 422 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
5,652,395 | 1,673 | 1,137 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Equity | Preferred Series B | 88,961 | 1,100 | 154 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 119,999 | 300 | 96 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 260,000 | 650 | 521 | |||||||||||||
| Surgical Devices | Equity | Preferred Series F | 100,200 | 500 | 471 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
569,160 | 2,550 | 1,242 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.33%)* |
|
4,223 | 2,379 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Equity Investments (9.40%)* |
|
59,217 | 67,442 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Warrant Investments |
||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||
| Labcyte, Inc.(14) |
Biotechnology Tools | Warrant | Preferred Series C | 1,127,624 | 323 | 187 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Biotechnology Tools (0.03%)* |
|
323 | 187 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-33
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Communications & Networking |
||||||||||||||||||
| Intelepeer, Inc.(14) |
Communications & Networking |
Warrant | Common Stock | 117,958 | $ | 102 | $ | — | ||||||||||
| OpenPeak, Inc. |
Communications & Networking |
Warrant | Common Stock | 108,982 | 149 | — | ||||||||||||
| PeerApp, Inc. |
Communications & Networking |
Warrant | Preferred Series B | 298,779 | 61 | 62 | ||||||||||||
| Peerless Network, Inc. |
Communications & Networking |
Warrant | Preferred Series A | 135,000 | 95 | 375 | ||||||||||||
| Ping Identity Corporation |
Communications & Networking |
Warrant | Preferred Series B | 1,136,277 | 52 | 236 | ||||||||||||
| SkyCross, Inc.(14) |
Communications & Networking |
Warrant | Preferred Series F | 9,762,777 | 394 | — | ||||||||||||
| Spring Mobile Solutions, Inc. |
Communications & Networking |
Warrant | Preferred Series D | 2,834,375 | 418 | 53 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Communications & Networking (0.10%)* |
|
1,271 | 726 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Consumer & Business Products |
||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(14) |
Consumer & Business Products |
Warrant | Preferred Series A | 1,662,441 | 228 | 2 | ||||||||||||
| Intelligent Beauty, Inc.(14) |
Consumer & Business Products |
Warrant | Preferred Series B | 190,234 | 230 | 214 | ||||||||||||
| IronPlanet, Inc. |
Consumer & Business Products |
Warrant | Preferred Series D | 1,155,821 | 1,076 | 651 | ||||||||||||
| Market Force Information, Inc. |
Consumer & Business Products |
Warrant | Preferred Series A-1 | 150,212 | 24 | 10 | ||||||||||||
| Nasty Gal(14) |
Consumer & Business Products |
Warrant | Preferred Series C | 845,194 | 23 | 20 | ||||||||||||
| The Neat Company(14) |
Consumer & Business Products |
Warrant | Preferred Series C-1 | 540,540 | 365 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Consumer & Business Products (0.13%)* |
|
1,946 | 897 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Diagnostic |
||||||||||||||||||
| Navidea Biopharmaceuticals, Inc. (p.k.a. Neoprobe)(3)(14) |
Diagnostic | Warrant | Common Stock | 333,333 | 244 | 17 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Diagnostic (0.00%)* |
|
244 | 17 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Delivery |
||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9)(14) |
Drug Delivery | Warrant | Common Stock | 176,730 | 786 | 238 | ||||||||||||
| Agile Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 180,274 | 730 | 680 | ||||||||||||
| BIND Therapeutics, Inc.(3)(14) |
Drug Delivery | Warrant | Common Stock | 152,586 | 488 | 6 | ||||||||||||
| BioQ Pharma Incorporated |
Drug Delivery | Warrant | Common Stock | 459,183 | 1 | 423 | ||||||||||||
| Celator Pharmaceuticals, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 210,675 | 138 | 59 | ||||||||||||
| Celsion Corporation(3) |
Drug Delivery | Warrant | Common Stock | 194,986 | 428 | 20 | ||||||||||||
| Dance Biopharm, Inc.(14) |
Drug Delivery | Warrant | Common Stock | 43,813 | 74 | 55 | ||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 78,595 | 390 | 417 | ||||||||||||
| Kaleo, Inc. (p.k.a. Intelliject, Inc.) |
Drug Delivery | Warrant | Preferred Series B | 82,500 | 594 | 1,217 | ||||||||||||
| Neos Therapeutics, Inc.(3)(14) |
Drug Delivery | Warrant | Common Stock | 70,833 | 285 | 275 | ||||||||||||
| Pulmatrix Inc.(3) |
Drug Delivery | Warrant | Common Stock | 25,150 | 116 | 12 | ||||||||||||
| ZP Opco, Inc. (p.k.a. Zosano Pharma)(3) |
Drug Delivery | Warrant | Common Stock | 72,379 | 266 | 4 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Delivery (0.47%)* |
|
4,296 | 3,406 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||
| ADMA Biologics, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 89,750 | 295 | 98 | ||||||||||||
| Anthera Pharmaceuticals, Inc.(3)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 40,178 | 984 | — | ||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9) |
Drug Discovery & Development |
Warrant | Common Stock | 608,696 | 194 | 216 | ||||||||||||
See notes to consolidated financial statements.
F-34
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Cerecor, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 22,328 | $ | 70 | $ | 10 | ||||||||||
| Cerulean Pharma, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 171,901 | 369 | 90 | ||||||||||||
| Chroma Therapeutics, Ltd.(4)(9) |
Drug Discovery & Development |
Warrant | Preferred Series D | 325,261 | 490 | — | ||||||||||||
| Cleveland BioLabs, Inc.(3)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 7,813 | 105 | 5 | ||||||||||||
| Concert Pharmaceuticals, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 70,796 | 367 | 368 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell |
Drug Discovery & Development |
Warrant | Common Stock | 292,398 | 165 | 59 | ||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 200 | 28 | — | ||||||||||||
| Epirus Biopharmaceuticals, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 64,194 | 276 | 55 | ||||||||||||
| Fortress Biotech, Inc. (p.k.a. Coronado
Biosciences, |
Drug Discovery & Development |
Warrant | Common Stock | 73,009 | 142 | 11 | ||||||||||||
| Genocea Biosciences, Inc.(3) |
Drug Discovery & Development |
Warrant | Common Stock | 73,725 | 266 | 92 | ||||||||||||
| Immune Pharmaceuticals(3) |
Drug Discovery & Development |
Warrant | Common Stock | 214,853 | 164 | 40 | ||||||||||||
| Mast Therapeutics, Inc.(3)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 1,524,389 | 203 | 215 | ||||||||||||
| Melinta Therapeutics |
Drug Discovery & Development |
Warrant | Preferred Series 3 | 1,382,323 | 626 | 130 | ||||||||||||
| Nanotherapeutics, Inc.(14) |
Drug Discovery & Development |
Warrant | Common Stock | 171,389 | 838 | 1,762 | ||||||||||||
| Neothetics, Inc. (p.k.a. Lithera, Inc.)(3)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 46,838 | 266 | 2 | ||||||||||||
| Neuralstem, Inc.(3)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 75,187 | 77 | 12 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, |
Drug Discovery & Development |
Warrant | Common Stock | 21,467 | 129 | 36 | ||||||||||||
| uniQure B.V.(3)(4)(9) |
Drug Discovery & Development |
Warrant | Common Stock | 37,174 | 218 | 183 | ||||||||||||
| XOMA Corporation(3)(9)(14) |
Drug Discovery & Development |
Warrant | Common Stock | 181,268 | 279 | 115 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Discovery & Development (0.49%)* |
|
6,551 | 3,499 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| Clustrix, Inc. |
Electronics & Computer Hardware |
Warrant | Common Stock | 50,000 | 12 | — | ||||||||||||
| Persimmon Technologies |
Electronics & Computer Hardware |
Warrant | Preferred Series C | 43,076 | 40 | 42 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.01%)* |
|
52 | 42 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||
| Agrivida, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series D | 471,327 | 120 | 38 | ||||||||||||
| Alphabet Energy, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series A | 86,329 | 82 | 159 | ||||||||||||
| American Superconductor Corporation(3) |
Sustainable and Renewable Technology |
Warrant | Common Stock | 58,823 | 39 | 82 | ||||||||||||
| Brightsource Energy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series 1 | 116,667 | 104 | 6 | ||||||||||||
| Calera, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 44,529 | 513 | — | ||||||||||||
See notes to consolidated financial statements.
F-35
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| EcoMotors, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series B | 437,500 | $ | 308 | $ | 176 | ||||||||||
| Fluidic, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series D | 61,804 | 102 | 43 | ||||||||||||
| Fulcrum Bioenergy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series C-1 | 280,897 | 275 | 152 | ||||||||||||
| GreatPoint Energy, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series D-1 | 393,212 | 548 | — | ||||||||||||
| Polyera Corporation(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 311,609 | 338 | 10 | ||||||||||||
| Proterra, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series 4 | 397,931 | 37 | 50 | ||||||||||||
| SCIEnergy, Inc. |
Sustainable and Renewable Technology |
Warrant | Common Stock | 530,811 | 181 | — | ||||||||||||
| Sustainable and Renewable Technology |
Warrant | Preferred Series 1 | 145,811 | 50 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total SCIEnergy, Inc. |
676,622 | 231 | — | |||||||||||||||
| Scifiniti (p.k.a. Integrated
Photovoltaics, |
Sustainable and Renewable Technology |
Warrant | Preferred Series A-1 | 390,000 | 82 | 48 | ||||||||||||
| Solexel, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 1,171,625 | 1,162 | 466 | ||||||||||||
| Stion Corporation(5) |
Sustainable and Renewable Technology |
Warrant | Preferred Series Seed |
2,154 | 1,378 | — | ||||||||||||
| Sungevity, Inc. |
Sustainable and Renewable Technology |
Warrant | Common Stock | 20,000,000 | 543 | 569 | ||||||||||||
| Sustainable and Renewable Technology |
Warrant | Preferred Series C | 32,472,222 | 902 | 525 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Sungevity, Inc. |
52,472,222 | 1,445 | 1,094 | |||||||||||||||
| TAS Energy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series AA | 428,571 | 299 | — | ||||||||||||
| Tendril Networks |
Sustainable and Renewable Technology |
Warrant | Preferred Series 3-A | 1,019,793 | 188 | 242 | ||||||||||||
| TPI Composites, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series B | 160 | 273 | 85 | ||||||||||||
| Trilliant, Inc.(14) |
Sustainable and Renewable Technology |
Warrant | Preferred Series A | 320,000 | 162 | 53 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.38%)* |
|
7,686 | 2,704 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||
| Chromadex Corporation(3)(14) |
Healthcare Services, Other |
Warrant | Common Stock | 419,020 | 157 | 164 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Healthcare Services, Other (0.02%)* |
|
157 | 164 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Information Services |
||||||||||||||||||
| Cha Cha Search, Inc.(14) |
Information Services | Warrant | Preferred Series G | 48,232 | 58 | — | ||||||||||||
| INMOBI Inc.(4)(9) |
Information Services | Warrant | Common Stock | 46,874 | 82 | 3 | ||||||||||||
| InXpo, Inc.(14) |
Information Services | Warrant | Preferred Series C | 648,400 | 98 | 2 | ||||||||||||
| Information Services | Warrant | Preferred Series C-1 | 1,032,416 | 74 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total InXpo, Inc. |
1,680,816 | 172 | 2 | |||||||||||||||
| RichRelevance, Inc.(14) |
Information Services | Warrant | Preferred Series E | 112,612 | 98 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Information Services (0.00%)* |
|
410 | 5 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||
| Aria Systems, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series E | 239,692 | 73 | 88 | ||||||||||||
| Blurb, Inc.(14) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 234,280 | 636 | 148 | ||||||||||||
See notes to consolidated financial statements.
F-36
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| CashStar, Inc.(14) |
Internet Consumer & Business Services |
Warrant | Preferred Series C-2 | 727,272 | $ | 130 | $ | 34 | ||||||||||
| Just Fabulous, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series B | 206,184 | 1,102 | 1,104 | ||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 245,610 | 20 | 82 | ||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services |
Warrant | Preferred Series G | 174,562 | 78 | 104 | ||||||||||||
| Prism Education Group, Inc.(14) |
Internet Consumer & Business Services |
Warrant | Preferred Series B | 200,000 | 43 | — | ||||||||||||
| ReachLocal(3) |
Internet Consumer & Business Services |
Warrant | Common Stock | 300,000 | 155 | 290 | ||||||||||||
| ShareThis, Inc.(14) |
Internet Consumer & Business Services |
Warrant | Preferred Series C | 493,502 | 547 | 93 | ||||||||||||
| Tapjoy, Inc. |
Internet Consumer & Business Services |
Warrant | Preferred Series D | 748,670 | 316 | 8 | ||||||||||||
| Tectura Corporation |
Internet Consumer & Business Services |
Warrant | Preferred Series B-1 | 253,378 | 51 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.27%)* |
|
3,151 | 1,951 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Media/Content/Info |
||||||||||||||||||
| Machine Zone, Inc. |
Media/Content/Info | Warrant | Common Stock | 143,626 | 1,802 | 2,086 | ||||||||||||
| Rhapsody International, Inc.(14) |
Media/Content/Info | Warrant | Common Stock | 715,755 | 384 | 218 | ||||||||||||
| Zoom Media Group, Inc. |
Media/Content/Info | Warrant | Preferred Series A | 1,204 | 348 | 23 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Media/Content/Info (0.32%)* |
|
2,534 | 2,327 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||
| Amedica Corporation(3)(14) |
Medical Devices & Equipment |
Warrant | Common Stock | 1,548,387 | 459 | 31 | ||||||||||||
| Aspire Bariatrics, Inc.(14) |
Medical Devices & Equipment |
Warrant | Preferred Series D | 395,000 | 455 | 236 | ||||||||||||
| Avedro, Inc.(14) |
Medical Devices & Equipment |
Warrant | Preferred Series AA | 300,000 | 401 | 142 | ||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment |
Warrant | Preferred Series E | 110,947 | 203 | 428 | ||||||||||||
| Gamma Medica, Inc. |
Medical Devices & Equipment |
Warrant | Preferred Series A | 357,500 | 170 | 144 | ||||||||||||
| Gelesis, Inc.(14) |
Medical Devices & Equipment |
Warrant | Preferred Series A-1 | 74,784 | 78 | 262 | ||||||||||||
| InspireMD, Inc.(3)(4)(9) |
Medical Devices & Equipment |
Warrant | Common Stock | 16,835 | 242 | — | ||||||||||||
| Medrobotics Corporation(14) |
Medical Devices & Equipment |
Warrant | Preferred Series E | 455,539 | 370 | 244 | ||||||||||||
| NetBio, Inc. |
Medical Devices & Equipment |
Warrant | Common Stock | 2,568 | 408 | 19 | ||||||||||||
| NinePoint Medical, Inc.(14) |
Medical Devices & Equipment |
Warrant | Preferred Series A-1 | 587,840 | 170 | 119 | ||||||||||||
| Novasys Medical, Inc. |
Medical Devices & Equipment |
Warrant | Common Stock | 109,449 | 2 | — | ||||||||||||
| Medical Devices & Equipment |
Warrant | Preferred Series D | 526,840 | 125 | — | |||||||||||||
| Medical Devices & Equipment |
Warrant | Preferred Series D-1 | 53,607 | 6 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Novasys Medical, Inc. |
689,896 | 133 | — | |||||||||||||||
| Optiscan Biomedical, Corp.(5)(14) |
Medical Devices & Equipment |
Warrant | Preferred Series D | 10,535,275 | 1,252 | 312 | ||||||||||||
| Oraya Therapeutics, Inc. |
Medical Devices & Equipment |
Warrant | Common Stock | 954 | 66 | — | ||||||||||||
| Medical Devices & Equipment |
Warrant | Preferred Series 1 | 1,632,084 | 676 | 63 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Oraya Therapeutics, Inc. |
1,633,038 | 742 | 63 | |||||||||||||||
See notes to consolidated financial statements.
F-37
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) |
Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment |
Warrant | Preferred Series A | 500,000 | $ | 402 | $ | 298 | ||||||||||
| Quanterix Corporation |
Medical Devices & Equipment |
Warrant | Preferred Series C | 115,618 | 156 | 60 | ||||||||||||
| SonaCare Medical, LLC (p.k.a. US HIFU, LLC) |
Medical Devices & Equipment |
Warrant | Preferred Series A | 6,464 | 188 | — | ||||||||||||
| Strata Skin Sciences, Inc. (p.k.a. MELA Sciences, Inc.)(3) |
Medical Devices & Equipment |
Warrant | Common Stock | 69,320 | 402 | — | ||||||||||||
| ViewRay, Inc.(3)(14) |
Medical Devices & Equipment |
Warrant | Common Stock | 128,231 | 333 | 84 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (0.34%)* |
|
6,564 | 2,442 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Semiconductors |
||||||||||||||||||
| Achronix Semiconductor Corporation(14) |
Semiconductors | Warrant | Preferred Series C | 360,000 | 160 | 27 | ||||||||||||
| Semiconductors | Warrant | Preferred Series D-1 | 500,000 | 6 | 6 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Achronix Semiconductor Corporation |
860,000 | 166 | 33 | |||||||||||||||
| Aquantia Corp. |
Semiconductors | Warrant | Preferred Series G | 196,831 | 4 | 39 | ||||||||||||
| Avnera Corporation |
Semiconductors | Warrant | Preferred Series E | 141,567 | 47 | 65 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Semiconductors (0.02%)* |
|
217 | 137 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| Actifio, Inc. |
Software | Warrant | Common Stock | 73,584 | 249 | 210 | ||||||||||||
| Braxton Technologies, LLC |
Software | Warrant | Preferred Series A | 168,750 | 188 | — | ||||||||||||
| CareCloud Corporation(14) |
Software | Warrant | Preferred Series B | 413,433 | 258 | 625 | ||||||||||||
| Clickfox, Inc.(14) |
Software | Warrant | Preferred Series B | 1,038,563 | 330 | 362 | ||||||||||||
| Software | Warrant | Preferred Series C | 592,019 | 730 | 272 | |||||||||||||
| Software | Warrant | Preferred Series C-A | 46,109 | 13 | 16 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Clickfox, Inc. |
1,676,691 | 1,073 | 650 | |||||||||||||||
| Hillcrest Laboratories, Inc.(14) |
Software | Warrant | Preferred Series E | 1,865,650 | 55 | 138 | ||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(14) |
Software | Warrant | Preferred Series E | 614,333 | 16 | — | ||||||||||||
| Message Systems, Inc.(14) |
Software | Warrant | Preferred Series B | 408,011 | 334 | 497 | ||||||||||||
| Mobile Posse, Inc.(14) |
Software | Warrant | Preferred Series C | 396,430 | 130 | 59 | ||||||||||||
| Neos, Inc.(14) |
Software | Warrant | Common Stock | 221,150 | 22 | 113 | ||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Warrant | Preferred Series E | 225,586 | 33 | 55 | ||||||||||||
| Poplicus, Inc.(14) |
Software | Warrant | Preferred Series C | 2,595,230 | — | 110 | ||||||||||||
| Soasta, Inc.(14) |
Software | Warrant | Preferred Series E | 410,800 | 691 | 561 | ||||||||||||
| Sonian, Inc.(14) |
Software | Warrant | Preferred Series C | 185,949 | 106 | 39 | ||||||||||||
| Touchcommerce, Inc.(14) |
Software | Warrant | Preferred Series E | 2,282,968 | 446 | 581 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (0.51%)* |
|
3,601 | 3,638 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||
| Alimera Sciences, Inc.(3) |
Specialty Pharmaceuticals |
Warrant | Common Stock | 660,377 | 729 | 435 | ||||||||||||
| QuatRx Pharmaceuticals Company |
Specialty Pharmaceuticals |
Warrant | Preferred Series E | 155,324 | 307 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.06%)* |
|
1,036 | 435 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
||||||||||||||||||
| Gynesonics, Inc.(14) |
Surgical Devices | Warrant | Preferred Series C | 180,480 | 75 | 12 | ||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 1,575,965 | 320 | 223 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
1,756,445 | 395 | 235 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Warrant | Preferred Series B | 40,436 | 224 | 2 | ||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 175,000 | 100 | 170 | |||||||||||||
| Surgical Devices | Warrant | Preferred Series F | 16,476 | 3 | 3 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
231,912 | 327 | 175 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.06%)* |
|
722 | 410 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Warrant Investments (3.21%)* |
|
40,761 | 22,987 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total Investments (167.42%)* |
|
$ | 1,252,281 | $ | 1,200,638 | |||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-38
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2015
(dollars in thousands)
| * | Value as a percent of net assets |
| (1) | Preferred and common stock, warrants, and equity interests are generally non-income producing. |
| (2) | Gross unrealized appreciation, gross unrealized depreciation, and net depreciation for federal income tax purposes totaled $29.3 million, $81.4 million and $52.1 million respectively. The tax cost of investments is $1.3 billion. |
| (3) | Except for warrants in 37 publicly traded companies and common stock in 20 publicly traded companies, all investments are restricted at December 31, 2015 and were valued at fair value as determined in good faith by the Board of Directors. No unrestricted securities of the same issuer are outstanding. The Company uses the Standard Industrial Code for classifying the industry grouping of its portfolio companies. |
| (4) | Non-U.S. company or the company’s principal place of business is outside the United States. |
| (5) | Affiliate investment as defined under the 1940 Act in which Hercules owns at least 5% but generally less than 25% of the company’s voting securities. |
| (6) | Control investment as defined under the 1940 Act in which Hercules owns at least 25% of the company’s voting securities or has greater than 50% representation on its board. There were no control investments at December 31, 2015. |
| (7) | Debt is on non-accrual status at December 31, 2015, and is therefore considered non-income producing. Note that at December 31, 2015, only the PIK interest is on non-accrual for the Company’s debt investment in SkyCross, Inc. and only the $2.1 million PIK loan is on non-accrual for the Company’s debt investment in One Planet Ops Inc. (p.k.a. Reply! Inc.). |
| (8) | Denotes that all or a portion of the debt investment is convertible senior debt. |
| (9) | Indicates assets that the Company deems not “qualifying assets” under section 55(a) of the 1940 Act. Qualifying assets must represent at least 70% of the Company’s total assets at the time of acquisition of any additional non-qualifying assets. |
| (10) | Denotes that all or a portion of the debt investment secures the notes offered in the Debt Securitizations. |
| (11) | Denotes that all or a portion of the debt investment is pledged as collateral under the Wells Facility. |
| (12) | Denotes that all or a portion of the debt investment principal includes accumulated PIK interest and is net of repayments. |
| (13) | Denotes that all or a portion of the debt investment includes an exit fee receivable. This fee ranges from 0.8% to 17.1% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| (14) | Denotes that all or a portion of the investment in this portfolio company is held by HT II or HT III, the Company’s wholly-owned SBIC subsidiaries. |
| (15) | The stated ‘maturity date’ for the Tectura assets reflects the last extension of the forbearance period on these loans. The borrower loans remain outstanding and management is continuing to work with the borrower to satisfy the obligations. The Company’s investment team and Investment Committee continue to closely monitor developments at the borrower company. |
| (16) | Repayment of debt investment is delinquent of the contractual maturity date. |
See notes to consolidated financial statements.
F-39
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Business and Basis of Presentation
Hercules Capital, Inc. (the “Company”) is a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences and sustainable and renewable technology industries. The Company sources its investments through its principal office located in Palo Alto, CA, as well as through its additional offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT, and San Diego, CA. The Company was incorporated under the General Corporation Law of the State of Maryland in December 2003.
The Company is an internally managed, non-diversified closed-end investment company that has elected to be regulated as a business development company under the Investment Company Act of 1940, as amended (the “1940 Act”). From incorporation through December 31, 2005, the Company was subject to tax as a corporation under Subchapter C of the Internal Revenue Code of 1986, as amended (the “Code”). Effective January 1, 2006, the Company elected to be treated for tax purposes as a regulated investment company, or RIC, under Subchapter M of the Code (see Note 5). As an investment company, the Company follows accounting and reporting guidance as set forth in Topic 946 (“Financial Services—Investment Companies”) of the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification, as amended (“ASC”).
Hercules Technology II, L.P. (“HT II”), Hercules Technology III, L.P. (“HT III”), and Hercules Technology IV, L.P. (“HT IV”), are Delaware limited partnerships that were formed in January 2005, September 2009 and December 2010, respectively. HT II and HT III were licensed to operate as small business investment companies (“SBICs”) under the authority of the Small Business Administration (“SBA”) on September 27, 2006 and May 26, 2010, respectively. As SBICs, HT II and HT III are subject to a variety of regulations concerning, among other things, the size and nature of the companies in which they may invest and the structure of those investments. HT IV was formed in anticipation of receiving an additional SBIC license; however, the Company has not received such license, and HT IV currently has no material assets or liabilities. The Company also formed Hercules Technology SBIC Management, LLC, or (“HTM”), a limited liability company in November 2003. HTM is a wholly owned subsidiary of the Company and serves as the limited partner and general partner of HT II and HT III (see Note 4 to the Company’s consolidated financial statements).
HT II and HT III hold approximately $100.0 million and $261.8 million in assets, respectively, and they accounted for approximately 5.3% and 13.9% of the Company’s total assets, respectively, prior to consolidation at December 31, 2016.
The Company also established wholly owned subsidiaries, all of which are structured as Delaware corporations and limited liability companies, to hold portfolio companies organized as limited liability companies, or LLCs (or other forms of pass-through entities). By investing through these wholly owned subsidiaries, the Company is able to benefit from the tax treatment of these entities and create a tax structure that is more advantageous with respect to the Company’s RIC status. These taxable subsidiaries are consolidated for financial reporting purposes and in accordance with U.S. generally accepted accounting principles (“GAAP”), and the portfolio investments held by these taxable subsidiaries are included in the Company’s consolidated financial statements and recorded at fair value. These taxable subsidiaries are not consolidated with Hercules for income tax purposes and may generate income tax expense, or benefit, and tax assets and liabilities as a result of their ownership of certain portfolio investments.
The consolidated financial statements include the accounts of the Company, its subsidiaries and its consolidated securitization VIE. All significant inter-company accounts and transactions have been eliminated in consolidation. In accordance with Articles 6 and 10 of Regulation S-X, the Company does not consolidate portfolio company investments. It is not appropriate for an investment company to consolidate a portfolio company that is not an investment company or that provides services to the Company. Rather, an investment company’s interest in portfolio companies that are not investment companies should be measured at fair value in accordance with ASC Topic 946.
F-40
Financial statements prepared on a GAAP basis require management to make estimates and assumptions that affect the amounts and disclosures reported in the consolidated financial statements and accompanying notes. Such estimates and assumptions could change in the future as more information becomes known, which could impact the amounts reported and disclosed herein.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The Consolidated Financial Statements include the accounts of the Company and its subsidiaries and all VIEs of which the Company is the primary beneficiary. All intercompany accounts and transactions have been eliminated in consolidation.
A VIE is an entity that either (i) has insufficient equity to permit the entity to finance its activities without additional subordinated financial support or (ii) has equity investors who lack the characteristics of a controlling financial interest. The primary beneficiary of a VIE is the party with both the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and the obligation to absorb the losses or the right to receive benefits that could be significant to the VIE.
To assess whether the Company has the power to direct the activities of a VIE that most significantly impact its economic performance, the Company considers all the facts and circumstances including its role in establishing the VIE and its ongoing rights and responsibilities. This assessment includes identifying the activities that most significantly impact the VIE’s economic performance and identifying which party, if any, has power over those activities. In general, the party that makes the most significant decisions affecting the VIE is determined to have the power to direct the activities of a VIE. To assess whether the Company has the obligation to absorb the losses or the right to receive benefits that could potentially be significant to the VIE, the Company considers all of its economic interests, including debt and equity interests, servicing rights and fee arrangements, and any other variable interests in the VIE. If the Company determines that it is the party with the power to make the most significant decisions affecting the VIE, and the Company has a potentially significant interest in the VIE, then it consolidates the VIE.
The Company performs periodic reassessments, usually quarterly, of whether it is the primary beneficiary of a VIE. The reassessment process considers whether the Company has acquired or divested the power to direct the activities of the VIE through changes in governing documents or other circumstances. The Company also reconsiders whether entities previously determined not to be VIEs have become VIEs, based on certain events, and therefore are subject to the VIE consolidation framework.
As of the date of this report, the only VIE consolidated by the Company is its securitization VIE formed in conjunction with the issuance of the 2021 Asset-Backed Notes (as defined herein). See “Note 4—Borrowings”.
Change in Accounting Principle
As of January 1, 2016, the Company adopted FASB Accounting Standards Update (“ASU”) 2015-03 “Simplifying the Presentation of Debt Issuance Costs” and ASU 2015-15 “Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements,” which collectively require debt issuance costs to be presented on the balance sheet as a direct deduction from the associated debt liability, except for debt issuance costs associated with line-of-credit arrangements. Adoption of these standards results in the reclassification of debt issuance costs from Other Assets and the presentation of the Company’s SBA debentures, 2019 Notes, 2024 Notes, and 2021 Asset-Backed Notes net of the associated debt issuance costs for each instrument in the liabilities section on the Consolidated Statement of Assets and Liabilities. In addition, the comparative Consolidated Statement of Assets and Liabilities as of December 31, 2015 has been adjusted to apply the change in accounting principle retrospectively. Specifically, the presentation of the Company’s Other
F-41
Assets, SBA debentures, 2019 Notes, 2024 Notes, 2021 Asset-Backed Notes, and 2016 Convertible Notes line items were adjusted by the amount of unamortized debt issuance costs for each instrument. There is no impact to the Company’s Consolidated Statement of Operations. In addition, there is no change to the presentation of the Wells Facility or Union Bank Facility as debt issuance costs are presented separately as an asset on the Consolidated Statement of Assets and Liabilities.
Debt issuance costs are fees and other direct incremental costs incurred by the Company in obtaining debt financing and are recognized as prepaid expenses and amortized over the life of the related debt instrument using the effective yield method or the straight line method, which closely approximates the effective yield method. In accordance with ASU 2015-03 and ASU 2015-15 debt issuance costs are presented as a reduction to the associated liability balance on the Consolidated Statement of Assets and Liabilities, except for debt issuance costs associated with line-of-credit arrangements. Debt issuance costs, net of accumulated amortization, were as follows as of December 31, 2016 and December 31, 2015.
| (in thousands) |
December 31, 2016 | December 31, 2015 | ||||||
| SBA Debentures |
$ | 2,699 | $ | 3,371 | ||||
| 2019 Notes |
1,546 | 2,185 | ||||||
| 2024 Notes |
7,482 | 2,872 | ||||||
| 2021 Asset-Backed Notes |
1,233 | 2,305 | ||||||
| 2016 Convertible Notes |
— | 44 | ||||||
| Wells Facility(1) |
501 | 669 | ||||||
| Union Bank Facility(1) |
768 | 229 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 14,229 | $ | 11,675 | ||||
|
|
|
|
|
|||||
| (1) | As the Wells Facility and Union Bank Facility are line-of-credit arrangements, the debt issuance costs associated with these instruments are presented separately as an asset on the Consolidated Statement of Assets and Liabilities in accordance with ASU 2015-15. As the Union Bank Facility was replaced on May 5, 2016, amounts included above prior to May 5, 2016 relate to the Prior Union Bank Facility (as defined herein, see Note 4). |
Reclassification
Certain balances from prior years have been reclassified in order to conform to the current year presentation.
Valuation of Investments
The most significant estimate inherent in the preparation of the Company’s consolidated financial statements is the valuation of investments and the related amounts of unrealized appreciation and depreciation of investments recorded.
At December 31, 2016, approximately 97.3% of the Company’s total assets represented investments in portfolio companies whose fair value is determined in good faith by the Board of Directors. Value, as defined in Section 2(a)(41) of the 1940 Act, is (i) the market price for those securities for which a market quotation is readily available and (ii) for all other securities and assets, fair value is as determined in good faith by the Board of Directors. The Company’s investments are carried at fair value in accordance with the 1940 Act and ASC Topic 946 and measured in accordance with ASC Topic 820 (“Fair Value Measurements”). The Company’s debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of the Company’s investments in these portfolio companies are considered Level 3 assets under ASC Topic 820 because there is no known or accessible market or market indexes for these investment securities to be traded or exchanged. As such, the Company values substantially all of its investments at fair value as determined in good faith pursuant to a consistent valuation policy by the Company’s Board of Directors in accordance with the provisions of ASC Topic 820 and the 1940 Act. Due to the inherent uncertainty in determining the fair value of investments that do not have a readily available market value, the fair value of the
F-42
Company’s investments determined in good faith by its Board of Directors may differ significantly from the value that would have been used had a readily available market existed for such investments, and the differences could be material.
The Company may from time to time engage an independent valuation firm to provide the Company with valuation assistance with respect to certain portfolio investments. The Company engages independent valuation firms on a discretionary basis. Specifically, on a quarterly basis, the Company will identify portfolio investments with respect to which an independent valuation firm will assist in valuing. The Company selects these portfolio investments based on a number of factors, including, but not limited to, the potential for material fluctuations in valuation results, credit quality and the time lapse since the last valuation of the portfolio investment by an independent valuation firm.
The Company intends to continue to engage an independent valuation firm to provide management with assistance regarding the Company’s determination of the fair value of selected portfolio investments each quarter unless directed by the Board of Directors to cancel such valuation services. The scope of services rendered by an independent valuation firm is at the discretion of the Board of Directors. The Company’s Board of Directors is ultimately and solely responsible for determining the fair value of the Company’s investments in good faith.
With respect to investments for which market quotations are not readily available or when such market quotations are deemed not to represent fair value, the Company’s Board of Directors has approved a multi-step valuation process each quarter, as described below:
(1) the Company’s quarterly valuation process begins with each portfolio company being initially valued by the investment professionals responsible for the portfolio investment;
(2) preliminary valuation conclusions are then documented and business based assumptions are discussed with the Company’s investment committee;
(3) the Audit Committee of the Board of Directors reviews the preliminary valuation of the investments in the portfolio as provided by the investment committee which incorporates the results of the independent valuation firm as appropriate; and
(4) the Board of Directors, upon the recommendation of the Audit Committee, discusses valuations and determines the fair value of each investment in the Company’s portfolio in good faith based on the input of, where applicable, the respective independent valuation firm and the investment committee.
ASC Topic 820 establishes a framework for measuring the fair value of assets and liabilities and outlines a fair value hierarchy which prioritizes the inputs used to measure fair value and the effect of fair value measures on earnings. ASC Topic 820 also requires disclosure for fair value measurements based on the level within the hierarchy of the information used in the valuation. ASC Topic 820 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value. ASC Topic 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The Company has categorized all investments recorded at fair value in accordance with ASC Topic 820 based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels, defined by ASC Topic 820 and directly related to the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities, are as follows:
Level 1—Inputs are unadjusted, quoted prices in active markets for identical assets at the measurement date. The types of assets carried at Level 1 fair value generally are equities listed in active markets.
Level 2—Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset in connection with market data at the measurement date and for the extent of the instrument’s anticipated life. Fair valued assets that are generally included in this category are publicly held debt investments and warrants held in a public company.
F-43
Level 3—Inputs reflect management’s best estimate of what market participants would use in pricing the asset at the measurement date. It includes prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. Generally, assets carried at fair value and included in this category are the debt investments and warrants and equities held in a private company.
Investments measured at fair value on a recurring basis are categorized in the tables below based upon the lowest level of significant input to the valuations as of December 31, 2016 and 2015. The Company transfers investments in and out of Level 1, 2 and 3 as of the beginning balance sheet date, based on changes in the use of observable and unobservable inputs utilized to perform the valuation for the period. During the year ended December 31, 2016, there were no transfers between Levels 1 or 2.
| (in thousands) Description |
Balance December 31, 2016 |
Quoted Prices In Active Markets For Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Senior Secured Debt |
$ | 1,328,803 | $ | — | $ | 4,825 | $ | 1,323,978 | ||||||||
| Preferred Stock |
39,418 | — | — | 39,418 | ||||||||||||
| Common Stock |
28,236 | 17,271 | — | 10,965 | ||||||||||||
| Warrants |
27,485 | — | 3,239 | 24,246 | ||||||||||||
| Escrow Receivable |
1,382 | — | — | 1,382 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,425,324 | $ | 17,271 | $ | 8,064 | $ | 1,399,989 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (in thousands) Description |
Balance December 31, 2015 |
Quoted Prices In Active Markets For Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Senior Secured Debt |
$ | 1,110,209 | $ | — | $ | 7,813 | $ | 1,102,396 | ||||||||
| Preferred Stock |
35,245 | — | — | 35,245 | ||||||||||||
| Common Stock |
32,197 | 30,670 | — | 1,527 | ||||||||||||
| Warrants |
22,987 | — | 4,422 | 18,565 | ||||||||||||
| Escrow Receivable |
2,967 | — | — | 2,967 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,203,605 | $ | 30,670 | $ | 12,235 | $ | 1,160,700 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The table below presents a reconciliation for all financial assets and liabilities measured at fair value on a recurring basis, excluding accrued interest components, using significant unobservable inputs (Level 3) for the years ended December 31, 2016 and December 31, 2015.
| (in thousands) |
Balance January 1, 2016 |
Net Realized Gains (Losses)(1) |
Net Change in Unrealized Appreciation (Depreciation)(2) |
Purchases(5) | Sales | Repayments(6) | Gross Transfers into Level 3(3) |
Gross Transfers out of Level 3(3) |
Balance December 31, 2016 |
|||||||||||||||||||||||||||
| Senior Debt |
$ | 1,102,396 | $ | (6,968 | ) | $ | (12,675 | ) | $ | 687,353 | $ | — | $ | (441,567 | ) | $ | — | $ | (4,561 | ) | $ | 1,323,978 | ||||||||||||||
| Preferred Stock |
35,245 | (334 | ) | (7,864 | ) | 13,873 | (1,367 | ) | — | 626 | (761 | ) | 39,418 | |||||||||||||||||||||||
| Common Stock |
1,527 | — | (1,404 | ) | 6,081 | — | — | 4,761 | — | 10,965 | ||||||||||||||||||||||||||
| Warrants |
18,565 | (116 | ) | 3,465 | 4,082 | (1,186 | ) | — | — | (564 | ) | 24,246 | ||||||||||||||||||||||||
| Escrow Receivable |
2,967 | (6 | ) | — | 2,009 | (3,588 | ) | — | — | — | 1,382 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 1,160,700 | $ | (7,424 | ) | $ | (18,478 | ) | $ | 713,398 | $ | (6,141 | ) | $ | (441,567 | ) | $ | 5,387 | $ | (5,886 | ) | $ | 1,399,989 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| (in thousands) |
Balance January 1, 2015 |
Net Realized Gains (Losses)(1) |
Net Change in Unrealized Appreciation (Depreciation)(2) |
Purchases(5) | Sales | Repayments(6) | Gross Transfers into Level 3(4) |
Gross Transfers out of Level 3(4) |
Balance December 31, 2015 |
|||||||||||||||||||||||||||
| Senior Debt |
$ | 923,906 | $ | (2,295 | ) | $ | (12,930 | ) | $ | 699,555 | $ | — | $ | (505,274 | ) | $ | — | $ | (566 | ) | $ | 1,102,396 | ||||||||||||||
| Preferred Stock |
57,548 | 2,598 | (1,539 | ) | 15,076 | (4,542 | ) | — | 685 | (34,581 | ) | 35,245 | ||||||||||||||||||||||||
| Common Stock |
1,387 | (298 | ) | 743 | — | (305 | ) | — | — | — | 1,527 | |||||||||||||||||||||||||
| Warrants |
21,923 | (3,849 | ) | (4,749 | ) | 5,311 | 1,220 | — | — | (1,291 | ) | 18,565 | ||||||||||||||||||||||||
| Escrow Receivable |
3,598 | 71 | — | 511 | (1,032 | ) | (181 | ) | — | — | 2,967 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 1,008,362 | $ | (3,773 | ) | $ | (18,475 | ) | $ | 720,453 | $ | (4,659 | ) | $ | (505,455 | ) | $ | 685 | $ | (36,438 | ) | $ | 1,160,700 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
F-44
| (1) | Included in net realized gains or losses in the accompanying Consolidated Statement of Operations. |
| (2) | Included in net change in unrealized appreciation (depreciation) in the accompanying Consolidated Statement of Operations. |
| (3) | Transfers out of Level 3 during the year ended December 31, 2016 relate to the exercise of warrants in TPI Composites, Inc. and Touchcommerce, Inc. to common stock in an initial public offering, or IPO, and acquisition, respectively; the exercise of warrants in Ping Identity Corporation to preferred stock; the conversion of debt to equity in Optiscan Biomedical Corp and Achilles Technology Management Co II, Inc. and the conversion of the Company’s preferred shares to common shares in SCIEnergy, Inc. Transfers into Level 3 during the year ended December 31, 2016 relate to the acquisition of preferred stock as a result of the exercise of warrants in Ping Identity Corporation, the conversion of debt to equity in Optiscan Biomedical Corp and Achilles Technology Management Co II, Inc. and the conversion of the Company’s preferred shares to common shares in SCIEnergy, Inc. |
| (4) | Transfers out of Level 3 during the year ended December 31, 2015 relate to the IPOs of Box, Inc., ZP Opco, Inc. (p.k.a. Zosano Pharma, Inc.), Neos Therapeutics, Edge Therapeutics Inc., ViewRay, Inc., and Cerecor, Inc. in addition to the exercise of warrants in both Forescout, Inc. and Atrenta, Inc. to preferred stock. Transfers into Level 3 during the year ended December 31, 2015 relate to the acquisition of preferred stock as a result of the exercise of warrants in both Forescout, Inc. and Atrenta, Inc. and the conversion of debt to equity in Home Dialysis Plus and Gynesonics. |
| (5) | Amounts listed above are inclusive of loan origination fees received at the inception of the loan which are deferred and amortized into fee income as well as the accretion of existing loan discounts and fees during the period. Escrow receivable purchases may include additions due to proceeds held in escrow from the liquidation of level 3 investments. |
| (6) | Amounts listed above include the acceleration and payment of loan discounts and loan fees due to early payoffs or restructures. |
For the year ended December 31, 2016, approximately $9.1 million and $1.4 million in net unrealized depreciation was recorded for preferred stock and common stock Level 3 investments, respectively, relating to assets still held at the reporting date. For the same period, approximately $25.7 million in net unrealized depreciation and $2.8 million in net unrealized appreciation was recorded for debt and warrant Level 3 investments, respectively, relating to assets still held at the reporting date.
For the year ended December 31, 2015, approximately $179,000 in net unrealized depreciation and $745,000 in net unrealized appreciation was recorded for preferred stock and common stock Level 3 investments, respectively, relating to assets still held at the reporting date. For the same period, approximately $13.7 million and $5.9 million in net unrealized depreciation was recorded for debt and warrant Level 3 investments, respectively, relating to assets still held at the reporting date.
The following tables provide quantitative information about the Company’s Level 3 fair value measurements as of December 31, 2016 and December 31, 2015. In addition to the techniques and inputs noted in the tables below, according to the Company’s valuation policy the Company may also use other valuation techniques and methodologies when determining the Company’s fair value measurements. The tables below are not intended to be all-inclusive, but rather provide information on the significant Level 3 inputs as they relate to the Company’s fair value measurements.
F-45
The significant unobservable input used in the fair value measurement of the Company’s escrow receivables is the amount recoverable at the contractual maturity date of the escrow receivable.
| Investment Type - Level Three Debt Investments |
Fair Value at December 31, 2016 (in thousands) |
Valuation
Techniques/ |
Unobservable Input(a) |
Range | Weighted Average(b) |
|||||||||
| Pharmaceuticals |
$ | 102,412 | Originated Within 6 Months |
Origination Yield |
12.24% - 14.59% | 13.64% | ||||||||
| 434,718 | Market Comparable Companies |
Hypothetical Market Yield |
9.07% - 15.62% | 12.44% | ||||||||||
| Premium/(Discount) |
(0.25%) - 0.75% | |||||||||||||
| 2,693 | Liquidation(c) | Probability weighting of alternative outcomes | 25.00% - 100.00% | |||||||||||
| Technology |
93,674 | Originated Within 6 Months |
Origination Yield |
7.29% - 16.53% | 13.69 | % | ||||||||
| 325,553 | Market Comparable Companies | Hypothetical Market Yield | 10.14% - 21.66% | 12.69 | % | |||||||||
| Premium/(Discount) |
(0.50%) - 0.50% | |||||||||||||
| 24,706 | Liquidation(c) | Probability weighting of alternative outcomes | 20.00% - 100.00% | |||||||||||
| Sustainable and Renewable Technology |
99,286 | Market Comparable Companies |
Hypothetical Market Yield |
11.77% - 16.84% |
13.45 | % | ||||||||
| Premium/(Discount) |
0.00% - 0.25% | |||||||||||||
| 44,626 | Liquidation(c) | Probability weighting of alternative outcomes | 10.00% - 40.00% | |||||||||||
| Medical Devices |
88,983 | Market Comparable Companies |
Hypothetical Market Yield |
10.25% - 18.60% | 14.01 | % | ||||||||
| Premium/(Discount) |
(0.25%) - 0.75% | |||||||||||||
| Lower Middle Market |
25,017 | Market Comparable Companies |
Hypothetical Market Yield |
8.85% - 15.79% | 10.10 | % | ||||||||
| Premium/(Discount) |
0.00% - 0.25% | |||||||||||||
| 13,148 | Liquidation(c) | Probability weighting of alternative outcomes |
100.00% | |||||||||||
| Debt Investments Where Fair Value Approximates Cost |
||||||||||||||
| 25,000 | Imminent Payoffs(d) | |||||||||||||
| 44,162 | Debt Investments Maturing in Less than One Year | |||||||||||||
|
|
|
|||||||||||||
| $ | 1,323,978 | Total Level Three Debt Investments | ||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s debt securities are hypothetical market yields and premiums/(discounts). The hypothetical market yield is defined as the exit price of an investment in a hypothetical market to hypothetical market participants where buyers and sellers are willing participants. The premiums (discounts) relate to company specific characteristics such as underlying investment performance, security liens, and other characteristics of the investment. Significant increases (decreases) in the inputs in isolation may result in a significantly lower (higher) fair value measurement, depending on the materiality of the investment. Debt investments in the industries noted in the Company’s Consolidated Schedule of Investments are included in the industries noted above as follows: |
| • | Pharmaceuticals, above, is comprised of debt investments in the Specialty Pharmaceuticals, Drug Discovery and Development, Drug Delivery and Biotechnology Tools industries in the Consolidated Schedule of Investments. |
| • | Technology, above, is comprised of debt investments in the Software, Semiconductors, Internet Consumer and Business Services, Consumer and Business Products, Information Services, and Communications and Networking industries in the Consolidated Schedule of Investments. |
| • | Sustainable and Renewable Technology, above, aligns with the Sustainable and Renewable Technology Industry in the Consolidated Schedule of Investments. |
| • | Medical Devices, above, is comprised of debt investments in the Surgical Devices and Medical Devices and Equipment industries in the Consolidated Schedule of Investments. |
| • | Lower Middle Market, above, is comprised of debt investments in the Communications and Networking, Electronics and Computer Hardware, Healthcare Services—Other, Information Services, Internet Consumer and Business Services, Media/Content/Info, and Specialty Pharmaceuticals industries in the Consolidated Schedule of Investments. |
| (b) | The weighted averages are calculated based on the fair market value of each investment. |
| (c) | The significant unobservable input used in the fair value measurement of impaired debt securities is the probability weighting of alternative outcomes. |
| (d) | Imminent payoffs represent debt investments that the Company expects to be fully repaid within the next three months, prior to their scheduled maturity date. |
F-46
| Investment Type - Level Three |
Fair Value at December 31, 2015 (in thousands) |
Valuation
Techniques/ |
Unobservable Input(a) |
Range | Weighted Average(b) |
|||||||||
| Pharmaceuticals |
$ | 72,981 | Originated Within 6 Months |
Origination Yield |
10.35% - 16.16% | 12.29% | ||||||||
| 406,590 | Market Comparable Companies | Hypothetical Market Yield | 9.55% - 16.75% | 12.67% | ||||||||||
| Premium/(Discount) |
(0.75%) - 0.00% | |||||||||||||
| Technology |
6,873 | Originated Within 6 Months | Origination Yield | 15.19% | 15.19% | |||||||||
| 283,045 | Market Comparable Companies |
Hypothetical Market Yield |
6.57% - 23.26% | 13.22% | ||||||||||
| Premium/(Discount) | (0.25%) - 0.50% | |||||||||||||
| 36,815 | Liquidation(c) | Probability weighting of alternative outcomes | 10.00% - 100.00% | |||||||||||
| Sustainable and Renewable |
11,045 | Originated Within 6 Months | Origination Yield | 19.74% | 19.74% | |||||||||
| Technology |
105,382 | Market Comparable Companies |
Hypothetical Market Yield |
10.62% - 27.31% | 15.91% | |||||||||
| Premium/(Discount) |
0.00% | |||||||||||||
| 1,013 | Liquidation(c) | Probability weighting of alternative outcomes | 100.00% | |||||||||||
| Medical Devices |
80,530 | Market Comparable Companies |
Hypothetical Market Yield |
11.65% - 19.90% | 15.26% | |||||||||
| Premium/(Discount) |
0.00% - 0.50% | |||||||||||||
| 3,764 | Liquidation(c) |
Probability weighting of alternative outcomes |
50.00% | |||||||||||
| Lower Middle Market |
17,811 | Originated Within 6 Months |
Origination Yield |
12.70% - 14.50% | 13.00% | |||||||||
| 15,151 | Liquidation(c) | Probability weighting of alternative outcomes | 25.00% - 75.00% | |||||||||||
| Debt Investments Where Fair Value Approximates Cost |
||||||||||||||
| 12,434 | Imminent Payoffs(d) | |||||||||||||
| 48,962 | Debt Investments Maturing in Less than One Year | |||||||||||||
|
|
|
|||||||||||||
| $ | 1,102,396 | Total Level Three Debt Investments | ||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s debt securities are hypothetical market yields and premiums/(discounts). The hypothetical market yield is defined as the exit price of an investment in a hypothetical market to hypothetical market participants where buyers and sellers are willing participants. The premiums (discounts) relate to company specific characteristics such as underlying investment performance, security liens, and other characteristics of the investment. Significant increases (decreases) in the inputs in isolation may result in a significantly lower (higher) fair value measurement, depending on the materiality of the investment. Debt investments in the industries noted in the Company’s Consolidated Schedule of Investments are included in the industries noted above as follows: |
| • | Pharmaceuticals, above, is comprised of debt investments in the Specialty Pharmaceuticals, Drug Discovery and Development and Drug Delivery industries in the Consolidated Schedule of Investments. |
| • | Technology, above, is comprised of debt investments in the Software, Semiconductors, Internet Consumer and Business Services, Consumer and Business Products, Information Services, and Communications and Networking industries in the Consolidated Schedule of Investments. |
| • | Sustainable and Renewable Technology, above, aligns with the Sustainable and Renewable Technology Industry in the Consolidated Schedule of Investments. |
| • | Medical Devices, above, is comprised of debt investments in the Surgical Devices, Medical Devices and Equipment and Biotechnology Tools industries in the Consolidated Schedule of Investments. |
| • | Lower Middle Market, above, is comprised of debt investments in the Communications and Networking, Electronics and Computer Hardware, Healthcare Services—Other, Information Services, Internet Consumer and Business Services, Media/Content/Info, and Specialty Pharmaceuticals industries in the Consolidated Schedule of Investments. |
| (b) | The weighted averages are calculated based on the fair market value of each investment. |
| (c) | The significant unobservable input used in the fair value measurement of impaired debt securities is the probability weighting of alternative outcomes. |
| (d) | Imminent payoffs represent debt investments that the Company expects to be fully repaid within the next three months, prior to their scheduled maturity date. |
F-47
| Investment Type - Level Three |
Fair Value at December 31, 2016 (in thousands) |
Valuation Techniques/ |
Unobservable Input(a) |
Range | Weighted Average(e) | |||||||||
| Equity Investments |
$ | 9,258 | Market Comparable Companies |
EBITDA Multiple(b) |
0.0x - 38.7x | 12.3x | ||||||||
| Revenue Multiple(b) |
0.9x - 8.7x | 3.1x | ||||||||||||
|
Discount for Lack of Marketability(c) |
13.75% - 25.97% | 16.73% | ||||||||||||
| Average Industry Volatility(d) |
45.54% - 113.16% | 61.06% | ||||||||||||
| Risk-Free Interest Rate |
0.79% - 1.50% | 0.91% | ||||||||||||
| Estimated Time to Exit (in months) |
10 - 38 | 15 | ||||||||||||
| 19,836 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) | 29.93% - 109.95% | 73.49% | ||||||||||
| Risk-Free Interest Rate |
0.65% - 1.44% | 0.92% | ||||||||||||
| Estimated Time to Exit (in months) |
10 - 34 | 15 | ||||||||||||
| 21,289 | Other(f) | |||||||||||||
| Warrant Investments |
8,959 | Market Comparable Companies |
EBITDA Multiple(b) |
2.6x - 51.4x | 13.8x | |||||||||
| Revenue Multiple(b) |
0.4x - 6.1x | 2.5x | ||||||||||||
|
Discount for Lack of Marketability(c) |
11.74% - 27.25% | 19.02% | ||||||||||||
| Average Industry Volatility(d) |
38.58% - 111.15% | 62.03% | ||||||||||||
| Risk-Free Interest Rate |
0.68% - 1.68% | 1.04% | ||||||||||||
| Estimated Time to Exit (in months) |
7 - 47 | 20 | ||||||||||||
| 9,713 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) |
29.93% - 116.29% | 67.20% | ||||||||||
| Risk-Free Interest Rate |
0.45% - 1.84% | 0.99% | ||||||||||||
| Estimated Time to Exit (in months) |
3 - 47 | 20 | ||||||||||||
| 5,574 | Other(f) | |||||||||||||
|
|
|
|||||||||||||
| Total Level Three Warrant and Equity Investments | $ | 74,629 | ||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s warrant and equity-related securities are revenue and/or EBITDA multiples and discounts for lack of marketability. Additional inputs used in the Black Scholes option pricing model (“OPM”) include industry volatility, risk free interest rate and estimated time to exit. Significant increases (decreases) in the inputs in isolation may result in a significantly higher (lower) fair value measurement, depending on the materiality of the investment. For some investments, additional consideration may be given to data from the last round of financing or merger/acquisition events near the measurement date. |
| (b) | Represents amounts used when the Company has determined that market participants would use such multiples when pricing the investments. |
| (c) | Represents amounts used when the Company has determined market participants would take into account these discounts when pricing the investments. |
| (d) | Represents the range of industry volatility used by market participants when pricing the investment. |
| (e) | Weighted averages are calculated based on the fair market value of each investment. |
| (f) | The fair market value of these investments is derived based on recent private market and merger and acquisition transaction prices. |
| Investment Type - Level Three |
Fair Value at December 31, 2015 (in thousands) |
Valuation Techniques/ |
Unobservable Input(a) |
Range | Weighted Average(e) | |||||||
| Equity Investments | $ | 5,898 | Market Comparable Companies | EBITDA Multiple(b) | 3.3x - 19.5x | 7.6x | ||||||
| Revenue Multiple(b) | 0.7x - 3.7x | 2.1x | ||||||||||
| Discount for Lack of Marketability(c) |
14.31% - 25.11% | 18.05% | ||||||||||
| Average Industry Volatility(d) | 37.72% - 109.64% | 60.27% | ||||||||||
| Risk-Free Interest Rate | 0.61% - 1.09% | 0.74% | ||||||||||
| Estimated Time to Exit (in months) | 10 - 26 | 15 | ||||||||||
| 30,874 | Market Adjusted OPM Backsolve |
Average Industry Volatility(d) |
28.52% - 86.41% | 65.40% | ||||||||
| Risk-Free Interest Rate | 0.36% - 1.51% | 0.80% | ||||||||||
| Estimated Time to Exit (in months) | 10 - 47 | 17 | ||||||||||
| Warrant Investments | 7,904 | Market Comparable Companies | EBITDA Multiple(b) | 5.1x - 57.9x | 16.0x | |||||||
| Revenue Multiple(b) | 0.4x - 9.6x | 3.0x | ||||||||||
| Discount for Lack of Marketability(c) |
10.09% - 31.37% | 23.11% | ||||||||||
| Average Industry Volatility(d) | 39.51% - 73.36% | 41.19% | ||||||||||
| Risk-Free Interest Rate | 0.32% - 1.51% | 0.87% | ||||||||||
| Estimated Time to Exit (in months) | 4 - 47 | 23 | ||||||||||
| 10,661 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) | 28.52% - 109.64% | 64.31% | ||||||||
| Risk-Free Interest Rate | 0.36% - 1.45% | 0.85% | ||||||||||
| Estimated Time to Exit (in months) | 10 - 44 | 20 | ||||||||||
|
|
|
|||||||||||
| Total Level Three Warrant and Equity Investments |
$ | 55,337 | ||||||||||
|
|
|
|||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s warrant and equity-related securities are revenue and/or EBITDA multiples and discounts for lack of marketability. Additional inputs used in the Black Scholes OPM include industry volatility, risk |
F-48
| free interest rate and estimated time to exit. Significant increases (decreases) in the inputs in isolation may result in a significantly higher (lower) fair value measurement, depending on the materiality of the investment. For some investments, additional consideration may be given to data from the last round of financing or merger/acquisition events near the measurement date. |
| (b) | Represents amounts used when the Company has determined that market participants would use such multiples when pricing the investments. |
| (c) | Represents amounts used when the Company has determined market participants would take into account these discounts when pricing the investments. |
| (d) | Represents the range of industry volatility used by market participants when pricing the investment. |
| (e) | Weighted averages are calculated based on the fair market value of each investment. |
Debt Investments
The Company follows the guidance set forth in ASC Topic 820 which establishes a framework for measuring the fair value of assets and liabilities and outlines a fair value hierarchy, which prioritizes the inputs used to measure fair value and the effect of fair value measures on earnings. The Company’s debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of the Company’s investments in these portfolio companies are considered Level 3 assets under ASC Topic 820 because there is no known or accessible market or market indexes for debt instruments for these investment securities to be traded or exchanged. In addition, the Company may, from time to time, invest in public debt of companies that meet the Company’s investment objectives. These investments are considered Level 2 assets.
In making a good faith determination of the value of the Company’s investments, the Company generally starts with the cost basis of the investment, which includes the value attributed to the original issue discount (“OID”), if any, and payment-in-kind (“PIK”) interest or other receivables which have been accrued as earned. The Company then applies the valuation methods as set forth below.
The Company applies a procedure for debt investments that assumes the sale of each investment in a hypothetical market to a hypothetical market participant where buyers and sellers are willing participants. The hypothetical market does not include scenarios where the underlying security was simply repaid or extinguished, but includes an exit concept. The Company determines the yield at inception for each debt investment. The Company then uses senior secured, leveraged loan yields provided by third party providers to determine the change in market yields between inception of the debt security and the measurement date. Industry specific indices and other relevant market data are used to benchmark/assess market based movements.
Under this process, the Company also evaluates the collateral for recoverability of the debt investments. The Company considers each portfolio company’s credit rating, security liens and other characteristics of the investment to adjust the baseline yield to derive a credit adjusted hypothetical yield for each investment as of the measurement date. The anticipated future cash flows from each investment are then discounted at the hypothetical yield to estimate each investment’s fair value as of the measurement date.
The Company’s process includes an analysis of, among other things, the underlying investment performance, the current portfolio company’s financial condition and market changing events that impact valuation, estimated remaining life, current market yield and interest rate spreads of similar securities as of the measurement date. The Company values its syndicated debt investments using broker quotes and bond indices amongst other factors. If there is a significant deterioration of the credit quality of a debt investment, the Company may consider other factors to estimate fair value, including the proceeds that would be received in a liquidation analysis.
The Company records unrealized depreciation on investments when it believes that an investment has decreased in value, including where collection of a debt investment is doubtful or, if under the in-exchange premise, when the value of a debt security is less than amortized cost of the investment. Conversely, where
F-49
appropriate, the Company records unrealized appreciation if it believes that the underlying portfolio company has appreciated in value and, therefore, that its investment has also appreciated in value or, if under the in-exchange premise, the value of a debt security is greater than amortized cost.
When originating a debt instrument, the Company generally receives warrants or other equity-related securities from the borrower. The Company determines the cost basis of the warrants or other equity-related securities received based upon their respective fair values on the date of receipt in proportion to the total fair value of the debt and warrants or other equity-related securities received. Any resulting discount on the debt investments from recordation of the warrant or other equity instruments is accreted into interest income over the life of the debt investment.
Debt investments that are traded on a public exchange are valued at the prevailing market price as of the valuation date.
Equity-Related Securities and Warrants
Securities that are traded in the over-the-counter markets or on a stock exchange will be valued at the prevailing bid price at period end. The Company has a limited amount of equity securities in public companies. In accordance with the 1940 Act, unrestricted publicly traded securities for which market quotations are readily available are valued at the closing market quote on the measurement date.
The Company estimates the fair value of warrants using a Black Scholes OPM. At each reporting date, privately held warrant and equity-related securities are valued based on an analysis of various factors including, but not limited to, the portfolio company’s operating performance and financial condition and general market conditions, price to enterprise value or price to equity ratios, discounted cash flow, valuation comparisons to comparable public companies or other industry benchmarks. When an external event occurs, such as a purchase transaction, public offering, or subsequent equity sale, the pricing indicated by that external event is utilized to corroborate the Company’s valuation of the warrant and equity-related securities. The Company periodically reviews the valuation of its portfolio companies that have not been involved in a qualifying external event to determine if the enterprise value of the portfolio company may have increased or decreased since the last valuation measurement date.
Escrow Receivables
Escrow receivables are collected in accordance with the terms and conditions of the escrow agreement. Escrow balances are typically distributed over a period greater than one year and may accrue interest during the escrow period. Escrow balances are measured for collectability on at least a quarterly basis and fair value is determined based on the amount of the estimated recoverable balances and the contractual maturity date. As of December 31, 2016 there were no material past due escrow receivables.
Portfolio Composition
As required by the 1940 Act, the Company classifies its investments by level of control. “Control investments” are defined in the 1940 Act as investments in those companies that the Company is deemed to “control.” Under the 1940 Act, the Company is generally deemed to “control” a company in which it has invested if it owns 25% or more of the voting securities of such company or has greater than 50% representation on its board. “Affiliate investments” are investments in those companies that are “affiliated companies” of the Company, as defined in the 1940 Act, which are not control investments. The Company is deemed to be an “affiliate” of a company in which it has invested if it owns 5% or more, but generally less than 25%, of the voting securities of such company. “Non-control/non-affiliate investments” are investments that are neither control investments nor affiliate investments.
F-50
The following table summarizes the Company’s realized and unrealized gain and loss and changes in the Company’s unrealized appreciation and depreciation on control and affiliate investments for the years ended December 31, 2016, 2015, and 2014. The Company did not hold any control investments at December 31, 2015 or 2014.
| (in thousands) | Year Ended December 31, 2016 | |||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at December 31, 2016 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation/ (Depreciation)(1) |
Realized Gain/(Loss) |
||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||
| SkyCross, Inc. |
Control | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | ||||||||||||
| Achilles Technology Management Co II, Inc. |
Control | 4,700 | 84 | (604 | ) | — | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Control Investments |
$ | 4,700 | $ | 84 | $ | (4,025 | ) | $ | — | $ | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | $ | 4,699 | $ | 12 | $ | (3,409 | ) | $ | — | $ | — | ||||||||||||
| Stion Corporation |
Affiliate | 333 | 148 | 539 | 648 | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Affiliate Investments |
$ | 5,032 | $ | 160 | $ | (2,870 | ) | $ | 648 | $ | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Control & Affiliate Investments |
$ | 9,732 | $ | 244 | $ | (6,895 | ) | $ | 648 | $ | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (in thousands) | Year Ended December 31, 2015 | |||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at December 31, 2015 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation/ (Depreciation)(1) |
Realized Gain/(Loss) |
||||||||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | $ | 6,973 | $ | — | $ | 901 | $ | — | $ | — | |||||||||||||
| Stion Corporation |
Affiliate | 1,013 | 348 | 206 | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total |
$ | 7,986 | $ | 348 | $ | 1,107 | $ | — | $ | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (in thousands) | Year Ended December 31, 2014 | |||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at December 31, 2014 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation/ (Depreciation)(1) |
Realized Gain/(Loss) |
||||||||||||||||||
| Gelesis, Inc. |
Affiliate | $ | 327 | $ | — | $ | (146 | ) | $ | — | $ | — | ||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | 6,072 | — | (24 | ) | — | — | |||||||||||||||||
| Stion Corporation |
Affiliate | 1,600 | 1,876 | (3,112 | ) | — | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total |
$ | 7,999 | $ | 1,876 | $ | (3,282 | ) | $ | — | $ | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (1) | Represents reversals of prior period collateral based impairments. |
In June 2016, the Company’s investments in SkyCross, Inc. became classified as a control investment as a result of obtaining more than 50% representation on the portfolio company’s board. In June 2016 the Company also acquired 100% ownership of the equity of Achilles Technology Management Co II, Inc. and classified it as a control investment in accordance with the requirements of the 1940 Act. In June 2016, Achilles Technology Management Co II, Inc. acquired the assets of a global antenna company that produces radio frequency system solutions as part of an article 9 consensual foreclosure and public auction for total consideration in the amount of $4.0 million. In September and November 2016, the Company made a $1.0 million and $250,000 debt
F-51
investment, respectively, in Achilles Technology Management II to provide working capital under the terms of a loan servicing agreement. The Company’s investments in Achilles Technology Management Co II, Inc. are carried on the consolidated statement of assets and liabilities at fair value.
As of December 31, 2015, changes to the capitalization structure of the portfolio company Gelesis, Inc. reduced the Company’s investment below the threshold for classification as an affiliate investment.
The following table shows the fair value of the Company’s portfolio of investments by asset class as of December 31, 2016 and December 31, 2015:
| December 31, 2016 | December 31, 2015 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| Senior Secured Debt with Warrants |
$ | 1,078,779 | 75.7 | % | $ | 961,464 | 80.1 | % | ||||||||
| Senior Secured Debt |
277,509 | 19.5 | % | 171,732 | 14.3 | % | ||||||||||
| Preferred Stock |
39,418 | 2.8 | % | 35,245 | 2.9 | % | ||||||||||
| Common Stock |
28,236 | 2.0 | % | 32,197 | 2.7 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,423,942 | 100.0 | % | $ | 1,200,638 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
A summary of the Company’s investment portfolio, at value, by geographic location as of December 31, 2016 and December 31, 2015 is shown as follows:
| December 31, 2016 | December 31, 2015 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| United States |
$ | 1,362,223 | 95.6 | % | $ | 1,167,281 | 97.2 | % | ||||||||
| Netherlands |
20,089 | 1.4 | % | 20,112 | 1.7 | % | ||||||||||
| England |
18,395 | 1.3 | % | 8,884 | 0.8 | % | ||||||||||
| Switzerland |
12,377 | 0.9 | % | — | 0.0 | % | ||||||||||
| Canada |
8,095 | 0.6 | % | 595 | 0.0 | % | ||||||||||
| Israel |
2,763 | 0.2 | % | 3,764 | 0.3 | % | ||||||||||
| India |
— | 0.0 | % | 2 | 0.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,423,942 | 100.0 | % | $ | 1,200,638 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-52
The following table shows the fair value of the Company’s portfolio by industry sector at December 31, 2016 and December 31, 2015:
| December 31, 2016 | December 31, 2015 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| Drug Discovery & Development |
$ | 422,550 | 29.7 | % | $ | 284,266 | 23.7 | % | ||||||||
| Software |
219,559 | 15.4 | % | 147,237 | 12.3 | % | ||||||||||
| Sustainable and Renewable Technology |
154,406 | 10.9 | % | 159,487 | 13.3 | % | ||||||||||
| Media/Content/Info |
137,567 | 9.7 | % | 95,488 | 7.9 | % | ||||||||||
| Drug Delivery |
109,834 | 7.7 | % | 164,665 | 13.7 | % | ||||||||||
| Medical Devices & Equipment |
107,695 | 7.6 | % | 90,560 | 7.5 | % | ||||||||||
| Internet Consumer & Business Services |
97,047 | 6.8 | % | 88,377 | 7.4 | % | ||||||||||
| Consumer & Business Products |
42,713 | 3.0 | % | 26,611 | 2.2 | % | ||||||||||
| Specialty Pharmaceuticals |
38,944 | 2.7 | % | 52,088 | 4.3 | % | ||||||||||
| Healthcare Services, Other |
30,200 | 2.1 | % | 15,131 | 1.3 | % | ||||||||||
| Communications & Networking |
18,019 | 1.3 | % | 33,213 | 2.8 | % | ||||||||||
| Surgical Devices |
12,553 | 0.9 | % | 11,185 | 0.9 | % | ||||||||||
| Semiconductors |
11,326 | 0.8 | % | 22,705 | 1.9 | % | ||||||||||
| Electronics & Computer Hardware |
7,664 | 0.5 | % | 6,928 | 0.6 | % | ||||||||||
| Biotechnology Tools |
7,200 | 0.5 | % | 719 | 0.1 | % | ||||||||||
| Information Services |
6,091 | 0.4 | % | 1,657 | 0.1 | % | ||||||||||
| Diagnostic |
574 | 0.0 | % | 321 | 0.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,423,942 | 100.0 | % | $ | 1,200,638 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
No single portfolio investment represents more than 10% of the fair value of the Company’s total investments as of December 31, 2016 and December 31, 2015.
Portfolio Activity
During the year ended December 31, 2016, the Company funded and or restructured investments in debt and equity securities totaling approximately $660.5 million and $20.2 million, respectively. During the year ended December 31, 2016, the Company converted approximately $4.6 million of debt to equity in two portfolio companies. During the year ended December 31, 2016, the Company converted approximately $512,000 of warrants to equity in two portfolio companies.
During the year ended December 31, 2015, the Company funded investments in debt securities and equity investments totaling approximately $694.1 million and $18.6 million, respectively. The Company converted approximately $566,000 of debt to equity in two portfolio companies in the year ended December 31, 2015. During the year ended December 31, 2015, the Company converted approximately $330,000 of warrants to equity in three portfolio companies.
During the year ended December 31, 2016, the Company recognized net realized gains of approximately $4.6 million on the portfolio. These net realized gains included gross realized gains of approximately $15.2 million primarily from the sale of investments in six portfolio companies, including Box, Inc. ($9.3 million), Celator Pharmaceuticals, Inc. ($1.5 million), TouchCommerce, Inc. ($1.5 million), Ping Identity Corporation ($1.3 million), ReachLocal ($610,000) and Hillcrest Laboratories, Inc. ($225,000). These gains were partially offset by gross realized losses of approximately $10.6 million primarily from the liquidation or write off of the Company’s warrant and equity investments in eight portfolio companies and the Company’s debt investments in five portfolio companies, including the settlement of the Company’s outstanding debt investment in The Neat Company ($6.2 million).
During the year ended December 31, 2015, the Company recognized net realized gains of approximately $5.1 million on the portfolio. These net realized gains included gross realized gains of approximately $12.6 million primarily from the sale of investments in seven portfolio companies, including Box, Inc. ($3.2 million), Atrenta, Inc. ($2.6 million), Cempra, Inc. ($2.0 million), Celladon Corporation ($1.4 million), Egalet
F-53
Corporation ($652,000), Everyday Health, Inc. ($387,000) and Identiv, Inc. ($304,000), and $1.5 million from subsequent recoveries received on two previously written-off debt investments. These gains were partially offset by gross realized losses of approximately $7.5 million primarily from the liquidation of the Company’s investments in sixteen portfolio companies.
Investment Collateral
In the majority of cases, the Company collateralizes its investments by obtaining a first priority security interest in a portfolio company’s assets, which may include its intellectual property. In other cases, the Company may obtain a negative pledge covering a company’s intellectual property. At December 31, 2016, approximately 91.8% of the Company’s debt investments were in a senior secured first lien position, with 42.0% secured by a first priority security in all of the assets of the portfolio company, including its intellectual property; 46.7% secured by a first priority security in all of the assets of the portfolio company and the portfolio company was prohibited from pledging or encumbering its intellectual property; and 3.1% secured by a first priority security in all of the assets of the portfolio company, including its intellectual property, with a second lien on the portfolio company’s cash and accounts receivable. The remaining 8.2% of the Company’s debt investments were secured by a second priority security interest in all of the portfolio company’s assets, other than intellectual property. At December 31, 2016 the Company had no equipment only liens on material investments in the Company’s portfolio companies.
Cash and Cash Equivalents
Cash and cash equivalents consists solely of funds deposited with financial institutions and short-term liquid investments in money market deposit accounts. Cash and cash equivalents are carried at cost, which approximates fair value.
Other Assets
Other Assets generally consists of prepaid expenses, deferred financing costs net of accumulated amortization, fixed assets net of accumulated depreciation, deferred revenues and deposits and other assets, including escrow receivable. The escrow receivable balance as of December 31, 2016 and December 31, 2015 was approximately $1.4 million and $3.0 million, respectively, and was fair valued and held in accordance with ASC Topic 820.
Income Recognition
The Company records interest income on an accrual basis and recognizes it as earned in accordance with the contractual terms of the loan agreement, to the extent that such amounts are expected to be collected. OID initially represents the value of detachable equity warrants obtained in conjunction with the acquisition of debt securities and is accreted into interest income over the term of the loan as a yield enhancement. When a loan becomes 90 days or more past due, or if management otherwise does not expect that principal, interest, and other obligations due will be collected in full, the Company will generally place the loan on non-accrual status and cease recognizing interest income on that loan until all principal and interest due has been paid or the Company believes the portfolio company has demonstrated the ability to repay the Company’s current and future contractual obligations. Any uncollected interest related to prior periods is reversed from income in the period that collection of the interest receivable is determined to be doubtful. However, the Company may make exceptions to this policy if the investment has sufficient collateral value and is in the process of collection.
At December 31, 2016, the Company had five debt investments on non-accrual with a cumulative investment cost and fair value of approximately $43.9 million and $6.2 million, respectively. At December 31, 2015, the Company had five debt investments on non-accrual at December 31, 2015 with a cumulative investment cost and fair value of approximately $47.4 million and $23.2 million, respectively. In addition, at December 31, 2015, the Company had one debt investment with an investment cost and fair value of
F-54
approximately $20.1 million and $14.9 million, respectively, for which only the PIK interest was on non-accrual. The decrease in the cumulative cost and fair value of debt investments on non-accrual between December 31, 2016 and December 31, 2015 is the result of placing two new debt investments on non-accrual status, offset by the liquidation of three debt investments that were on non-accrual at December 31, 2015. For the year ended December 31, 2016, the Company recognized a realized loss of approximately $6.2 million on the settlement of one debt investment that was on non-accrual at December 31, 2015. In addition, the Company recognized realized losses of $419,000 and $430,000 on the liquidation and partial write off, respectively, of two debt investments that were on non-accrual as of December 31, 2015.
Fee income, generally collected in advance, includes loan commitment and facility fees for due diligence and structuring, as well as fees for transaction services and management services rendered by the Company to portfolio companies and other third parties. Loan and commitment fees are amortized into income over the contractual life of the loan. Management fees are generally recognized as income when the services are rendered. Loan origination fees are capitalized and then amortized into interest income using the effective interest rate method. In certain loan arrangements, warrants or other equity interests are received from the borrower as additional origination fees. The Company had approximately $38.2 million of unamortized fees at December 31, 2016, of which approximately $35.8 million was included as an offset to the cost basis of its current debt investments and approximately $2.4 million was deferred contingent upon the occurrence of a funding or milestone. At December 31, 2015 the Company had approximately $26.1 million of unamortized fees, of which approximately $23.6 million was included as an offset to the cost basis of its current debt investments and approximately $2.5 million was deferred contingent upon the occurrence of a funding or milestone.
The Company recognizes nonrecurring fees amortized over the remaining term of the loan commencing in the quarter relating to specific loan modifications. Certain fees may still be recognized as one-time fees, including prepayment penalties, fees related to select covenant default waiver fees and acceleration of previously deferred loan fees and OID related to early loan pay-off or material modification of the specific debt outstanding.
In addition, the Company may also be entitled to an exit fee that is amortized into income over the life of the loan. Loan exit fees to be paid at the termination of the loan are accreted into interest income over the contractual life of the loan. At December 31, 2016, the Company had approximately $32.8 million in exit fees receivable, of which approximately $30.3 million was included as an offset to the cost basis of its current debt investments and approximately $2.5 million was deferred related to expired commitments. At December 31, 2015 the Company had approximately $22.7 million in exit fees receivable, of which approximately $17.4 million was included as an offset to the cost basis of its current debt investments and approximately $5.3 million was related to expired commitments.
The Company has debt investments in its portfolio that contain a PIK provision. Contractual PIK interest, which represents contractually deferred interest added to the loan balance that is generally due at the end of the loan term, is generally recorded on the accrual basis to the extent such amounts are expected to be collected. The Company will generally cease accruing PIK interest if there is insufficient value to support the accrual or management does not expect the portfolio company to be able to pay all principal and interest due. The Company recorded approximately $7.8 million and $4.7 million in PIK income in the years ended December 31, 2016 and 2015, respectively.
To maintain the Company’s status as a RIC, PIK and exit fee income must be paid out to stockholders in the form of dividend distributions even though the cash has not yet been collected. Amounts necessary to pay these distributions may come from available cash or the liquidation of certain investments.
In certain investment transactions, the Company may provide advisory services. For services that are separately identifiable and external evidence exists to substantiate fair value, income is recognized as earned, which is generally when the investment transaction closes. The Company had no income from advisory services in the years ended December 31, 2016 and December 31, 2015.
F-55
Other Income (Loss)
Other Income (loss) generally consists of income or losses generated from sources other than the Company’s investment portfolio. For the year ended December 31, 2016 it consists of litigation settlement proceeds (refer to “Note 10—Commitments and Contingencies”). For the years ended December 31, 2015 and December 31, 2014 it consists of loss on extinguishment of debt (refer to “Note 4—Borrowings”). Other income (loss) is classified as a component of net investment income in the Company’s Consolidated Statement of Operations.
Equity Offering Expenses
The Company’s offering costs are charged against the proceeds from equity offerings when received.
Stock Based Compensation
The Company has issued and may, from time to time, issue additional stock options and restricted stock to employees under the Company’s 2004 Equity Incentive Plan and members of the Board of Directors under the Company’s 2006 Equity Incentive Plan. Management follows the guidelines set forth under ASC Topic 718, (“Compensation—Stock Compensation”) to account for stock options granted. Under ASC Topic 718, compensation expense associated with stock-based compensation is measured at the grant date based on the fair value of the award and is recognized over the vesting period. Determining the appropriate fair value model and calculating the fair value of stock-based awards at the grant date requires judgment, including estimating stock price volatility, forfeiture rate and expected option life.
Income Taxes
The Company intends to operate so as to qualify to be subject to tax as a RIC under Subchapter M of the Code and, as such, will not be subject to federal income tax on the portion of taxable income and gains distributed to stockholders. Taxable income includes the Company’s taxable interest, dividend and fee income, reduced by certain deductions, as well as taxable net realized securities gains. Taxable income generally differs from net income for financial reporting purposes due to temporary and permanent differences in the recognition of income and expenses, and generally excludes net unrealized appreciation or depreciation, as such gains or losses are not included in taxable income until they are realized.
As a RIC, the Company will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income unless the Company makes distributions treated as dividends for U.S. federal income tax purposes in a timely manner to its stockholders in respect of each calendar year of an amount at least equal to the sum of (1) 98% of the Company’s ordinary income (taking into account certain deferrals and elections) for each calendar year, (2) 98.2% of the Company’s capital gain net income (adjusted for certain ordinary losses) for the 1-year period ending October 31 of each such calendar year and (3) any ordinary income and capital gain net income realized, but not distributed, in preceding calendar years. The Company will not be subject to this excise tax on any amount on which the Company incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains).
Depending on the level of taxable income earned in a taxable year, the Company may choose to carry over taxable income in excess of current taxable year distributions treated as dividends for U.S. federal income tax purposes from such taxable income into the next taxable year and incur a 4% excise tax on such taxable income, as required. The maximum amount of excess taxable income that may be carried over for distribution in the next taxable year under the Code is the total amount of distributions treated as dividends for U.S. federal income tax purposes paid in the following taxable year, subject to certain declaration and payment guidelines. To the extent the Company chooses to carry over taxable income into the next taxable year, distributions declared and paid by the Company in a taxable year may differ from the Company’s taxable income for that taxable year as such distributions may include the distribution of current taxable year taxable income, the distribution of prior taxable year taxable income carried over into and distributed in the current taxable year, or returns of capital.
F-56
The Company intends to distribute 100% of its spillover earnings, which consists of ordinary income and capital gains, from the taxable year ended December 31, 2016 to the Company’s shareholders during 2017. The Company distributed 100% of its spillover earnings from ordinary income from its taxable year ended December 31, 2015 to the Company’s stockholders during 2016.
Because federal income tax regulations differ from accounting principles generally accepted in the United States, distributions in accordance with tax regulations may differ from net investment income and net realized securities gains recognized for financial reporting purposes. Differences may be permanent or temporary. Permanent differences are reclassified among capital accounts in the financial statements to reflect their appropriate tax character. Permanent differences may also result from the change in the classification of certain items, such as the treatment of short-term gains as ordinary income for tax purposes. Temporary differences arise when certain items of income, expense, gain or loss are recognized at some time in the future.
Earnings Per Share (“EPS”)
Basic EPS is calculated by dividing net earnings applicable to common shareholders by the weighted average number of common shares outstanding. Common shares outstanding includes common stock and restricted stock for which no future service is required as a condition to the delivery of the underlying common stock. Diluted EPS includes the determinants of basic EPS and, in addition, reflects the dilutive effect of the common stock deliverable pursuant to stock options and to restricted stock for which future service is required as a condition to the delivery of the underlying common stock.
Comprehensive Income
The Company reports all changes in comprehensive income in the Consolidated Statement of Operations. The Company did not have other comprehensive income in 2016, 2015, or 2014. The Company’s comprehensive income is equal to its net increase in net assets resulting from operations.
Distributions
Distributions to common stockholders are approved by the Board of Directors on a quarterly basis and the distribution payable is recorded on the ex-dividend date.
The Company maintains an “opt out” dividend reinvestment plan that provides for reinvestment of the Company’s distribution on behalf of the Company’s stockholders, unless a stockholder elects to receive cash. As a result, if the Company declares a distribution, cash distributions will be automatically reinvested in additional shares of its common stock unless the stockholder specifically “opts out” of the dividend reinvestment plan and chooses to receive cash distributions. During 2016, 2015, and 2014, the Company issued approximately 144,308, 199,894, and 96,976 shares, respectively, of common stock to shareholders in connection with the dividend reinvestment plan.
Segments
The Company lends to and invests in portfolio companies in various technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology. The Company separately evaluates the performance of each of its lending and investment relationships. However, because each of these loan and investment relationships has similar business and economic characteristics, they have been aggregated into a single lending and investment segment.
Recent Accounting Pronouncements
In January 2016, the FASB issued ASU 2016-01, “Financial Instruments—Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities,” which, among other things, requires
F-57
that (i) all equity investments, other than equity-method investments, in unconsolidated entities generally be measured at fair value through earnings and (ii) an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments. Additionally, the ASU changes the disclosure requirements for financial instruments. ASU 2016-01 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2017. Early adoption is permitted for certain provisions. The Company does not believe that ASU 2016-01 will have a material impact on its consolidated financial statements and disclosures.
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842),” which, among other things, requires recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous GAAP. Additionally, the ASU requires the classification of all cash payments on leases within operating activities in the Consolidated Statement of Cash Flows. ASU 2016-02 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2018. Early adoption is permitted. The Company does not believe that ASU 2016-02 will have a material impact on its consolidated financial statements and disclosures.
In March 2016, the FASB issued ASU 2016-09, “Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting,” which, among other things, simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. ASU 2016-09 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2016. Early adoption is permitted. The Company does not believe that ASU 2016-09 will have a material impact on its consolidated financial statements and disclosures.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments,” which addresses eight specific cash flow issues including, among other things, the classification of debt prepayment or debt extinguishment costs. ASU 2016-15 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2017. Early adoption is permitted. The Company does not believe that ASU 2016-15 will have a material impact on its consolidated financial statements and disclosures.
In November 2016, the FASB issued ASU 2016-18, “Statement of Cash Flows (Topic 230),” which requires that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The new guidance is effective for interim and annual periods beginning after December 15, 2017 and early adoption is permitted. The amendment should be adopted retrospectively. The Company does not believe that ASU 2016-18 will have a material impact on its consolidated financial statements and disclosures.
3. Fair Value of Financial Instruments
Fair value estimates are made at discrete points in time based on relevant information. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and, therefore, cannot be determined with precision. The Company believes that the carrying amounts of its financial instruments, consisting of cash and cash equivalents, receivables including escrow receivables, accounts payable and accrued liabilities, approximate the fair values of such items due to the short maturity of such instruments. The April 2019 Notes, the September 2019 Notes (together with the April 2019 Notes, the “2019 Notes”), which the Company has publicly announced its intention to redeem on February 24, 2017, the 2024 Notes, the 2021 Asset-Backed Notes, and the SBA debentures, provide a strategic advantage as sources of liquidity due to their flexible structure, long-term duration, and low fixed interest rates. At December 31, 2016, the April 2019 Notes were trading on the New York Stock Exchange, or “NYSE” for $25.55 per share at par value, the September 2019
F-58
Notes were trading on the NYSE for $25.57 per share at par value and the 2024 Notes were trading on the NYSE for $25.40 per share at par value. The par value at underwriting for each of these notes was $25.00 per share. Based on market quotations on or around December 31, 2016, the 2021 Asset-Backed Notes were quoted for 1.002 per dollar at par value. Calculated based on the net present value of payments over the term of the notes using estimated market rates for similar notes and remaining terms, the fair value of the SBA debentures would be approximately $202.4 million, compared to the principal outstanding amount of $190.2 million as of December 31, 2016. The fair value of the outstanding borrowings under the Wells Facility at December 31, 2016 is equal to its transaction price as the Company renegotiated the terms of the agreement with Wells Fargo Capital Finance, LLC in December 2015 and added two additional lenders to the facility at the same terms during 2016.
See the accompanying Consolidated Schedule of Investments for the fair value of the Company’s investments. The methodology for the determination of the fair value of the Company’s investments is discussed in Note 2.
The liabilities of the Company are recorded at amortized cost and not at fair value on the Consolidated Statement of Assets and Liabilities. The following tables provide additional information about the fair value and level in the fair value hierarchy of the Company’s liabilities at December 31, 2016 and December 31, 2015:
| (in thousands) Description(1) |
December 31, 2016 | Identical Assets (Level 1) |
Observable Inputs (Level 2) |
Unobservable Inputs (Level 3) |
||||||||||||
| Wells Facility |
5,016 | — | — | 5,016 | ||||||||||||
| 2021 Asset-Backed Notes |
109,376 | — | 109,376 | — | ||||||||||||
| April 2019 Notes |
65,909 | — | 65,909 | — | ||||||||||||
| September 2019 Notes |
46,920 | — | 46,920 | — | ||||||||||||
| 2024 Notes |
256,919 | — | 256,919 | — | ||||||||||||
| SBA Debentures |
202,364 | — | — | 202,364 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 686,504 | $ | — | $ | 479,124 | $ | 207,380 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (in thousands) Description |
December 31, 2015 | Identical Assets (Level 1) |
Observable Inputs (Level 2) |
Unobservable Inputs (Level 3) |
||||||||||||
| 2016 Convertible Notes(1) |
$ | 19,540 | $ | — | $ | 19,540 | $ | — | ||||||||
| Wells Facility |
50,000 | — | — | 50,000 | ||||||||||||
| 2021 Asset-Backed Notes |
128,775 | — | 128,775 | — | ||||||||||||
| April 2019 Notes |
65,573 | — | 65,573 | — | ||||||||||||
| September 2019 Notes |
46,297 | — | 46,297 | — | ||||||||||||
| 2024 Notes |
104,401 | — | 104,401 | — | ||||||||||||
| SBA Debentures |
194,121 | — | — | 194,121 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 608,707 | $ | — | $ | 364,586 | $ | 244,121 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016. |
4. Borrowings
Outstanding Borrowings
At December 31, 2016 and December 31, 2015, the Company had the following available and outstanding borrowings:
| December 31, 2016 | December 31, 2015 | |||||||||||||||||||||||
| (in thousands) |
Total Available | Principal | Carrying Value(1) | Total Available | Principal | Carrying Value(1) | ||||||||||||||||||
| SBA Debentures(2) |
$ | 190,200 | $ | 190,200 | $ | 187,501 | $ | 190,200 | $ | 190,200 | $ | 186,829 | ||||||||||||
| 2019 Notes |
110,364 | 110,364 | 108,818 | 110,364 | 110,364 | 108,179 | ||||||||||||||||||
| 2024 Notes |
252,873 | 252,873 | 245,490 | 103,000 | 103,000 | 100,128 | ||||||||||||||||||
| 2021 Asset-Backed Notes |
109,205 | 109,205 | 107,972 | 129,300 | 129,300 | 126,995 | ||||||||||||||||||
| 2016 Convertible Notes(3) |
— | — | — | 17,604 | 17,604 | 17,478 | ||||||||||||||||||
| Wells Facility(4) |
120,000 | 5,016 | 5,016 | 75,000 | 50,000 | 50,000 | ||||||||||||||||||
| Union Bank Facility(4) |
75,000 | — | — | 75,000 | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 857,642 | $ | 667,658 | $ | 654,797 | $ | 700,468 | $ | 600,468 | $ | 589,609 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
F-59
| (1) | Except for the Wells Facility and Union Bank Facility, all carrying values represent the principal amount outstanding less the remaining unamortized debt issuance costs and unaccreted premium or discount, if any, associated with the loan as of the balance sheet date. See “Note 2—Summary of Significant Accounting Policies” for the amount of debt issuance cost associated with each borrowing. |
| (2) | At both December 31, 2016 and December 31, 2015, the total available borrowings under the SBA debentures were $190.2 million, of which $41.2 million was available in HT II and $149.0 million was available in HT III. |
| (3) | The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016. |
| (4) | Availability subject to the Company meeting the borrowing base requirements. As the Union Bank Facility was replaced on May 5, 2016, amounts included above prior to May 5, 2016 relate to the Prior Union Bank Facility (as defined herein). |
Long-Term SBA Debentures
On September 27, 2006, HT II received a license to operate as a SBIC under the SBIC program and is able to borrow funds from the SBA against eligible investments and additional contributions to regulatory capital. Under the Small Business Investment Company Act and current SBA policy applicable to SBICs, a SBIC can have outstanding at any time SBA guaranteed debentures up to twice the amount of its regulatory capital. With the Company’s net investment of $44.0 million in HT II as of December 31, 2016, HT II has the capacity to issue a total of $41.2 million of SBA guaranteed debentures, subject to SBA approval, of which $41.2 million was outstanding as of December 31, 2016. As of December 31, 2016, HT II has paid the SBA commitment fees and facility fees of approximately $1.5 million and $3.6 million, respectively. As of December 31, 2016, the Company held investments in HT II in 36 companies with a fair value of approximately $84.3 million, accounting for approximately 5.9% of the Company’s total investment portfolio. HT II held approximately $100.0 million in assets and accounted for approximately 5.3% of the Company’s total assets prior to consolidation at December 31, 2016.
On May 26, 2010, HT III received a license to operate as a SBIC under the SBIC program and is able to borrow funds from the SBA against eligible investments and additional contributions to regulatory capital. With the Company’s net investment of $74.5 million in HT III as of December 31, 2016, HT III has the capacity to issue a total of $149.0 million of SBA guaranteed debentures, subject to SBA approval, of which $149.0 million was outstanding as of December 31, 2016. As of December 31, 2016, HT III has paid the SBA commitment fees and facility fees of approximately $1.5 million and $3.6 million, respectively. As of December 31, 2016, the Company held investments in HT III in 51 companies with a fair value of approximately $261.2 million, accounting for approximately 18.3% of the Company’s total portfolio. HT III held approximately $261.8 million in assets and accounted for approximately 13.9% of the Company’s total assets prior to consolidation at December 31, 2016.
SBICs are designed to stimulate the flow of private equity capital to eligible small businesses. Under present SBA regulations, eligible small businesses include businesses that have a tangible net worth not exceeding $19.5 million and have average annual fully taxed net income not exceeding $6.5 million for the two most recent fiscal years. In addition, SBICs must devote 25.0% of its investment activity to “smaller” enterprises as defined by the SBA. A smaller enterprise is one that has a tangible net worth not exceeding $6.0 million and has average annual fully taxed net income not exceeding $2.0 million for the two most recent fiscal years. SBA regulations also provide alternative size standard criteria to determine eligibility, which depend on the industry in which the business is engaged and are based on such factors as the number of employees and gross sales. According to SBA regulations, SBICs may make long-term loans to small businesses, invest in the equity securities of such businesses and provide them with consulting and advisory services. Through the Company’s wholly-owned subsidiaries HT II and HT III, the Company plans to provide long-term loans to qualifying small businesses, and in connection therewith, make equity investments.
HT II and HT III are periodically examined and audited by the SBA’s staff to determine their compliance with SBA regulations. If HT II or HT III fails to comply with applicable SBA regulations, the SBA could, depending on the severity of the violation, limit or prohibit HT II’s or HT III’s use of debentures, declare outstanding debentures immediately due and payable, and/or limit HT II or HT III from making new investments. In addition, HT II or HT III may also be limited in their ability to make distributions to the Company if they do
F-60
not have sufficient capital in accordance with SBA regulations. Such actions by the SBA would, in turn, negatively affect the Company because HT II and HT III are the Company’s wholly owned subsidiaries. HT II and HT III were in compliance with the terms of the SBIC’s leverage as of December 31, 2016 as a result of having sufficient capital as defined under the SBA regulations.
The rates of borrowings under various draws from the SBA beginning in March 2009 are set semiannually in March and September and range from 2.25% to 4.62% excluding annual fees. Interest payments on SBA debentures are payable semiannually. There are no principal payments required on these issues prior to maturity and no prepayment penalties. Debentures under the SBA generally mature ten years after being borrowed. Based on the initial draw down date of March 2009, the initial maturity of SBA debentures will occur in March 2019. In addition, the SBA charges a fee that is set annually, depending on the Federal fiscal year the leverage commitment was delegated by the SBA, regardless of the date that the leverage was drawn by the SBIC. The annual fees related to HT II debentures that pooled on September 22, 2010 were 0.406% and 0.285%, depending upon the year in which the underlying commitment was closed. The annual fees on other debentures have been set at 0.906%. The annual fees related to HT III debentures that pooled on March 27, 2013 were 0.804%. The annual fees on other debentures have been set at 0.515%. The rates of borrowings on the Company’s SBA debentures range from 3.05% to 5.53% when including these annual fees.
The average amount of debentures outstanding for the year ended December 31, 2016 for HT II was approximately $41.2 million with an average interest rate of approximately 4.52%. The average amount of debentures outstanding for the year ended December 31, 2016 for HT III was approximately $149.0 million with an average interest rate of approximately 3.43%.
For the years ended December 31, 2016 and 2015, the components of interest expense and related fees and cash paid for interest expense for the SBA debentures are as follows:
| Year Ended December 31, | ||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 6,988 | $ | 6,969 | ||||
| Amortization of debt issuance cost (loan fees) |
671 | 667 | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 7,659 | $ | 7,636 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 6,961 | $ | 6,942 | ||||
As of December 31, 2016, the maximum statutory limit on the dollar amount of combined outstanding SBA guaranteed debentures is $350.0 million, subject to periodic adjustments by the SBA. In aggregate, at December 31, 2016, with the Company’s net investment of $118.5 million, HT II and HT III have the capacity to issue a total of $190.2 million of SBA-guaranteed debentures, subject to SBA approval. At December 31, 2016, the Company has issued $190.2 million in SBA-guaranteed debentures in the Company’s SBIC subsidiaries.
The Company reported the following SBA debentures outstanding principal balances as of December 31, 2016 and December 31, 2015:
| (in thousands) Issuance/Pooling Date |
Maturity Date | Interest Rate(1) | December 31, 2016 | December 31, 2015 | ||||||||||||
| March 25, 2009 |
March 1, 2019 | 5.53 | % | $ | 18,400 | $ | 18,400 | |||||||||
| September 23, 2009 |
September 1, 2019 | 4.64 | % | 3,400 | 3,400 | |||||||||||
| September 22, 2010 |
September 1, 2020 | 3.62 | % | 6,500 | 6,500 | |||||||||||
| September 22, 2010 |
September 1, 2020 | 3.50 | % | 22,900 | 22,900 | |||||||||||
| March 29, 2011 |
March 1, 2021 | 4.37 | % | 28,750 | 28,750 | |||||||||||
| September 21, 2011 |
September 1, 2021 | 3.16 | % | 25,000 | 25,000 | |||||||||||
| March 21, 2012 |
March 1, 2022 | 3.28 | % | 25,000 | 25,000 | |||||||||||
| March 21, 2012 |
March 1, 2022 | 3.05 | % | 11,250 | 11,250 | |||||||||||
| September 19, 2012 |
September 1, 2022 | 3.05 | % | 24,250 | 24,250 | |||||||||||
| March 27, 2013 |
March 1, 2023 | 3.16 | % | 24,750 | 24,750 | |||||||||||
|
|
|
|
|
|||||||||||||
| Total SBA Debentures |
$ | 190,200 | $ | 190,200 | ||||||||||||
|
|
|
|
|
|||||||||||||
| (1) | Interest rate includes annual charge |
F-61
2019 Notes
On March 6, 2012, the Company and U.S. Bank National Association (the “2019 Trustee”) entered into an indenture (the “Base Indenture”). On April 17, 2012, the Company and the 2019 Trustee entered into the First Supplemental Indenture to the Base Indenture (the “First Supplemental Indenture”), dated April 17, 2012, relating to the Company’s issuance, offer and sale of $43.0 million aggregate principal amount of 7.00% notes due 2019 (the “April 2019 Notes”).
In July 2012, the Company reopened the Company’s April 2019 Notes and issued an additional $41.5 million in aggregate principal amount of April 2019 Notes, which included the exercise of an over-allotment option, bringing the total amount of the April 2019 Notes issued to approximately $84.5 million in aggregate principal amount.
On September 24, 2012, the Company and the 2019 Trustee, entered into the Second Supplemental Indenture to the Base Indenture (the “Second Supplemental Indenture”), dated as of September 24, 2012, relating to the Company’s issuance, offer and sale of $75.0 million aggregate principal amount of 7.00% notes due 2019 (the “September 2019 Notes”).
In October 2012, the underwriters exercised their over-allotment option for an additional $10.9 million of the September 2019 Notes, bringing the total amount of the September 2019 Notes issued to approximately $85.9 million in aggregate principal outstanding.
In April 2015, the Company redeemed $20.0 million of the $84.5 million issued and outstanding aggregate principal amount of April 2019 Notes, as previously approved by the Board of Directors. In December 2015, the Company redeemed $40.0 million of the $85.9 million issued and outstanding aggregate principal amount of September 2019 Notes, as previously approved by the Board of Directors. The Company has publicly announced its intention to redeem the remaining outstanding 2019 Notes on February 24, 2017. See “Note 14—Subsequent Events”.
As of December 31, 2016 and December 31, 2015, the 2019 Notes payable is comprised of:
| (in thousands) |
December 31, 2016 | December 31, 2015 | ||||||
| April 2019 Notes |
$ | 64,490 | $ | 64,490 | ||||
| September 2019 Notes |
45,874 | 45,874 | ||||||
|
|
|
|
|
|||||
| Total 2019 Notes principal outstanding |
$ | 110,364 | $ | 110,364 | ||||
|
|
|
|
|
|||||
April 2019 Notes
The April 2019 Notes will mature on April 30, 2019 and may be redeemed in whole or in part at the Company’s option at any time or from time to time on or after April 30, 2015, upon not less than 30 days nor more than 60 days written notice by mail prior to the date fixed for redemption thereof, at a redemption price of 100% of the outstanding principal amount thereof plus accrued and unpaid interest payments otherwise payable for the then-current quarterly interest period accrued to but not including the date fixed for redemption. The April 2019 Notes bear interest at a rate of 7.00% per year payable quarterly on January 30, April 30, July 30 and October 30 of each year, commencing on July 30, 2012, and trade on the NYSE under the trading symbol “HTGZ.”
The April 2019 Notes are the Company’s direct unsecured obligations and rank: (i) pari passu with the Company’s other outstanding and future senior unsecured indebtedness; (ii) senior to any of the Company’s future indebtedness that expressly provides it is subordinated to the April 2019 Notes; (iii) effectively subordinated to all the Company’s existing and future secured indebtedness (including indebtedness that is initially unsecured to which the Company subsequently grant security), to the extent of the value of the assets
F-62
securing such indebtedness; (iv) structurally subordinated to all existing and future indebtedness and other obligations of any of the Company’s subsidiaries.
The Base Indenture, as supplemented by the First Supplemental Indenture, contains certain covenants including covenants requiring the Company’s compliance with (regardless of whether it is subject to) the asset coverage requirements set forth in Section 18 (a)(1)(A) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act to comply with the restrictions on dividends and other distributions as well as the purchase of capital stock set forth in Section 18(a)(1)(B) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act and to provide financial information to the holders of the April 2019 Notes and the 2019 Trustee if the Company should no longer be subject to the reporting requirements under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These covenants are subject to important limitations and exceptions that are described in the Base Indenture, as supplemented by the First Supplemental Indenture. The Base Indenture provides for customary events of default and further provides that the 2019 Trustee or the holders of 25% in aggregate principal amount of the outstanding April 2019 Notes in a series may declare such April 2019 Notes immediately due and payable upon the occurrence of any event of default after expiration of any applicable grace period.
September 2019 Notes
The September 2019 Notes will mature on September 30, 2019 and may be redeemed in whole or in part at the Company’s option at any time or from time to time on or after September 30, 2015, upon not less than 30 days nor more than 60 days written notice by mail prior to the date fixed for redemption thereof, at a redemption price of 100% of the outstanding principal amount thereof plus accrued and unpaid interest payments otherwise payable for the then-current quarterly interest period accrued to but not including the date fixed for redemption. The September 2019 Notes bear interest at a rate of 7.00% per year payable quarterly on March 30, June 30, September 30 and December 30 of each year, commencing on December 30, 2012, and trade on the NYSE under the trading symbol “HTGY.”
The September 2019 Notes are the Company’s direct unsecured obligations and rank: (i) pari passu with the Company’s other outstanding and future senior unsecured indebtedness; (ii) senior to any of the Company’s future indebtedness that expressly provides it is subordinated to the September 2019 Notes; (iii) effectively subordinated to all the Company’s existing and future secured indebtedness (including indebtedness that is initially unsecured to which the Company subsequently grants security), to the extent of the value of the assets securing such indebtedness; (iv) structurally subordinated to all existing and future indebtedness and other obligations of any of the Company’s subsidiaries.
The Base Indenture, as supplemented by the Second Supplemental Indenture, contains certain covenants including covenants requiring the Company to comply with (regardless of whether it is subject to) the asset coverage requirements set forth in Section 18 (a)(1)(A) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act to comply with the restrictions on dividends and other distributions as well as the purchase of capital stock set forth in Section 18(a)(1)(B) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act and to provide financial information to the holders of the September 2019 Notes and the 2019 Trustee if the Company should no longer be subject to the reporting requirements under the Exchange Act. These covenants are subject to important limitations and exceptions that are described in the Base Indenture, as supplemented by the Second Supplemental Indenture. The Base Indenture provides for customary events of default and further provides that the 2019 Trustee or the holders of 25% in aggregate principal amount of the outstanding September 2019 Notes in a series may declare such September 2019 Notes immediately due and payable upon the occurrence of any event of default after expiration of any applicable grace period.
F-63
For the years ended December 31, 2016 and 2015, the components of interest expense and related fees and cash paid for interest expense for the 2019 Notes are as follows:
| Year Ended December 31, |
||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 7,725 | $ | 10,899 | ||||
| Amortization of debt issuance cost (loan fees) |
639 | 2,167 | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 8,364 | $ | 13,066 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 7,726 | $ | 11,132 | ||||
As of December 31, 2016, the Company was in compliance with the terms of the Base Indenture, and respective supplemental indentures thereto, governing the April 2019 Notes and September 2019 Notes.
2024 Notes
On July 14, 2014, the Company and U.S. Bank, N.A. (the “2024 Trustee”), entered into the Third Supplemental Indenture (the “Third Supplemental Indenture”) to the Base Indenture between the Company and the 2024 Trustee, dated July 14, 2014, relating to the Company’s issuance, offer and sale of $100.0 million aggregate principal amount of 6.25% unsecured notes due 2024 (the “2024 Notes”). On August 6, 2014, the underwriters issued notification to exercise their over-allotment option for an additional $3.0 million in aggregate principal amount of the 2024 Notes.
On May 2, 2016, the Company closed an underwritten public offering of an additional $72.9 million in aggregate principal amount of the 2024 Notes. The $72.9 million in aggregate principal amount includes $65.4 million from the initial offering on April 21, 2016 and $7.5 million as a result of underwriters exercising a portion of their option to purchase up to an additional $9.8 million in aggregate principal to cover overallotments on April 29, 2016.
On June 27, 2016, the Company closed an underwritten public offering of an additional $60.0 million in aggregate principal amount of the 2024 Notes. On June 30, 2016, the underwriters exercised their option to purchase up to an additional $9.0 million in aggregate principal to cover overallotments, resulting in total aggregate principal of $69.0 million from the offering.
On October 11, 2016, the Company entered into a debt distribution agreement, pursuant to which it may offer for sale, from time to time, up to $150.0 million in aggregate principal amount of 2024 Notes through FBR Capital Markets & Co. acting as its sales agent (the “2024 Notes Agent”). Sales of the 2024 Notes may be made in negotiated transactions or transactions that are deemed to be “at the market offerings” as defined in Rule 415 under the Securities Act, including sales made directly on the NYSE, or similar securities exchange or sales made through a market maker other than on an exchange at prices related to prevailing market prices or at negotiated prices.
The 2024 Notes Agent receives a commission from the Company equal to up to 2.00% of the gross sales of any 2024 Notes sold through the 2024 Notes Agent under the debt distribution agreement. The 2024 Notes Agent is not required to sell any specific principal amount of 2024 Notes, but will use its commercially reasonable efforts consistent with its sales and trading practices to sell the 2024 Notes. The 2024 Notes are expected to trade “flat,” which means that purchasers in the secondary market will not pay, and sellers will not receive, any accrued and unpaid interest on the 2024 Notes that is not reflected in the trading price.
Subsequent to October 11, 2016 and as of December 31, 2016, the Company sold 317,125 notes for approximately $7.9 million in aggregate principal amount. As of December 31, 2016 approximately $142.1 million in aggregate principal amount remains available for issuance and sale under the debt distribution agreement. See “Note 14—Subsequent Events”.
F-64
All issuances of 2024 Notes rank equally in right of payment and form a single series of notes.
The 2024 Notes will mature on July 30, 2024 and may be redeemed in whole or in part at the Company’s option at any time or from time to time on or after July 30, 2017, upon not less than 30 days nor more than 60 days written notice by mail prior to the date fixed for redemption thereof, at a redemption price of 100% of the outstanding principal amount thereof plus accrued and unpaid interest payments otherwise payable for the then-current quarterly interest period accrued to but not including the date fixed for redemption. The 2024 Notes bear interest at a rate of 6.25% per year payable quarterly on January 30, April 30, July 30 and October 30 of each year, commencing on July 30, 2014, and trade on the NYSE under the trading symbol “HTGX.”
The 2024 Notes are the Company’s direct unsecured obligations and rank: (i) pari passu with the Company’s other outstanding and future senior unsecured indebtedness; (ii) senior to any of the Company’s future indebtedness that expressly provides it is subordinated to the 2024 Notes; (iii) effectively subordinated to all the Company’s existing and future secured indebtedness (including indebtedness that is initially unsecured to which the Company subsequently grants security), to the extent of the value of the assets securing such indebtedness; (iv) structurally subordinated to all existing and future indebtedness and other obligations of any of the Company’s subsidiaries.
The Base Indenture, as supplemented by the Third Supplemental Indenture, contains certain covenants including covenants requiring the Company to comply with (regardless of whether it is subject to) the asset coverage requirements set forth in Section 18 (a)(1)(A) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act and to comply with the restrictions on dividends and other distributions as well as the purchase of capital stock set forth in Section 18(a)(1)(B) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act. These covenants are subject to important limitations and exceptions that are described in the Base Indenture, as supplemented by the Third Supplemental Indenture. The Base Indenture, as supplemented by the Third Supplemental Indenture, also contains certain reporting requirements, including a requirement that the Company provide financial information to the holders of the 2024 Notes and the 2024 Trustee if the Company should no longer be subject to the reporting requirements under the Exchange Act. The Base Indenture provides for customary events of default and further provides that the 2024 Trustee or the holders of 25% in aggregate principal amount of the outstanding 2024 Notes in a series may declare such 2024 Notes immediately due and payable upon the occurrence of any event of default after expiration of any applicable grace period. As of December 31, 2016, the Company was in compliance with the terms of the Base Indenture as supplemented by the Third Supplemental Indenture.
As of December 31, 2016 and December 31, 2015, the components of the carrying value of the 2024 Notes were as follows:
| (in thousands) |
December 31, 2016 | December 31, 2015 | ||||||
| Principal amount of debt |
$ | 252,873 | $ | 103,000 | ||||
| Unamortized debt issuance cost |
(7,482 | ) | (2,872 | ) | ||||
| Original issue premium, net of accretion |
99 | — | ||||||
|
|
|
|
|
|||||
| Carrying value of 2024 Notes |
$ | 245,490 | $ | 100,128 | ||||
|
|
|
|
|
|||||
For the years ended December 31, 2016 and 2015, the components of interest expense and related fees and cash paid for interest expense for the 2024 Notes are as follows:
| Year Ended December 31, |
||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 11,775 | $ | 6,437 | ||||
| Amortization of debt issuance cost (loan fees) |
686 | 333 | ||||||
| Accretion of original issue premium |
3 | — | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 12,464 | $ | 6,770 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 10,873 | $ | 6,437 | ||||
F-65
2021 Asset-Backed Notes
On November 13, 2014, the Company completed a $237.4 million term debt securitization in connection with which an affiliate of the Company made an offer of $129.3 million in aggregate principal amount of fixed-rate asset-backed notes (the “2021 Asset-Backed Notes”), which were rated A(sf) by Kroll Bond Rating Agency, Inc. The 2021 Asset-Backed Notes were sold by Hercules Capital Funding Trust 2014-1 pursuant to a note purchase agreement, dated as of November 13, 2014, by and among the Company, Hercules Capital Funding 2014-1, LLC as trust depositor (the “2014 Trust Depositor”), Hercules Capital Funding Trust 2014-1 as issuer (the “2014 Securitization Issuer”), and Guggenheim Securities, LLC, as initial purchaser, and are backed by a pool of senior loans made to certain of the Company’s portfolio companies and secured by certain assets of those portfolio companies and are to be serviced by the Company. The securitization has an 18-month reinvestment period during which time principal collections may be reinvested into additional eligible loans. Interest on the 2021 Asset-Backed Notes is paid, to the extent of funds available, at a fixed rate of 3.524% per annum. The 2021 Asset-Backed Notes have a stated maturity of April 16, 2021.
As part of this transaction, the Company entered into a sale and contribution agreement with the 2014 Trust Depositor under which the Company has agreed to sell or have contributed to the 2014 Trust Depositor certain senior loans made to certain of the Company’s portfolio companies (the “2014 Loans”). The Company has made customary representations, warranties and covenants in the sale and contribution agreement with respect to the 2014 Loans as of the date of their transfer to the 2014 Trust Depositor.
In connection with the issuance and sale of the 2021 Asset-Backed Notes, the Company has made customary representations, warranties and covenants in the note purchase agreement. The 2021 Asset-Backed Notes are secured obligations of the 2014 Securitization Issuer and are non-recourse to the Company. The 2014 Securitization Issuer also entered into an indenture governing the 2021 Asset-Backed Notes, which includes customary representations, warranties and covenants. The 2021 Asset-Backed Notes were sold without being registered under the Securities Act of 1933, as amended, (the “Securities Act”) (A) in the United States to “qualified institutional buyers” as defined in Rule 144A under the Securities Act and to institutional “accredited investors” (as defined in Rules 501(a)(1), (2), (3) or (7) under the Securities Act) who in each case, are “qualified purchasers” as defined in Sec. 2(a)(51)(A) of the 1940 Act and pursuant to an exemption under the Securities Act and (B) to non-U.S. purchasers acquiring interest in the 2021 Asset-Backed Notes outside the United States in accordance with Regulation S under the Securities Act. The 2014 Securitization Issuer is not registered under the 1940 Act in reliance on an exemption provided by Section 3(c)(7) thereof and Rule 3a-7 thereunder. In addition, the 2014 Trust Depositor entered into an amended and restated trust agreement in respect of the 2014 Securitization Issuer, which includes customary representation, warranties and covenants.
The 2014 Loans are serviced by the Company pursuant to a sale and servicing agreement, which contains customary representations, warranties and covenants. The Company performs certain servicing and administrative functions with respect to the 2014 Loans. The Company is entitled to receive a monthly fee from the 2014 Securitization Issuer for servicing the 2014 Loans. This servicing fee is equal to the product of one-twelfth (or in the case of the first payment date, a fraction equal to the number of days from and including October 5, 2014 through and including December 5, 2014 over 360) of 2.00% and the aggregate outstanding principal balance of the 2014 Loans plus collections on deposit in the 2014 Securitization Issuer’s collections account, as of the first day of the related collection period (the period from the 5th day of the immediately preceding calendar month through the 4th day of the calendar month in which a payment date occurs, and for the first payment date, the period from and including October 5, 2014, to the close of business on December 5, 2014).
The Company also serves as administrator to the 2014 Securitization Issuer under an administration agreement, which includes customary representations, warranties and covenants.
At December 31, 2016 and December 31, 2015, the 2021 Asset-Backed Notes had an outstanding principal balance of $109.2 million and $129.3 million, respectively.
F-66
For the years ended December 31, 2016 and 2015, the components of interest expense and related fees and cash paid for interest expense for the 2021 Asset-Backed Notes are as follows:
| Year Ended December 31, |
||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 4,366 | $ | 4,557 | ||||
| Amortization of debt issuance cost (loan fees) |
1,071 | 902 | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 5,437 | $ | 5,459 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 4,396 | $ | 4,557 | ||||
Under the terms of the 2021 Asset-Backed Notes, the Company is required to maintain a reserve cash balance, funded through interest and principal collections from the underlying securitized debt portfolio, which may be used to pay monthly interest and principal payments on the 2021 Asset-Backed Notes. The Company has segregated these funds and classified them as restricted cash. There was approximately $8.3 million and $9.2 million of restricted cash as of December 31, 2016 and December 31, 2015, respectively, funded through interest collections.
2016 Convertible Notes
In April 2011, the Company issued $75.0 million in aggregate principal amount of 6.00% convertible notes due 2016 (the “2016 Convertible Notes”). The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016.
Prior to the close of business on October 14, 2015, holders were able to convert their 2016 Convertible Notes only under certain circumstances set forth in the indenture governing the 2016 Convertible Notes. On or after October 15, 2015 until the close of business on the scheduled trading day immediately preceding the maturity date, holders were able to convert their 2016 Convertible Notes at any time. Throughout the life of the 2016 Convertible Notes, holders of approximately $74.8 million of the 2016 Convertible Notes exercised their conversion rights. These 2016 Convertible Notes were settled with a combination of cash equal to the outstanding principal amount of the 2016 Convertible Notes and approximately 1.6 million shares of the Company’s common stock, or $24.3 million.
The Company recorded a loss on extinguishment of debt for the proportionate amount of unamortized debt issuance costs and original issue discount. The loss was partially offset by a gain in the amount of the difference between the outstanding principal balance of the 2016 Convertible Notes and the fair value of the debt instrument. The net loss on extinguishment of debt the Company recorded for the year ended December 31, 2015 was $1,000. The Company did not record a loss on extinguishment of debt for the year ended December 31, 2016. The loss on extinguishment of debt was classified as a component of net investment income in the Company’s Consolidated Statement of Operations.
The 2016 Convertible Notes were accounted for in accordance with ASC Subtopic 470-20 (“Debt Instruments with Conversion and Other Options”). In accounting for the 2016 Convertible Notes, the Company estimated at the time of issuance that the values of the debt and the embedded conversion feature of the 2016 Convertible Notes were approximately 92.8% and 7.2%, respectively. The original issue discount of 7.2% attributable to the conversion feature was recorded in “capital in excess of par value” in the Consolidated Statement of Assets and Liabilities. As a result, the Company recorded interest expense comprised of both stated interest expense as well as accretion of the original issue discount resulting in an estimated effective interest rate of approximately 8.1%.
F-67
As of December 31, 2015, the components of the carrying value of the 2016 Convertible Notes were as follows:
| (in thousands) |
December 31, 2015 | |||
| Principal amount of debt |
$ | 17,604 | ||
| Unamortized debt issuance cost |
(44 | ) | ||
| Original issue discount, net of accretion |
(82 | ) | ||
|
|
|
|||
| Carrying value of 2016 Convertible Notes |
$ | 17,478 | ||
|
|
|
|||
For the years ended December 31, 2016 and 2015, the components of interest expense, fees and cash paid for interest expense for the 2016 Convertible Notes were as follows:
| Year Ended December 31, | ||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 352 | $ | 1,007 | ||||
| Accretion of original issue discount |
82 | 246 | ||||||
| Amortization of debt issuance cost (loan fees) |
44 | 131 | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 478 | $ | 1,384 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 440 | $ | 1,057 | ||||
The estimated effective interest rate of the debt component of the 2016 Convertible Notes, equal to the stated interest of 6.0% plus the accretion of the original issue discount, was approximately 8.1% for the years ended December 31, 2016 and December 31, 2015.
Credit Facilities
As of December 31, 2016 and December 31, 2015, the Company has two available secured credit facilities, the Wells Facility and the Union Bank Facility.
Wells Facility
On June 29, 2015, the Company, through a special purpose wholly-owned subsidiary, Hercules Funding II LLC (“Hercules Funding II”), entered into an Amended and Restated Loan and Security Agreement (the “Wells Facility”) with Wells Fargo Capital Finance, LLC, as a lender and as the arranger and the administrative agent, and the lenders party thereto from time to time.
The Wells Facility matures on August 2, 2019, unless terminated sooner in accordance with its terms.
Under the Wells Facility, Wells Fargo Capital Finance, LLC made commitments of $75.0 million. Alostar Bank of Commerce made commitments of $20.0 million, and Everbank Commercial Finance Inc. made commitments of $25.0 million. The Wells Facility contains an accordion feature, in which the Company can increase the credit line up to an aggregate of $300.0 million, funded by additional lenders and with the agreement of Wells Fargo and subject to other customary conditions. The Company expects to continue discussions with various other potential lenders to join the facility; however, there can be no assurances that additional lenders will join the Wells Facility. Borrowings under the Wells Facility generally bear interest at a rate per annum equal to LIBOR plus 3.25%, and the Wells Facility has an advance rate of 50% against eligible debt investments. The Wells Facility is secured by all of the assets of Hercules Funding II. The Wells Facility requires payment of a non-use fee on a scale of 0.0% to 0.50% depending on the average monthly outstanding balance under the facility relative to the maximum amount of commitments at such time. For the years ended December 31, 2016 and 2015, this non-use fee was approximately $483,000 and $294,000, respectively.
The Wells Facility also includes various financial and other covenants applicable to the Company and the Company’s subsidiaries, in addition to those applicable to Hercules Funding II, including covenants relating to certain changes of control of the Company and Hercules Funding II. Among other things, these covenants also
F-68
require the Company to maintain certain financial ratios, including a maximum debt to worth ratio, minimum interest coverage ratio, minimum portfolio funding liquidity, and a minimum tangible net worth in an amount, when added to outstanding subordinated indebtedness, that is in excess of $500.0 million plus 90% of the cumulative amount of equity raised after June 30, 2014. As of December 31, 2016, the minimum tangible net worth covenant has increased to $675.9 million as a result of the March 2015 follow-on public offering of 7.6 million shares of common stock for total gross proceeds of approximately $100.4 million and the 7.3 million shares of common stock issued under the At-The-Market (“ATM”) equity distribution agreement (the “Equity Distribution Agreement”) with JMP Securities (“JMP”) for gross proceeds of $95.0 million during the year ended December 31, 2016. The Wells Facility provides for customary events of default, including, without limitation, with respect to payment defaults, breach of representations and covenants, certain key person provisions, cross acceleration provisions to certain other debt, lien and judgment limitations, and bankruptcy.
On June 20, 2011 the Company paid $1.1 million in structuring fees in connection with the original Wells Facility. In connection with an amendment to the original Wells Facility in August 2014, the Company paid an additional $750,000 in structuring fees and in connection with the amendment in December 2015, the Company paid an additional $188,000 in structuring fees. These fees are being amortized through the end of the term of the Wells Facility.
The Company had aggregate draws of $195.9 million on the available facility during the year ended December 31, 2016 offset by repayments of $240.9 million. There was $5.0 million and $50.0 million of borrowings outstanding on this facility at December 31, 2016 and 2015, respectively.
For the years ended December 31, 2016 and 2015, the components of interest expense and related fees and cash paid for interest expense for the Wells Facility are as follows:
| Year Ended December 31, | ||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 539 | $ | 578 | ||||
| Amortization of debt issuance cost (loan fees) |
492 | 361 | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 1,031 | $ | 939 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 577 | $ | 402 | ||||
Union Bank Facility
On May 5, 2016, the Company, through a special purpose wholly owned subsidiary, Hercules Funding III LLC (“Hercules Funding III”), as borrower, entered into the credit facility (the “Union Bank Facility”) with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Union Bank Facility from time to time. The Union Bank Facility replaced the company’s credit facility (the “Prior Union Bank Facility”) entered into on August 14, 2014 (as amended and restated from time to time) with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Prior Union Bank Facility from time to time. Any references to amounts related to the Union Bank Facility prior to May 5, 2016 were incurred and relate to the Prior Union Bank Facility.
On July 18, 2016, the Company entered into the First Amendment to the Loan and Security Agreement, dated as of May 5, 2016 with MUFG Union Bank, N.A. The Amendment amends certain definitions relating to borrowings which accrue interest based on the London Interbank Offered Rate (“LIBOR Loans”) and (ii) the method(s) for calculating interest on and the paying of certain fees related to such LIBOR Loans.
Under the Union Bank Facility, MUFG Union Bank made commitments of $75.0 million. The Union Bank Facility contains an accordion feature, in which the Company can increase the credit line up to an aggregate of $200.0 million, funded by additional lenders and with the agreement of MUFG Union Bank and subject to other customary conditions. There can be no assurances that additional lenders will join the Union Bank Facility to
F-69
increase available borrowings. Borrowings under the Union Bank Facility generally bear interest at either (i) if such borrowing is a base rate loan, a base rate per annum equal to the federal funds rate plus 1.00%, LIBOR plus 1.00% or MUFG Union Bank’s prime rate, in each case, plus a margin of 1.25% or (ii) if such borrowing is a LIBOR loan, a rate per annum equal to LIBOR plus 3.25%, and the Union Bank Facility generally has an advance rate of 50% against eligible debt investments. The Union Bank Facility is secured by all of the assets of Hercules Funding III.
The Union Bank Facility requires payment of a non-use fee during the revolving credit availability period on a scale of 0.25% to 0.50% depending on the average monthly outstanding balance under the facility relative to the maximum amount of commitments at such time. The Company paid a one-time $562,500 structuring fee in connection with the Union Bank Facility. Although the Company did not incur any non-use fees under the Union Bank Facility prior to May 5, 2016, for the years ended December 31, 2016 and 2015, the Company incurred non-use fees under the existing and previous Union Bank Facility of $356,000 and $380,000, respectively.
The Union Bank Facility also includes various financial and other covenants applicable to the Company and the Company’s subsidiaries, in addition to those applicable to Hercules Funding III, including covenants relating to certain changes of control of the Company and Hercules Funding III. Among other things, these covenants also require the Company to maintain certain financial ratios, including a maximum debt to worth ratio, minimum interest coverage ratio, minimum portfolio funding liquidity, and a minimum tangible net worth in an amount that is in excess of $500.0 million plus 90% of the cumulative amount of equity raised after June 30, 2014. As of December 31, 2016, the minimum tangible net worth covenant increased to $723.6 million as a result of the March 2015 follow-on public offering of 7.6 million shares of common stock for total net proceeds of approximately $100.1 million and the 7.3 million shares of common stock issued under the Equity Distribution Agreement with JMP for net proceeds of $92.8 million during the year ended December 31, 2016. The Union Bank Facility provides for customary events of default, including with respect to payment defaults, breach of representations and covenants, servicer defaults, certain key person provisions, cross default provisions to certain other debt, lien and judgment limitations, and bankruptcy.
The Union Bank Facility matures on May 5, 2020, unless sooner terminated in accordance with its terms.
In connection with the Union Bank Facility, the Company and Hercules Funding III also entered into the Sale Agreement, by and among Hercules Funding III, as borrower, the Company, as originator and servicer, and MUFG Union Bank, as agent. Under the Sale Agreement, the Company agrees to (i) sell or transfer certain loans to Hercules Funding III under the Union Bank Facility and (ii) act as servicer for the loans sold or transferred.
The Company had aggregate draws of $90.0 million on the available facility during the year ended December 31, 2016 offset by repayments of $90.0 million. At December 31, 2016 there were no borrowings outstanding on the Union Bank Facility.
For the years ended December 31, 2016 and 2015, the components of interest expense and related fees and cash paid for interest expense for the previous and current Union Bank Facility are as follows:
| Year Ended December 31, | ||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Interest expense |
$ | 189 | $ | — | ||||
| Amortization of debt issuance cost (loan fees) |
356 | 61 | ||||||
|
|
|
|
|
|||||
| Total interest expense and fees |
$ | 545 | $ | 61 | ||||
|
|
|
|
|
|||||
| Cash paid for interest expense and fees |
$ | 38 | $ | — | ||||
F-70
Citibank Credit Facility
The Company, through Hercules Funding Trust I, an affiliated statutory trust, had a securitized credit facility (the “Citibank Credit Facility”) with Citigroup Global Markets Realty Corp. (“Citigroup”), which expired under normal terms. During the first quarter of 2009, the Company paid off all principal and interest owed under the Citibank Credit Facility. Citigroup has an equity participation right through a warrant participation agreement on the pool of debt investments and warrants collateralized under the Citibank Credit Facility. Pursuant to the warrant participation agreement, the Company granted to Citigroup a 10% participation in all warrants held as collateral. However, no additional warrants were included in collateral subsequent to the facility amendment on May 2, 2007. As a result, Citigroup is entitled to 10% of the realized gains on the warrants until the realized gains paid to Citigroup pursuant to the agreement equal $3,750,000 (the “Maximum Participation Limit”). The obligations under the warrant participation agreement continue even after the Citibank Credit Facility is terminated until the Maximum Participation Limit has been reached.
During the year ended December 31, 2016, the Company reduced its realized gain by approximately $146,000 for Citigroup’s participation from the acquisition proceeds received on equity exercised from warrants that were included in the collateral pool. The Company also recorded a decrease in participation liability and an increase in unrealized appreciation by a net amount of approximately $16,000 primarily due to depreciation of fair value on the pool of warrants collateralized under the warrant participation and the acquisition proceeds received on the Company’s Ping Identity Corporation equity investment. The remaining value of Citigroup’s participation right on unrealized gains in the related equity investments is approximately $127,000 as of December 31, 2016 and is included in accrued liabilities. There can be no assurances that the unrealized appreciation of the warrants will not be higher or lower in future periods due to fluctuations in the value of the warrants, thereby increasing or reducing the effect on the cost of borrowing. Since inception of the agreement, the Company has paid Citigroup approximately $2.4 million under the warrant participation agreement thereby reducing realized gains by this amount. The Company will continue to pay Citigroup under the warrant participation agreement until the Maximum Participation Limit is reached or the warrants expire. The remaining warrants subject to the Citigroup participation agreement are set to expire in January 2017.
5. Income Taxes
The Company intends to operate so as to qualify to be subject to tax as a RIC under Subchapter M of the Code and, as such, will not be subject to U.S. federal income tax on the portion of taxable income and gains distributed to stockholders. Taxable income includes the Company’s taxable interest, dividend and fee income, reduced by certain deductions, as well as taxable net realized securities gains. Taxable income generally differs from net income for financial reporting purposes due to temporary and permanent differences in the recognition of income and expenses, and generally excludes net unrealized appreciation or depreciation, as such gains or losses are not included in taxable income until they are realized.
To qualify and be subject to tax as a RIC, the Company is required to meet certain income and asset diversification tests in addition to distributing dividends of an amount generally at least equal to 90% of its investment company taxable income, as defined by the Code and determined without regard to any deduction for distributions paid, to its stockholders. The amount to be paid out as a distribution is determined by the Board of Directors each quarter and is based upon the annual earnings estimated by the management of the Company. To the extent that the Company’s earnings fall below the amount of dividend distributions declared, however, a portion of the total amount of the Company’s distributions for the fiscal year may be deemed a return of capital for tax purposes to the Company’s stockholders.
Because federal income tax regulations differ from accounting principles generally accepted in the United States, distributions in accordance with tax regulations may differ from net investment income and realized gains recognized for financial reporting purposes. Differences may be permanent or temporary in nature. Permanent differences are reclassified among capital accounts in the financial statements to reflect their appropriate tax
F-71
character. Permanent differences may also result from the change in the classification of short-term gains as ordinary income for tax purposes. Temporary differences arise when certain items of income, expense, gain or loss are recognized at some time in the future.
During the year ended December 31, 2016 and 2015, the Company reclassified for book purposes amounts arising from permanent book/tax differences primarily related to accelerated revenue recognition for income tax purposes, respectively, as follows:
| Year Ended December 31, | ||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Undistributed net investment income (distributions in excess of investment income) |
$ | 1,644 | $ | (994 | ) | |||
| Accumulated realized gains |
$ | 5,034 | $ | 8,767 | ||||
| Additional paid-in capital |
$ | (7,020 | ) | $ | (7,773 | ) | ||
For income tax purposes, distributions paid to shareholders are reported as ordinary income, return of capital, long-term capital gains or a combination thereof. The tax character of distributions paid for the year ended December 31, 2016 was ordinary income in the amount of $91.1 million. The tax character of distributions paid for the year ended December 31, 2015 was ordinary income in the amount of $70.6 million and long term capital gains in the amount of $15.3 million.
The aggregate gross unrealized appreciation of the Company’s investments over cost for U.S. federal income tax purposes was $24.7 million and $29.3 million as of December 31, 2016 and 2015, respectively. The aggregate gross unrealized depreciation of the Company’s investments under cost for U.S. federal income tax purposes was $114.5 million and $81.4 million as of December 31, 2016 and 2015, respectively. The net unrealized depreciation over cost for U.S. federal income tax purposes was $89.8 million and $52.1 million as of December 31, 2016 and 2015, respectively. The aggregate cost of securities for U.S. federal income tax purposes was $1.5 billion and $1.3 billion as of December 31, 2016 and 2015, respectively.
At December 31, 2016 and 2015, the components of distributable earnings on a tax basis detailed below differ from the amounts reflected in the Company’s Consolidated Statements of Assets and Liabilities by temporary book/tax differences primarily arising from the treatment of loan related yield enhancements.
| Year Ended December 31, | ||||||||
| (in thousands) |
2016 | 2015 | ||||||
| Accumulated Capital Gains |
$ | 14,893 | $ | 7,962 | ||||
| Other Temporary Differences |
1,306 | 4,117 | ||||||
| Undistributed Ordinary Income |
19,283 | 236 | ||||||
| Unrealized Depreciation |
(87,275 | ) | (47,498 | ) | ||||
|
|
|
|
|
|||||
| Components of Distributable Earnings |
$ | (51,793 | ) | $ | (35,183 | ) | ||
|
|
|
|
|
|||||
As a RIC, the Company will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income unless the Company makes distributions treated as dividends for U.S. federal income tax purposes in a timely manner to its stockholders in respect of each calendar year of an amount at least equal to the sum of (1) 98% of the Company’s ordinary income (taking into account certain deferrals and elections) for each calendar year, (2) 98.2% of the Company’s capital gain net income (adjusted for certain ordinary losses) for the 1-year period ending October 31 of each such calendar year and (3) any ordinary income and capital gain net income realized, but not distributed, in preceding calendar years. The Company will not be subject to this excise tax on any amount on which the Company incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains).
The Company has taxable subsidiaries which are designed to hold certain portfolio investments in an effort to limit potential legal liability and/or comply with source-income type requirements contained in the RIC tax provisions of the Code. These taxable subsidiaries are consolidated for U.S. GAAP financial reporting purposes and the portfolio investments held by the taxable subsidiaries are included in the Company’s consolidated
F-72
financial statements, and recorded at fair value. These taxable subsidiaries are not consolidated with the Company for income tax purposes and may generate income tax expense, or benefit, and tax assets and liabilities as a result of their ownership of certain portfolio investments. Any income generated by these taxable subsidiaries would be taxed at normal corporate tax rates based on its taxable income.
For the year ended December 31, 2016, the Company paid approximately $184,000 of income tax and had approximately $652,000 of accrued but unpaid income tax as of December 31, 2016. For the year ended December 31, 2015, the Company paid approximately $751,000 of income tax and did not have an accrued but unpaid amount as of the balance sheet date.
The Company evaluates tax positions taken in the course of preparing the Company’s tax returns to determine whether the tax positions are “more-likely-than-not” to be sustained by the applicable tax authority. Tax benefits of positions not deemed to meet the more-likely-than-not threshold, or uncertain tax positions, would be recorded as a tax expense in the current year. It is the Company’s policy to recognize accrued interest and penalties, if any, related to unrecognized tax benefits as a component of provision for income taxes.
Based on an analysis of the Company’s tax position, there are no uncertain tax positions that met the recognition or measurement criteria. The Company is currently not undergoing any tax examinations. The Company does not anticipate any significant increase or decrease in unrecognized tax benefits for the next twelve months. The 2013- 2015 federal tax years for the Company remain subject to examination by the Internal Revenue Service. The 2012-2015 state tax years for the Company remain subject to examination by the state taxing authorities.
6. Shareholders’ Equity
On August 16, 2013, the Company entered into the Equity Distribution Agreement with JMP and on March 7, 2016, the Company renewed the Equity Distribution Agreement. The Equity Distribution Agreement provides that the Company may offer and sell up to 8.0 million shares of its common stock from time to time through JMP, as the sales agent. On December 21, 2016 the Equity Distribution Agreement was further amended to increase the numbers of shares by 4.0 million to a total of up to 12.0 million shares available. Sales of the Company’s common stock, if any, may be made in negotiated transactions or transactions that are deemed to be “at the market,” as defined in Rule 415 under the Securities Act, including sales made directly on the NYSE or similar securities exchange or sales made to or through a market maker other than on an exchange, at prices related to the prevailing market prices or at negotiated prices.
During the year ended December 31, 2016 the Company sold 7.3 million shares of common stock for total accumulated net proceeds of approximately $92.8 million, including $2.2 million of offering expenses. The Company did not sell any shares under the program during the year ended December 31, 2015. The Company generally uses net proceeds from these offerings to make investments, to repurchase or pay down liabilities and for general corporate purposes. As of December 31, 2016 approximately 4.1 million shares remain available for issuance and sale under the equity distribution agreement. See “Note 14—Subsequent Events”.
On February 24, 2015, the Company’s Board of Directors authorized a stock repurchase plan permitting the Company to repurchase up to $50.0 million of its common stock. This plan expired on August 24, 2015. On August 27, 2015, the Company’s Board of Directors authorized a replacement stock repurchase plan permitting the Company to repurchase up to $50.0 million of its common stock and on February 17, 2016, the Company’s Board of Directors extended the program until August 23, 2016, after which the plan expired. During the month of January 2016, the Company repurchased 449,588 shares of its common stock at an average price per share of $10.64 per share and a total cost of approximately $4.8 million. The Company did not make any repurchases in subsequent months during 2016. During the year ended December 31, 2015, the Company repurchased 437,006 shares of its common stock at an average price per share of $10.61 per share and a total cost of approximately $4.6 million.
F-73
On March 27, 2015, the Company raised approximately $100.1 million, after deducting offering expenses of $323,000, in a public offering of 7,590,000 shares of its common stock.
At the 2015 Annual Meeting of Stockholders on July 7, 2015, the Company’s common stockholders approved a proposal to allow the Company to issue common stock at a discount from its then current net asset value (“NAV”) per share, which was effective until the 2016 annual meeting of stockholders on July 7, 2016. Such authorization was not sought at the 2016 annual meeting of stockholders. During the year ended December 31, 2016, the Company has not issued common stock at a discount to NAV.
The Company has issued stock options for common stock subject to future issuance, of which 668,171 and 622,171 were outstanding at December 31, 2016 and December 31, 2015, respectively.
7. Equity Incentive Plan
The Company and its stockholders have authorized and adopted the 2004 Equity Incentive Plan (the “2004 Plan”) for purposes of attracting and retaining the services of its executive officers and key employees. Under the 2004 Plan, the Company is authorized to issue 12.0 million shares of common stock.
The Company and its stockholders have authorized and adopted the 2006 Non-Employee Director Plan (the “2006 Plan” and, together with the 2004 Plan, the “Plans”) for purposes of attracting and retaining the services of its Board of Directors. Under the 2006 Plan, the Company is authorized to issue 1.0 million shares of common stock. The Company filed an exemptive relief request with the Securities and Exchange Commission (“SEC”) to allow options to be issued under the 2006 Plan which was approved on October 10, 2007.
On June 21, 2007, the stockholders approved amendments to the 2004 Plan and the 2006 Plan allowing for the grant of restricted stock. The amended Plans limit the combined maximum amount of restricted stock that may be issued under both Plans to 10% of the outstanding shares of the Company’s stock on the effective date of the Plans plus 10% of the number of shares of stock issued or delivered by the Company during the terms of the Plans. The amendments further specify that no one person shall be granted awards of restricted stock relating to more than 25% of the shares available for issuance under the 2004 Plan. Further, the amount of voting securities that would result from the exercise of all of the Company’s outstanding warrants, options and rights, together with any restricted stock issued pursuant to the Plans, at the time of issuance shall not exceed 25% of its outstanding voting securities, except that if the amount of voting securities that would result from such exercise of all of the Company’s outstanding warrants, options and rights issued to the Company’s directors, officers and employees, together with any restricted stock issued pursuant to the Plans, would exceed 15% of the Company’s outstanding voting securities, then the total amount of voting securities that would result from the exercise of all outstanding warrants, options and rights, together with any restricted stock issued pursuant to the Plans, at the time of issuance shall not exceed 20% of the Company’s outstanding voting securities.
On December 29, 2016, the Company’s Board of Directors approved a further amendment and restatement of the 2004 Plan. The amended plan provides, in addition to the preexisting types of awards available for grant thereunder and among other things, (1) for the grant of restricted stock units; (2) for the deferral of the receipt of the shares of the Company’s common stock underlying vested restricted stock units; (3) that grantees may receive up to 10% of the value of the tentative restricted stock unit grants proposed for any grantee in the form of an option to acquire shares of the Company’s common stock; (4) that awards of restricted stock units may include performance vesting conditions; (5) that awards may require that all or a portion of the shares of the Company’s common stock delivered in respect of any vested restricted stock unit award be subject to a specified post-delivery holding period; and (6) that restricted stock unit awards may accrue dividend equivalents in respect of the Company’s common stock underlying any restricted stock unit award payable in the form of cash or additional shares of the Company’s common stock to the extent, and in respect of, any vested restricted stock units. As of December 31, 2016, the Company has not issued any restricted stock units based on the December 2016 amended terms.
F-74
A summary of the restricted stock activity under the Company’s 2006 and 2004 Plans for each of the three periods ended December 31 2016, 2015, and 2014 is as follows:
| 2006 Plan | 2004 Plan |
|||||||
| Outstanding at December 31, 2013 |
36,668 | 2,395,778 | ||||||
| Granted |
8,333 | 981,550 | ||||||
| Cancelled |
— | (152,277 | ) | |||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2014 |
45,001 | 3,225,051 | ||||||
| Granted |
19,999 | 656,341 | ||||||
| Cancelled |
— | (312,564 | ) | |||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2015 |
65,000 | 3,568,828 | ||||||
| Granted |
61,666 | 493,881 | ||||||
| Cancelled |
(3,333 | ) | (33,610 | ) | ||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2016 |
123,333 | 4,029,099 | ||||||
|
|
|
|
|
|||||
In 2016, 2015, and 2014, the Company granted approximately 555,547, 676,340 and 989,883 shares, respectively, of restricted stock pursuant to the Plans. All restricted stock grants under the 2004 Plan made prior to March 4, 2013 will continue to vest on a monthly basis following their one year anniversary over the succeeding 36 months. During 2012, the Compensation Committee adopted a policy that provided for awards with different vesting schedules for short and long-term awards. Under the 2004 Plan, restricted stock awarded subsequent to March 3, 2013 will vest subject to continued employment based on two vesting schedules: short-term awards vest one-half on the one year anniversary of the date of the grant and quarterly over the succeeding 12 months, and long-term awards vest one-fourth on the one year anniversary of the date of grant and quarterly over the succeeding 36 months. No restricted stock was granted pursuant to the 2004 Plan prior to 2009. See “Note 14—Subsequent Events.”
The Company determined that the fair value of restricted stock granted under the 2006 and 2004 Plans during the years ended December 31, 2016, 2015, and 2014 was approximately $6.7 million, $9.2 million and $13.7 million, respectively based on the grant date close price and vesting period of each grant. During the years ended December 31, 2016, 2015, and 2014 the Company expensed approximately $7.0 million, $9.2 million and $9.2 million of compensation expense related to restricted stock, respectively. As of December 31, 2016, there was approximately $7.7 million of total unrecognized compensation costs related to restricted stock. These costs are expected to be recognized over a weighted average period of 1.66 years.
The following table summarizes the activities for the Company’s unvested restricted stock for the years ended December 31, 2016, 2015, and 2014:
| Unvested Restricted Stock Awards | ||||||||
| Restricted Stock Awards |
Weighted Average Grant Date Fair Value |
|||||||
| Unvested at December 31, 2013 |
1,035,897 | $ | 11.94 | |||||
| Granted |
989,883 | $ | 13.82 | |||||
| Vested |
(570,723 | ) | $ | 12.00 | ||||
| Forfeited |
(152,277 | ) | $ | 12.82 | ||||
|
|
|
|||||||
| Unvested at December 31, 2014 |
1,302,780 | $ | 13.23 | |||||
| Granted |
676,340 | $ | 13.67 | |||||
| Vested |
(816,484 | ) | $ | 13.26 | ||||
| Forfeited |
(312,564 | ) | $ | 13.16 | ||||
|
|
|
|||||||
| Unvested at December 31, 2015 |
850,072 | $ | 13.59 | |||||
| Granted |
555,547 | $ | 12.02 | |||||
| Vested |
(569,118 | ) | $ | 13.58 | ||||
| Forfeited |
(36,943 | ) | $ | 12.70 | ||||
|
|
|
|||||||
| Unvested at December 31, 2016 |
799,558 | $ | 12.54 | |||||
|
|
|
|||||||
F-75
The SEC, through an exemptive order granted on June 22, 2010, approved amendments to the Plans which allow participants to elect to have the Company withhold shares of the Company’s common stock to pay for the exercise price and applicable taxes with respect to an option exercise (“net issuance exercise”). The exemptive order also permits the holders of restricted stock to elect to have the Company withhold shares of the Company’s stock to pay the applicable taxes due on restricted stock at the time of vesting. Each individual can make a cash payment at the time of option exercise or to pay taxes on restricted stock.
The following table summarizes the common stock options activities under the Company’s 2006 and 2004 Plans for each of the three periods ended December 31 2016, 2015, and 2014:
| Common Stock Options |
Weighted Average Exercise Price |
|||||||
| Shares Outstanding at December 31, 2013 |
833,923 | $ | 12.53 | |||||
| Granted |
426,000 | $ | 15.54 | |||||
| Exercised |
(353,547 | ) | $ | 10.76 | ||||
| Forfeited |
(208,344 | ) | $ | 14.80 | ||||
| Expired |
(2,360 | ) | $ | 13.78 | ||||
|
|
|
|||||||
| Shares Outstanding at December 31, 2014 |
695,672 | $ | 14.58 | |||||
| Granted |
163,500 | $ | 12.68 | |||||
| Exercised |
(36,331 | ) | $ | 10.81 | ||||
| Forfeited |
(190,006 | ) | $ | 14.83 | ||||
| Expired |
(10,664 | ) | $ | 13.21 | ||||
|
|
|
|||||||
| Shares Outstanding at December 31, 2015 |
622,171 | $ | 14.25 | |||||
|
|
|
|||||||
| Granted |
230,000 | $ | 12.16 | |||||
| Exercised |
(36,500 | ) | $ | 11.05 | ||||
| Forfeited |
(82,895 | ) | $ | 13.41 | ||||
| Expired |
(64,605 | ) | $ | 15.05 | ||||
|
|
|
|||||||
| Shares Outstanding at December 31, 2016 |
668,171 | $ | 13.73 | |||||
|
|
|
|||||||
| Shares Expected to Vest at December 31, 2016 |
281,421 | $ | 13.73 | |||||
The following table summarizes stock options outstanding and exercisable at December 31, 2016:
| (Dollars in thousands, except exercise price) |
Options outstanding | Options exercisable | ||||||||||||||||||||||||||||||
| Range of exercise prices |
Number of shares |
Weighted Average Remaining Contractual Life |
Aggregate Intrinsic Value |
Weighted Average Exercise Price |
Number of shares |
Weighted Average Remaining Contractual Life |
Aggregate Intrinsic Value |
Weighted Average Exercise Price |
||||||||||||||||||||||||
| $9.25 - $14.02 |
332,587 | 6.27 | $ | 703,067 | $ | 12.00 | 103,201 | 4.89 | $ | 275,444 | $ | 11.44 | ||||||||||||||||||||
| $14.60 - $16.34 |
335,584 | 4.64 | — | $ | 15.45 | 283,549 | 4.51 | — | $ | 15.45 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| $9.25 - $16.34 |
668,171 | 5.45 | $ | 703,067 | $ | 13.73 | 386,750 | 4.61 | $ | 275,444 | $ | 14.38 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
Options generally vest 33% one year after the date of grant and ratably over the succeeding 24 months. All options may be exercised for a period ending seven years after the date of grant. At December 31, 2016, options for approximately 386,750 shares were exercisable at a weighted average exercise price of approximately $14.38 per share with weighted average of remaining contractual term of 4.61 years.
The Company determined that the fair value of options granted under the 2006 and 2004 Plans during the years ended December 31, 2016, 2015, and 2014 was approximately $837,000, $57,000 and $211,000, respectively. During the years ended December 31, 2016, 2015, and 2014, approximately $169,000, $265,000 and $395,000 of share-based cost due to stock option grants was expensed, respectively. As of December 31, 2016, there was $117,000 of total unrecognized compensation costs related to stock options. These costs are expected to be recognized over a weighted average period of 2.04 years.
F-76
The Company follows ASC Topic 718 (“Compensation – Stock Compensation”) to account for stock options granted. Under ASC Topic 718, compensation expense associated with stock-based compensation is measured at the grant date based on the fair value of the award and is recognized over the vesting period. Determining the appropriate fair value model and calculating the fair value of stock-based awards at the grant date requires judgment, including estimating stock price volatility, forfeiture rate and expected option life. The fair value of options granted is based upon a Black Scholes option pricing model using the assumptions in the following table for each of the three periods ended December 31, 2016, 2015, and 2014 is as follows:
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Expected Volatility |
23.73 | % | 18.94 | % | 19.90 | % | ||||||
| Expected Dividends |
10 | % | 10 | % | 10 | % | ||||||
| Expected term (in years) |
4.5 | 4.5 | 4.5 | |||||||||
| Risk-free rate |
0.87% - 1.98 | % | 1.08% - 1.70 | % | 1.21% - 1.66 | % | ||||||
8. Earnings Per Share
Shares used in the computation of the Company’s basic and diluted earnings per share are as follows:
| Year Ended December 31, | ||||||||||||
| (in thousands, except per share data) |
2016 | 2015 | 2014 | |||||||||
| Numerator |
||||||||||||
| Net increase in net assets resulting from operations |
$ | 68,703 | $ | 42,916 | $ | 71,188 | ||||||
| Less: Distributions declared-common and restricted shares |
(92,333 | ) | (87,438 | ) | (78,562 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Undistributed earnings |
(23,630 | ) | (44,522 | ) | (7,374 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Undistributed earnings-common shares |
(23,630 | ) | (44,522 | ) | (7,374 | ) | ||||||
| Add: Distributions declared-common shares |
91,065 | 85,959 | 76,953 | |||||||||
|
|
|
|
|
|
|
|||||||
| Numerator for basic and diluted change in net assets per common share |
$ | 67,435 | $ | 41,437 | $ | 69,579 | ||||||
|
|
|
|
|
|
|
|||||||
| Denominator |
||||||||||||
| Basic weighted average common shares outstanding |
73,753 | 69,479 | 61,862 | |||||||||
| Common shares issuable |
22 | 184 | 1,363 | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding assuming dilution |
73,775 | 69,663 | 63,225 | |||||||||
| Change in net assets per common share |
||||||||||||
| Basic |
$ | 0.91 | $ | 0.60 | $ | 1.12 | ||||||
| Diluted |
$ | 0.91 | $ | 0.59 | $ | 1.10 | ||||||
In the table above, unvested share-based payment awards that have non-forfeitable rights to distributions or distribution equivalents are treated as participating securities for calculating earnings per share.
Unvested common stock options are also included in the denominator for the purpose of calculating diluted earnings per share. For the year ended December 31, 2015, the dilutive effect of the 2016 Convertible Notes under the treasury stock method was also included in this calculation because the Company’s share price was greater than the conversion price in effect ($11.03 as of December 31, 2015) for the 2016 Convertible Notes for such period. The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016, as such there is no potential additional dilutive effect for the year ended December 31, 2016.
The calculation of change in net assets resulting from operations per common share—assuming dilution, excludes all anti-dilutive shares. For the years ended December 31, 2016, 2015, and 2014, the number of anti-dilutive shares, as calculated based on the weighted average closing price of the Company’s common stock for the periods, was 676,133, 627,483 and 727,733 shares, respectively.
At December 31, 2016, the Company was authorized to issue 200 million shares of common stock with a par value of $0.001. Each share of common stock entitles the holder to one vote.
F-77
9. Financial Highlights
Following is a schedule of financial highlights for the three years ended December 31, 2016.
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Per share data(1): |
||||||||||||
| Net asset value at beginning of period |
$ | 9.94 | $ | 10.18 | $ | 10.51 | ||||||
| Net investment income |
1.36 | 1.06 | 1.16 | |||||||||
| Net realized gain on investments |
0.06 | 0.07 | 0.32 | |||||||||
| Net unrealized depreciation on investments |
(0.49 | ) | (0.51 | ) | (0.33 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total from investment operations |
0.93 | 0.62 | 1.15 | |||||||||
| Net increase (decrease) in net assets from capital share transactions(1) |
0.18 | 0.26 | (0.37 | ) | ||||||||
| Distributions of net investment income(6) |
(1.25 | ) | (1.26 | ) | (1.27 | ) | ||||||
| Stock-based compensation expense included in investment income(2) |
0.10 | 0.14 | 0.16 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net asset value at end of period |
$ | 9.90 | $ | 9.94 | $ | 10.18 | ||||||
|
|
|
|
|
|
|
|||||||
| Ratios and supplemental data: |
||||||||||||
| Per share market value at end of period |
$ | 14.11 | $ | 12.19 | $ | 14.88 | ||||||
| Total return(3) |
26.87 | % | (9.70 | %) | (1.75 | %) | ||||||
| Shares outstanding at end of period |
79,555 | 72,118 | 64,715 | |||||||||
| Weighted average number of common shares outstanding |
73,753 | 69,479 | 61,862 | |||||||||
| Net assets at end of period |
$ | 787,944 | $ | 717,134 | $ | 658,864 | ||||||
| Ratio of total expense to average net assets(4) |
11.25 | % | 11.55 | % | 10.97 | % | ||||||
| Ratio of net investment income before investment gains and losses to average net assets(4) |
13.65 | % | 10.15 | % | 10.94 | % | ||||||
| Portfolio turnover rate(5) |
36.22 | % | 46.34 | % | 56.15 | % | ||||||
| Average debt outstanding |
$ | 635,365 | $ | 615,198 | $ | 535,127 | ||||||
| Weighted average debt per common share |
$ | 8.61 | $ | 8.85 | $ | 8.65 | ||||||
| (1) | All per share activity is calculated based on the weighted average shares outstanding for the relevant period, except net increase (decrease) in net assets from capital share transactions, which is based on the common shares outstanding as of the relevant balance sheet date. |
| (2) | Stock option expense is a non-cash expense that has no effect on net asset value. Pursuant to ASC Topic 718, net investment income includes the expense associated with the granting of stock options which is offset by a corresponding increase in paid-in capital. |
| (3) | The total return for the years ended December 31, 2016, 2015 and 2014 equals the change in the ending market value over the beginning of the period price per share plus distributions paid per share during the period, divided by the beginning price assuming the distribution is reinvested on the date of the distribution. The total return does not reflect any sales load that must be paid by investors. |
| (4) | All ratios are calculated based on weighted average net assets for the relevant period. |
| (5) | The portfolio turnover rate for the years ended December 31, 2016, 2015 and 2014 equals the lesser of investment portfolio purchases or sales during the period, divided by the average investment portfolio value during the period. |
| (6) | Includes distributions on unvested shares. |
10. Commitments and Contingencies
The Company’s commitments and contingencies consist primarily of unused commitments to extend credit in the form of loans to the Company’s portfolio companies. A portion of these unfunded contractual commitments as of December 31, 2016 are dependent upon the portfolio company reaching certain milestones before the debt commitment becomes available. Furthermore, the Company’s credit agreements contain customary lending provisions which allow the Company relief from funding obligations for previously made commitments in instances where the underlying company experiences materially adverse events that affect the financial condition or business outlook for the Company. Since a portion of these commitments may expire without being drawn, unfunded contractual commitments do not necessarily represent future cash requirements. As such, the Company’s disclosure of unfunded contractual commitments includes only those which are available at the request of the portfolio company and unencumbered by milestones.
At December 31, 2016, the Company had approximately $59.7 million of unfunded commitments, including undrawn revolving facilities, which were available at the request of the portfolio company and unencumbered by milestones.
The Company also had approximately $55.0 million of non-binding term sheets outstanding at December 31, 2016. Non-binding outstanding term sheets are subject to completion of the Company’s due
F-78
diligence and final investment committee approval process, as well as the negotiation of definitive documentation with the prospective portfolio companies. These non-binding term sheets generally convert to contractual commitments in approximately 90 days from signing. Not all non-binding term sheets are expected to close and do not necessarily represent future cash requirements.
The fair value of the Company’s unfunded commitments is considered to be immaterial as the yield determined at the time of underwriting is expected to be materially consistent with the yield upon funding, given that interest rates are generally pegged to a market indices and given the existence of milestones, conditions and/or obligations imbedded in the borrowing agreements.
As of December 31, 2016, the Company’s unfunded contractual commitments available at the request of the portfolio company, including undrawn revolving facilities, and unencumbered by milestones are as follows:
| (in thousands) Portfolio Company |
Unfunded Commitments(1) |
|||
| NewVoiceMedia Limited |
$ | 15,000 | ||
| Evernote Corporation |
14,000 | |||
| Aquantia Corp. |
11,500 | |||
| WP Technology, Inc. (Wattpad, Inc.) |
7,500 | |||
| Edge Therapeutics, Inc. |
5,000 | |||
| Achronix Semiconductor Corporation |
3,318 | |||
| Druva, Inc. |
3,000 | |||
| RedSeal Inc. |
365 | |||
|
|
|
|||
| Total |
$ | 59,683 | ||
|
|
|
|||
| (1) | Amount represents unfunded commitments, including undrawn revolving facilities, which are available at the request of the portfolio company. Amount excludes unfunded commitments which are unavailable due to the borrower having not met certain milestones. |
Certain premises are leased under agreements which expire at various dates through March 2020. Total rent expense amounted to approximately $1.7 million, $1.7 million and $1.6 million, during the years ended December 31, 2016, 2015, and 2014, respectively. The Company’s contractual obligations as of December 31, 2016 include:
| Payments due by period (in thousands) | ||||||||||||||||||||
| Contractual Obligations(1)(2) |
Total | Less than 1 year | 1 - 3 years | 3 - 5 years | After 5 years | |||||||||||||||
| Borrowings(3)(4)(6) |
$ | 667,658 | $ | 110,364 | $ | 136,021 | $ | 83,150 | $ | 338,123 | ||||||||||
| Operating Lease Obligations(5) |
3,362 | 1,699 | 1,604 | 59 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 671,020 | $ | 112,063 | $ | 137,625 | $ | 83,209 | $ | 338,123 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Excludes commitments to extend credit to the Company’s portfolio companies. |
| (2) | The Company also has a warrant participation agreement with Citigroup. See Note 4 to the Company’s consolidated financial statements. |
| (3) | Includes $190.2 million in principal outstanding under the SBA debentures, $110.4 million of the 2019 Notes, $252.9 million of the 2024 Notes, $109.2 million of the 2021 Asset-Backed Notes, and $5.0 million in outstanding borrowings on the Wells Facility as of December 31, 2016. |
| (4) | Amounts represent future principal repayments and not the carrying value of each liability. See Note 4 to the Company’s consolidated financial statements. |
| (5) | Long-Term facility leases. |
| (6) | Reflects announced redemption of the remaining 2019 Notes in 2017. See “Note 14—Subsequent Events.” |
The Company may, from time to time, be involved in litigation arising out of its operations in the normal course of business or otherwise. Furthermore, third parties may try to seek to impose liability on the Company in connection with the activities of its portfolio companies. While the outcome of any current legal proceedings cannot at this time be predicted with certainty, the Company does not expect any current matters will materially affect the Company’s financial condition or results of operations; however, there can be no assurance whether any pending legal proceedings will have a material adverse effect on the Company’s financial condition or results of operations in any future reporting period.
F-79
On December 19, 2016, the Company entered into a Confidential Settlement Agreement (the “Settlement Agreement”) with all defendants in connection with a litigation matter (“the Action”) filed in November 2014. In connection with the Settlement Agreement, the Action was settled among the parties and the Company received a settlement payment in the amount of $8.0 million. The Settlement Agreement also provides a mutual release by the Company and the defendants of any and all claims and cross-claims that were asserted in the Action, the circumstances and events underlying the Action and attorney’s fees and costs related thereto. The Settlement Agreement does not constitute an admission of liability, fault, or wrongdoing by any party. The settlement payment was classified as a component of net investment income in the Company’s Consolidated Statement of Operations.
11. Indemnification
The Company has entered into indemnification agreements with its directors. The indemnification agreements are intended to provide the Company’s directors the maximum indemnification permitted under Maryland law and the 1940 Act. Each indemnification agreement provides that the Company shall indemnify the director who is a party to the agreement, or an “Indemnitee,” including the advancement of legal expenses, if, by reason of his or her corporate status, the Indemnitee is, or is threatened to be, made a party to or a witness in any threatened, pending, or completed proceeding, to the maximum extent permitted by Maryland law and the 1940 Act.
The Company and its executives and directors are covered by Directors and Officers Insurance, with the directors and officers being indemnified by the Company to the maximum extent permitted by Maryland law subject to the restrictions in the 1940 Act.
12. Concentrations of Credit Risk
The Company’s customers are primarily privately held companies and public companies which are active in the drug discovery and development, software, sustainable and renewable technology, media/content/info, drug delivery, medical devices and equipment, internet consumer and business services, consumer and business products, specialty pharmaceuticals, healthcare services, communications and networking, surgical devices, semiconductors, electronics and computer hardware, biotechnology tools, information services, and diagnostic industry sectors. These sectors are characterized by high margins, high growth rates, consolidation and product and market extension opportunities. Value for companies in these sectors is often vested in intangible assets and intellectual property.
Industry and sector concentrations vary as new loans are recorded and loans pay off. Loan revenue, consisting of interest, fees, and recognition of gains on equity and warrant or other equity-related interests, can fluctuate materially when a loan is paid off or a related warrant or equity interest is sold. Revenue recognition in any given year can be highly concentrated among several portfolio companies.
For the years ended December 31, 2016 and December 31, 2015, the Company’s ten largest portfolio companies represented approximately 34.0% and 32.1% of the total fair value of the Company’s investments in portfolio companies, respectively. At December 31, 2016 and December 31, 2015, the Company had seven and two investments, respectively, that represented 5% or more of the Company’s net assets. At December 31, 2016, the Company had seven equity investments representing approximately 54.7% of the total fair value of the Company’s equity investments, and each represented 5% or more of the total fair value of the Company’s equity investments. At December 31, 2015, the Company had four equity investments which represented approximately 53.2% of the total fair value of the Company’s equity investments, and each represented 5% or more of the total fair value of such investments.
F-80
13. Selected Quarterly Data (Unaudited)
The following tables set forth certain quarterly financial information for each of the last eight quarters ended December 31, 2016. This information was derived from the Company’s unaudited consolidated financial statements. Results for any quarter are not necessarily indicative of results for the full year or for any further quarter.
| Quarter Ended | ||||||||||||||||
| (in thousands, except per share data) |
March 31, 2016 | June 30, 2016 | September 30, 2016 | December 31, 2016 | ||||||||||||
| Total investment income |
$ | 38,939 | $ | 43,538 | $ | 45,102 | $ | 47,472 | ||||||||
| Net investment income |
20,097 | 23,354 | 23,776 | 33,117 | ||||||||||||
| Net increase in net assets resulting from operations |
14,295 | 9,475 | 30,812 | 14,121 | ||||||||||||
| Change in net assets resulting from operations per common share (basic) |
$ | 0.20 | $ | 0.13 | $ | 0.41 | $ | 0.18 | ||||||||
| Quarter Ended | ||||||||||||||||
| March 31, 2015 | June 30, 2015 | September 30, 2015 | December 31, 2015 | |||||||||||||
| Total investment income |
$ | 32,494 | $ | 38,126 | $ | 47,132 | $ | 39,380 | ||||||||
| Net investment income |
12,993 | 16,781 | 23,590 | 20,137 | ||||||||||||
| Net increase in net assets resulting from operations |
21,919 | 2,752 | 4,075 | 14,170 | ||||||||||||
| Change in net assets resulting from operations per common share (basic) |
$ | 0.33 | $ | 0.03 | $ | 0.05 | $ | 0.20 | ||||||||
14. Subsequent Events
Distribution Declaration
On February 16, 2017 the Company’s Board of Directors declared a cash distribution of $0.31 per share to be paid on March 13, 2017 to shareholders of record as of March 6, 2017. This distribution represents the Company’s forty-sixth consecutive distribution since the Company’s initial public offering, bringing the total cumulative distribution to date to $12.78 per share.
Restricted Stock Unit Grants
In January 2017, the Company granted 600,461 restricted stock units pursuant to the Plans.
4.375% Convertible Notes due 2022
On January 25, 2017, the Company issued $230.0 million in aggregate principal amount of 4.375% Convertible Notes due 2022 (the “2022 Convertible Notes”), which amount includes the additional $30.0 million aggregate principal amount of 2022 Convertible Notes issued pursuant to the initial purchaser’s exercise in full of its overallotment option. The 2022 Convertible Notes were issued pursuant to an Indenture, dated January 25, 2017 (the “2022 Convertible Notes Indenture”), between the Company and U.S. Bank, National Association, as trustee (the “2022 Trustee”). The sale of the 2022 Convertible Notes generated net proceeds of approximately $224.3 million. Aggregate estimated offering expenses in connection with the transaction, including the initial purchaser’s discount of approximately $5.2 million, were approximately $5.7 million.
The Company intends to use the net proceeds from this offering (i) to repurchase or otherwise redeem all of its 2019 Notes, (ii) to fund investments in debt and equity securities in accordance with its investment objective and (iii) for working capital and other general corporate purposes. The 2022 Convertible Notes will mature on February 1, 2022, unless previously converted or repurchased in accordance with their terms. The 2022 Convertible Notes bear interest at a rate of 4.375% per year payable semiannually in arrears on February 1 and August 1 of each year, commencing on August 1, 2017.
The 2022 Convertible Notes will be unsecured obligations of the Company and will rank senior in right of payment to the Company’s future indebtedness that is expressly subordinated in right of payment to the 2022
F-81
Convertible Notes; equal in right of payment to the Company’s existing and future indebtedness that is not so subordinated; effectively junior in right of payment to any of the Company’s secured indebtedness (including unsecured indebtedness that the Company later secures) to the extent of the value of the assets securing such indebtedness; and structurally junior to all existing and future indebtedness (including trade payables) incurred by the Company’s subsidiaries, financing vehicles or similar facilities.
Prior to the close of business on the business day immediately preceding August 1, 2021, holders may convert their 2022 Convertible Notes only under certain circumstances set forth in the 2022 Convertible Notes Indenture. On or after August 1, 2021 until the close of business on the scheduled trading day immediately preceding the Maturity Date, holders may convert their 2022 Convertible Notes at any time. Upon conversion, the Company will pay or deliver, as the case may be, at its election, cash, shares of its common stock or a combination of cash and shares of its common stock. The conversion rate is initially 60.9366 shares of common stock per $1,000 principal amount of 2022 Convertible Notes (equivalent to an initial conversion price of approximately $16.41 per share of common stock). The conversion rate will be subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, if certain corporate events occur prior to the maturity date, the Company will increase the conversion rate for a holder who elects to convert its 2022 Convertible Notes in connection with such a corporate event in certain circumstances.
The Company may not redeem the 2022 Convertible Notes at its option prior to maturity. No sinking fund is provided for the 2022 Convertible Notes. In addition, if certain corporate events occur in respect of the Company, holders of the 2022 Convertible Notes may require the Company to repurchase for cash all or part of their 2022 Convertible Notes at a repurchase price equal to 100% of the principal amount of the 2022 Convertible Notes to be repurchased, plus accrued and unpaid interest through, but excluding, the required repurchase date.
The 2022 Convertible Notes Indenture contains certain covenants, including covenants requiring the Company to comply with Section 18(a)(1)(A) as modified by Section 61(a)(1) of the 1940 Act and to provide financial information to the holders of the 2022 Convertible Notes and the 2022 Trustee if the Company ceases to be subject to the reporting requirements of the Exchange Act. These covenants are subject to important limitations and exceptions that are described in the 2022 Convertible Notes Indenture. The Company offered and sold the 2022 Convertible Notes to the initial purchaser in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act, for resale by the initial purchaser to qualified institutional buyers (as defined in the Securities Act) pursuant to the exemption from registration provided by Rule 144A under the Securities Act. The Company relied on these exemptions from registration based in part on representations made by the initial purchaser in connection with the sale of the 2022 Convertible Notes.
2019 Notes—Redemption
On January 25, 2017, the Company’s Board of Directors approved a redemption of the remaining $110.4 million of outstanding aggregate principal amount of the 2019 Notes, and notice for such redemption has been provided. The Company has publicly announced its intention to redeem the remaining 2019 Notes on February 24, 2017.
ATM Debt Program Issuances
Subsequent to December 31, 2016 and as of February 17, 2017, the Company sold 225,457 notes of the 2024 Notes through the 2024 Notes Agent for approximately $5.6 million in aggregate principal amount under the ATM debt distribution agreement with FBR Capital Markets & Co. As of February 17, 2017, approximately $136.4 million in aggregate principal amount remains available for issuance and sale under the debt distribution agreement.
F-82
ATM Equity Program Issuances
Subsequent to December 31, 2016 and as of February 17, 2017, the Company sold 3.3 million shares of common stock for total accumulated net proceeds of approximately $47.1 million, including $356,000 of offering expenses, under the Equity Distribution Agreement with JMP. As of February 17, 2017 approximately 751,000 shares remain available for issuance and sale under the equity distribution agreement.
Portfolio Company Developments
As of February 17, 2017, the Company held warrants or equity positions in six companies that have filed registration statements on Form S-1 with the SEC in contemplation of potential initial public offerings. All six companies filed confidentially under the Jumpstart Our Business Startups Act of 2012. There can be no assurance that these companies will complete their initial public offerings in a timely manner or at all. In addition, subsequent to December 31, 2016, the Company’s portfolio companies announced the following events:
| 1. | In February 2017, the Company’s portfolio company Jaguar Animal Health, Inc. entered into a binding agreement to merge with Napo Pharmaceuticals, a company that focuses on the development and commercialization of proprietary pharmaceuticals for the global marketplace in collaboration with local partners. |
F-83
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS IN AND ADVANCES TO AFFILIATES
As of and for the year ended December 31, 2016
(in thousands)
| Portfolio Company |
Investment(1) |
Amount of Interest Credited to Income(2) |
As of December 31, 2015 Fair Value |
Gross Additions(3) |
Gross Reductions(4) |
Net Change in Unrealized Appreciation/ (Depreciation) |
As of December 31, 2016 Fair Value |
|||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||||
| Majority Owned Control Investments |
||||||||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(5) |
Senior Debt | 78 | — | 1,304 | — | — | 1,304 | |||||||||||||||||||
| Common Stock |
— | — | 4,000 | — | (604 | ) | 3,396 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Majority Owned Control Investments |
$ | 78 | $ | — | $ | 5,304 | $ | — | $ | (604 | ) | $ | 4,700 | |||||||||||||
| Other Control Investments |
||||||||||||||||||||||||||
| SkyCross, Inc.(5) |
Senior Debt | $ | — | $ | — | $ | 16,900 | $ | (13,479 | ) | $ | (3,421 | ) | $ | — | |||||||||||
| Preferred Warrants |
— | — | 394 | (394 | ) | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Other Control Investments |
$ | — | $ | — | $ | 17,294 | $ | (13,873 | ) | $ | (3,421 | ) | $ | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Control Investments |
$ | 78 | $ | — | $ | 22,598 | $ | (13,873 | ) | $ | (4,025 | ) | $ | 4,700 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Senior Debt | $ | 12 | $ | — | $ | 431 | $ | (431 | ) | $ | — | $ | — | ||||||||||||
| Preferred Stock |
— | 6,661 | 1,135 | — | (3,267 | ) | 4,529 | |||||||||||||||||||
| Preferred Warrants |
— | 312 | — | — | (142 | ) | 170 | |||||||||||||||||||
| Stion Corporation |
Senior Debt | 148 | 1,013 | — | (1,866 | ) | 1,187 | 333 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Affiliate Investments |
$ | 160 | $ | 7,986 | $ | 1,566 | $ | (2,297 | ) | $ | (2,222 | ) | $ | 5,032 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Control and Affiliate Investments |
$ | 238 | $ | 7,986 | $ | 24,164 | $ | (16,170 | ) | $ | (6,247 | ) | $ | 9,732 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (1) | Stock and warrants are generally non-income producing and restricted. The principal amount for debt is shown in the Consolidated Schedule of Investments as of December 31, 2016. |
| (2) | Represents the total amount of interest or dividends credited to income for the year an investment was an affiliate or control investment. |
| (3) | Gross additions include increases in the cost basis of investments resulting from new portfolio investments, paid-in-kind interest or dividends, the amortization of discounts and closing fees and the exchange of one or more existing securities for one or more new securities. |
| (4) | Gross reductions include decreases in the cost basis of investments resulting from principal repayments or sales and the exchange of one or more existing securities for one or more new securities. Gross reductions also include previously recognized depreciation on investments that become control or affiliate investments during the period. |
| (5) | In June 2016, the Company’s investments in SkyCross, Inc. became classified as a control investment as a result of obtaining more than 50% representation on a portfolio company’s board. In June 2016 the Company also acquired 100% ownership of the equity of Achilles Technology Management Co II, Inc. and classified it as a control investment in accordance with the requirements of the 1940 Act. In June 2016, Achilles Technology Management Co II, Inc. acquired the assets of a global antenna company that produces radio frequency system solutions as part of an article 9 consensual foreclosure and public auction for total consideration in the amount of $4.0 million. In September and November 2016 the Company made a $1.0 million and $250,000 debt investment, respectively, in Achilles Technology Management II to provide working capital under the terms of a loan servicing agreement. The Company’s investments in Achilles Technology Management Co II, Inc. are carried on the consolidated statement of assets and liabilities at fair value. |
F-84
HERCULES CAPITAL, INC.
CONSOLIDATED STATEMENT OF ASSETS AND LIABILITIES
(unaudited)
(dollars in thousands, except per share data)
| June 30, 2017 | December 31, 2016 | |||||||
| Assets |
||||||||
| Investments: |
||||||||
| Non-control/Non-affiliate investments (cost of $1,385,401 and $1,475,918 respectively) |
$ | 1,357,914 | $ | 1,414,210 | ||||
| Control investments (cost of $102,888 and $22,598, respectively) |
31,564 | 4,700 | ||||||
| Affiliate investments (cost of $12,850 and $13,010, respectively) |
5,991 | 5,032 | ||||||
|
|
|
|
|
|||||
| Total investments, at value (cost of $1,501,139 and $1,511,526 respectively) |
1,395,469 | 1,423,942 | ||||||
| Cash and cash equivalents |
160,412 | 13,044 | ||||||
| Restricted cash |
17,226 | 8,322 | ||||||
| Interest receivable |
10,204 | 11,614 | ||||||
| Other assets |
5,398 | 7,282 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 1,588,709 | $ | 1,464,204 | ||||
|
|
|
|
|
|||||
| Liabilities |
||||||||
| Accounts payable and accrued liabilities |
$ | 22,193 | $ | 21,463 | ||||
| Credit Facilities |
— | 5,016 | ||||||
| 2021 Asset-Backed Notes, net (principal of $87,678 and $109,205, respectively)(1) |
86,865 | 107,972 | ||||||
| Convertible Notes, net (principal of $230,000 and $0, respectively)(1) |
222,898 | — | ||||||
| 2019 Notes, net (principal of $0 and $110,364, respectively)(1) |
— | 108,818 | ||||||
| 2024 Notes, net (principal of $258,510 and $252,873, respectively)(1) |
251,478 | 245,490 | ||||||
| SBA Debentures, net (principal of $190,200 and $190,200, respectively)(1) |
187,824 | 187,501 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
$ | 771,258 | $ | 676,260 | ||||
| Net assets consist of: |
||||||||
| Common stock, par value |
83 | 80 | ||||||
| Capital in excess of par value |
892,930 | 839,657 | ||||||
| Unrealized depreciation on investments(2) |
(106,941 | ) | (89,025 | ) | ||||
| Accumulated undistributed realized gains on investments |
35,128 | 37,603 | ||||||
| Distributions in excess of net investment income |
(3,749 | ) | (371 | ) | ||||
|
|
|
|
|
|||||
| Total net assets |
$ | 817,451 | $ | 787,944 | ||||
|
|
|
|
|
|||||
| Total liabilities and net assets |
$ | 1,588,709 | $ | 1,464,204 | ||||
|
|
|
|
|
|||||
| Shares of common stock outstanding ($0.001 par value, 200,000,000 authorized) |
82,819 | 79,555 | ||||||
| Net asset value per share |
$ | 9.87 | $ | 9.90 | ||||
| (1) | The Company’s 2021 Asset-Backed Notes, Convertible Notes, 2019 Notes, 2024 Notes and SBA Debentures, as each term is defined herein, are presented net of the associated debt issuance costs for each instrument. See “Note 4—Borrowings”. |
| (2) | Amounts include $1.3 million and $1.4 million in net unrealized depreciation on other assets and accrued liabilities, including escrow receivables, estimated taxes payable and warrant participation agreement liabilities as of June 30, 2017 and December 31, 2016, respectively. |
See notes to consolidated financial statements.
F-85
The following table presents the assets and liabilities of our consolidated securitization trust for the 2021 Asset-Backed Notes (see Note 4), which is a variable interest entity (“VIE”). The assets of our securitization VIE can only be used to settle obligations of our consolidated securitization VIE, these liabilities are only the obligations of our consolidated securitization VIE, and the creditors (or beneficial interest holders) do not have recourse to our general credit. These assets and liabilities are included in the Consolidated Statement of Assets and Liabilities above.
| (Dollars in thousands) |
June 30, 2017 | December 31, 2016 | ||||||
| Assets |
||||||||
| Restricted Cash |
$ | 17,226 | $ | 8,322 | ||||
| Total investments, at value (cost of $190,276 and $244,695, respectively) |
190,168 | 242,349 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 207,394 | $ | 250,671 | ||||
|
|
|
|
|
|||||
| Liabilities |
||||||||
| 2021 Asset-Backed Notes, net (principal of $87,678 and $109,205, respectively)(1) |
$ | 86,865 | $ | 107,972 | ||||
|
|
|
|
|
|||||
| Total liabilities |
$ | 86,865 | $ | 107,972 | ||||
|
|
|
|
|
|||||
| (1) | The Company’s 2021 Asset-Backed Notes are presented net of the associated debt issuance costs. See “Note 4—Borrowings”. |
See notes to consolidated financial statements.
F-86
HERCULES CAPITAL, INC.
CONSOLIDATED STATEMENT OF OPERATIONS
(unaudited)
(in thousands, except per share data)
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| 2017 | 2016 | 2017 | 2016 | |||||||||||||
| Investment income: |
||||||||||||||||
| Interest and PIK interest income |
||||||||||||||||
| Interest income: |
||||||||||||||||
| Non-control/Non-affiliate investments |
$ | 37,715 | $ | 37,736 | $ | 78,027 | $ | 72,436 | ||||||||
| Control investments |
340 | — | 672 | — | ||||||||||||
| Affiliate investments |
— | 50 | 2 | 115 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest income |
38,055 | 37,786 | 78,701 | 72,551 | ||||||||||||
| PIK interest income: |
||||||||||||||||
| Non-control/Non-affiliate investments |
2,264 | 1,835 | 4,297 | 3,544 | ||||||||||||
| Control investments |
187 | — | 369 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total PIK interest income |
2,451 | 1,835 | 4,666 | 3,544 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest and PIK interest income |
40,506 | 39,621 | 83,367 | 76,095 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fee income |
||||||||||||||||
| Commitment, facility and loan fee income: |
||||||||||||||||
| Non-control/Non-affiliate investments |
2,440 | 3,126 | 5,374 | 5,426 | ||||||||||||
| Control investments |
5 | — | 10 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total commitment, facility and loan fee income |
2,445 | 3,126 | 5,384 | 5,426 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| One-time fee income: |
||||||||||||||||
| Non-control/Non-affiliate investments |
5,501 | 791 | 6,066 | 956 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total one-time fee income |
5,501 | 791 | 6,066 | 956 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total fee income |
7,946 | 3,917 | 11,450 | 6,382 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total investment income |
48,452 | 43,538 | 94,817 | 82,477 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Interest |
9,254 | 7,572 | 18,861 | 14,589 | ||||||||||||
| Loan fees |
1,348 | 1,278 | 4,186 | 2,267 | ||||||||||||
| General and administrative |
4,750 | 4,401 | 8,814 | 7,980 | ||||||||||||
| Employee compensation: |
||||||||||||||||
| Compensation and benefits |
5,916 | 5,331 | 11,262 | 10,016 | ||||||||||||
| Stock-based compensation |
1,909 | 1,602 | 3,742 | 4,174 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total employee compensation |
7,825 | 6,933 | 15,004 | 14,190 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
23,177 | 20,184 | 46,865 | 39,026 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net investment income |
25,275 | 23,354 | 47,952 | 43,451 | ||||||||||||
| Net realized gain (loss) on investments |
||||||||||||||||
| Non-control/Non-affiliate investments |
(5,319 | ) | 25 | (2,030 | ) | (4,443 | ) | |||||||||
| Control investments |
(394 | ) | — | (445 | ) | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total net realized gain (loss) on investments |
(5,713 | ) | 25 | (2,475 | ) | (4,443 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net change in unrealized appreciation (depreciation) on investments |
||||||||||||||||
| Non-control/Non-affiliate investments |
66,255 | (8,159 | ) | 34,100 | (9,618 | ) | ||||||||||
| Control investments |
(53,349 | ) | (3,421 | ) | (53,135 | ) | (3,421 | ) | ||||||||
| Affiliate investments |
681 | (2,324 | ) | 1,119 | (2,199 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total net unrealized appreciation (depreciation) on investments |
13,587 | (13,904 | ) | (17,916 | ) | (15,238 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total net realized and unrealized gain (loss) |
7,874 | (13,879 | ) | (20,391 | ) | (19,681 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase in net assets resulting from operations |
$ | 33,149 | $ | 9,475 | $ | 27,561 | $ | 23,770 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net investment income before investment gains and losses per common share: |
||||||||||||||||
| Basic |
$ | 0.31 | $ | 0.32 | $ | 0.58 | $ | 0.59 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Change in net assets resulting from operations per common share: |
||||||||||||||||
| Basic |
$ | 0.40 | $ | 0.13 | $ | 0.33 | $ | 0.32 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | 0.40 | $ | 0.13 | $ | 0.33 | $ | 0.32 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average shares outstanding |
||||||||||||||||
| Basic |
82,292 | 72,746 | 81,858 | 71,959 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
82,395 | 72,762 | 81,953 | 71,965 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Distributions declared per common share: |
||||||||||||||||
| Basic |
$ | 0.31 | $ | 0.31 | $ | 0.62 | $ | 0.62 | ||||||||
See notes to consolidated financial statements.
F-87
HERCULES CAPITAL, INC.
CONSOLIDATED STATEMENT OF CHANGES IN NET ASSETS
(unaudited)
(dollars and shares in thousands)
| Common Stock | Capital in excess of par value |
Unrealized Appreciation (Depreciation) on Investments |
Accumulated Undistributed Realized Gains (Losses) on Investments |
Undistributed Net Investment Income/ (Distributions in Excess of Investment Income) |
Net Assets |
|||||||||||||||||||||||
| Shares | Par Value | |||||||||||||||||||||||||||
| Balance at December 31, 2015 |
72,118 | $ | 73 | $ | 751,902 | $ | (52,808 | ) | $ | 27,993 | $ | (10,026 | ) | $ | 717,134 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net increase (decrease) in net assets resulting from operations |
— | — | — | (15,238 | ) | (4,443 | ) | 43,451 | 23,770 | |||||||||||||||||||
| Public offering, net of offering expenses |
2,201 | 2 | 23,668 | — | — | — | 23,670 | |||||||||||||||||||||
| Acquisition of common stock under repurchase plan |
(450 | ) | (1 | ) | (4,789 | ) | — | — | — | (4,790 | ) | |||||||||||||||||
| Issuance of common stock due to stock option exercises |
11 | — | 118 | — | — | — | 118 | |||||||||||||||||||||
| Issuance of common stock under restricted stock plan |
547 | 1 | (1 | ) | — | — | — | — | ||||||||||||||||||||
| Retired shares for restricted stock vesting |
(192 | ) | — | (2,122 | ) | — | — | — | (2,122 | ) | ||||||||||||||||||
| Distributions reinvested in common stock |
85 | — | 997 | — | — | — | 997 | |||||||||||||||||||||
| Distributions |
— | — | — | — | — | (45,206 | ) | (45,206 | ) | |||||||||||||||||||
| Stock-based compensation(1) |
— | — | 4,224 | — | — | — | 4,224 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at June 30, 2016 |
74,320 | $ | 75 | $ | 773,997 | $ | (68,046 | ) | $ | 23,550 | $ | (11,781 | ) | $ | 717,795 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2016 |
79,555 | $ | 80 | $ | 839,657 | $ | (89,025 | ) | $ | 37,603 | $ | (371 | ) | $ | 787,944 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net increase (decrease) in net assets resulting from operations |
— | — | — | (17,916 | ) | (2,475 | ) | 47,952 | 27,561 | |||||||||||||||||||
| Public offering, net of offering expenses |
3,309 | 3 | 46,908 | — | — | — | 46,911 | |||||||||||||||||||||
| Issuance of common stock due to stock option exercises |
27 | — | 211 | — | — | — | 211 | |||||||||||||||||||||
| Retired shares from net issuance |
(18 | ) | — | (170 | ) | — | — | — | (170 | ) | ||||||||||||||||||
| Issuance of common stock under restricted stock plan |
10 | — | — | — | — | — | — | |||||||||||||||||||||
| Retired shares for restricted stock vesting |
(145 | ) | — | (1,988 | ) | — | — | — | (1,988 | ) | ||||||||||||||||||
| Distributions reinvested in common stock |
81 | — | 1,122 | — | — | — | 1,122 | |||||||||||||||||||||
| Issuance of Convertible Notes |
— | — | 3,413 | — | — | — | 3,413 | |||||||||||||||||||||
| Distributions |
— | — | — | — | — | (51,330 | ) | (51,330 | ) | |||||||||||||||||||
| Stock-based compensation(1) |
— | — | 3,777 | — | — | — | 3,777 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at June 30, 2017 |
82,819 | $ | 83 | $ | 892,930 | $ | (106,941 | ) | $ | 35,128 | $ | (3,749 | ) | $ | 817,451 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (1) | Stock-based compensation includes $35 and $50 of restricted stock and option expense related to director compensation for the six months ended June 30, 2017 and 2016, respectively. |
See notes to consolidated financial statements.
F-88
HERCULES CAPITAL, INC.
CONSOLIDATED STATEMENT OF CASH FLOWS
(unaudited)
(dollars in thousands)
| For the Six Months Ended June 30, |
||||||||
| 2017 | 2016 | |||||||
| Cash flows from operating activities: |
||||||||
| Net increase (decrease) in net assets resulting from operations |
$ | 27,561 | $ | 23,770 | ||||
| Adjustments to reconcile net increase in net assets resulting from operations to net cash provided by (used in) operating activities: |
||||||||
| Purchase of investments |
(340,632 | ) | (330,750 | ) | ||||
| Principal and fee payments received on investments |
349,519 | 221,331 | ||||||
| Proceeds from the sale of investments |
18,450 | 6,041 | ||||||
| Net unrealized depreciation on investments |
17,916 | 15,238 | ||||||
| Net realized loss (gain) on investments |
2,475 | 4,443 | ||||||
| Accretion of paid-in-kind principal |
(4,656 | ) | (3,243 | ) | ||||
| Accretion of loan discounts |
(3,776 | ) | (3,776 | ) | ||||
| Accretion of loan discount on Convertible Notes |
280 | 82 | ||||||
| Accretion of loan exit fees |
(10,653 | ) | (10,968 | ) | ||||
| Change in deferred loan origination revenue |
19 | (44 | ) | |||||
| Unearned fees related to unfunded commitments |
769 | (113 | ) | |||||
| Amortization of debt fees and issuance costs |
3,557 | 1,839 | ||||||
| Depreciation |
105 | 104 | ||||||
| Stock-based compensation and amortization of restricted stock grants(1) |
3,777 | 4,224 | ||||||
| Change in operating assets and liabilities: |
||||||||
| Interest and fees receivable |
1,410 | (214 | ) | |||||
| Prepaid expenses and other assets |
589 | (9,041 | ) | |||||
| Accounts payable |
— | 56 | ||||||
| Accrued liabilities |
898 | (879 | ) | |||||
|
|
|
|
|
|||||
| Net cash provided by (used in) operating activities |
67,608 | (81,900 | ) | |||||
| Cash flows from investing activities: |
||||||||
| Purchases of capital equipment |
(89 | ) | (146 | ) | ||||
| Reduction of (increase in) restricted cash |
(8,904 | ) | 5,586 | |||||
|
|
|
|
|
|||||
| Net cash (used in) provided by investing activities |
(8,993 | ) | 5,440 | |||||
| Cash flows from financing activities: |
||||||||
| Issuance of common stock, net |
46,911 | 23,670 | ||||||
| Repurchase of common stock, net |
— | (4,790 | ) | |||||
| Retirement of employee shares |
(1,947 | ) | (2,004 | ) | ||||
| Distributions paid |
(50,208 | ) | (44,209 | ) | ||||
| Issuance of Convertible Notes |
230,000 | — | ||||||
| Issuance of 2024 Notes Payable |
5,637 | 141,945 | ||||||
| Repayments of 2019 Notes Payable |
(110,364 | ) | — | |||||
| Repayments of 2021 Asset-Backed Notes |
(21,527 | ) | — | |||||
| Borrowings of credit facilities |
8,497 | 170,985 | ||||||
| Repayments of credit facilities |
(13,513 | ) | (220,985 | ) | ||||
| Cash paid for debt issuance costs |
(4,480 | ) | (4,722 | ) | ||||
| Cash paid for redemption of convertible notes |
— | (17,604 | ) | |||||
| Fees paid for credit facilities and debentures |
(253 | ) | (1,307 | ) | ||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
88,753 | 40,979 | ||||||
| Net increase (decrease) in cash and cash equivalents |
147,368 | (35,481 | ) | |||||
|
|
|
|
|
|||||
| Cash and cash equivalents at beginning of period |
13,044 | 95,196 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents at end of period |
$ | 160,412 | $ | 59,715 | ||||
|
|
|
|
|
|||||
| Supplemental non-cash investing and financing activities: |
||||||||
| Distributions reinvested |
1,122 | 997 | ||||||
| (1) | Stock-based compensation includes $35 and $50 of restricted stock and option expense related to director compensation for the six months ended June 30, 2017 and 2016, respectively. |
See notes to consolidated financial statements.
F-89
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Debt Investments |
|
|||||||||||||||||||
| Biotechnology Tools |
|
|||||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Exicure, Inc.(11)(14A) |
Biotechnology Tools | Senior Secured |
September 2019 |
Interest rate PRIME + 6.45% or Floor rate of 9.95% |
$ | 6,000 | $6,046 | $6,124 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
6,046 | 6,124 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Biotechnology Tools (0.75%)* |
|
6,046 | 6,124 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Communications & Networking |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(13)(14B) |
Communications & Networking | Senior Secured |
August 2017 | PIK Interest 10.50% | $ | 819 | 928 | 928 | ||||||||||||
| OpenPeak, Inc.(7) |
Communications & Networking | Senior Secured |
April 2018 |
Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 11,464 | 8,228 | — | ||||||||||||
| SkyCross, Inc.(6)(7)(13)(14B)(15) |
Communications & Networking | Senior Secured |
January 2018 | Interest rate FIXED 10.95%, PIK Interest 5.00% |
$ | 14,916 | 15,058 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
24,214 | 928 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Spring Mobile Solutions, Inc.(12)(14B) |
Communications & Networking | Senior Secured |
January 2019 | Interest rate PRIME + 6.70% or Floor rate of 9.95% |
$ | 2,739 | 2,826 | 2,827 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
2,826 | 2,827 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Communications & Networking (0.46%)* |
|
27,040 | 3,755 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(14A)(15) |
Consumer & Business Products | Senior Secured |
December 2019 | Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 20,000 | 19,988 | 20,146 | ||||||||||||
| Consumer & Business Products | Senior Secured |
December 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.50% |
$ | 1,000 | 1,000 | 1,000 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Antenna79 (p.k.a. Pong Research Corporation) |
$ | 21,000 | 20,988 | 21,146 | ||||||||||||||||
| Second Time Around (Simplify Holdings, LLC)(7)(14A)(15) |
Consumer & Business Products | Senior Secured |
February 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ | 1,886 | 1,920 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
22,908 | 21,146 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Consumer & Business Products (2.59%)* |
|
22,908 | 21,146 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Delivery |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| BioQ Pharma Incorporated(10)(14A)(14B) |
Drug Delivery | Senior Secured |
May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 6,356 | 6,850 | 6,850 | ||||||||||||
| Drug Delivery | Senior Secured |
May 2018 | Interest rate PRIME + 7.00% or Floor rate of 10.25% |
$ | 1,898 | 1,979 | 1,979 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total BioQ Pharma Incorporated |
$ | 8,254 | 8,829 | 8,829 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
8,829 | 8,829 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(9)(10)(14C)(15) |
Drug Delivery | Senior Secured |
March 2020 | Interest rate PRIME + 6.05% or Floor rate of 9.55% |
$ | 20,466 | 21,340 | 21,425 | ||||||||||||
| Agile Therapeutics, Inc.(10)(14A) |
Drug Delivery | Senior Secured |
December 2018 | Interest rate PRIME + 4.75% or Floor rate of 9.00% |
$ | 14,004 | 14,234 | 14,150 | ||||||||||||
| Antares Pharma Inc.(9)(14A)(15) |
Drug Delivery | Senior Secured |
July 2022 | Interest rate PRIME + 4.50% or Floor rate of 9.50% |
$ | 25,000 | 24,862 | 24,862 | ||||||||||||
| Aprecia Pharmaceuticals Company(11)(14A) |
Drug Delivery | Senior Secured |
January 2020 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 15,000 | 15,221 | 15,215 | ||||||||||||
See notes to consolidated financial statements.
F-90
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| Edge Therapeutics, Inc.(11)(14A) |
Drug Delivery | |
Senior Secured |
|
February 2020 | Interest rate PRIME + 4.65% or Floor rate of 9.15% |
$ | 20,000 | $ | 20,131 | $ | 20,226 | ||||||||||
| Pulmatrix Inc.(8)(10)(14A) |
Drug Delivery | |
Senior Secured |
|
July 2018 | Interest rate PRIME + 6.25% or Floor rate of 9.50% |
$ | 4,639 | 4,772 | 4,807 | ||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(10)(14A) |
Drug Delivery | |
Senior Secured |
|
December 2018 | Interest rate PRIME + 2.70% or Floor rate of 7.95% |
$ | 9,277 | 9,495 | 9,465 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
110,055 | 110,150 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Drug Delivery (14.55%)* |
|
118,884 | 118,979 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Drug Discovery & Development |
|
|||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| Cerecor, Inc.(11)(14A) |
Drug Discovery & Development |
|
Senior Secured |
|
August 2017 |
Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 607 | 792 | 792 | ||||||||||||
| Epirus Biopharmaceuticals, Inc.(7)(14A) |
Drug Discovery & Development |
|
Senior Secured |
|
April 2018 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 3,066 | 3,349 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
4,141 | 792 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Auris Medical Holding, AG(4)(9)(14B) |
Drug Discovery & Development |
|
Senior Secured |
|
January 2020 |
Interest rate PRIME + 6.05% or Floor rate of 9.55% |
$ | 12,500 | 12,547 | 12,586 | ||||||||||||
| Aveo Pharmaceuticals, Inc.(9)(12)(14A)(14B) |
Drug Discovery & Development |
|
Senior Secured |
|
December 2019 |
Interest rate PRIME + 6.90% or Floor rate of 11.90% |
$ | 10,000 | 10,339 | 10,377 | ||||||||||||
| Drug Discovery & Development |
|
Senior Secured |
|
December 2019 |
Interest rate PRIME + 6.90% or Floor rate of 11.90% |
$ | 10,000 | 9,842 | 9,858 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Aveo Pharmaceuticals, Inc. |
$ | 20,000 | 20,181 | 20,235 | ||||||||||||||||||
| Axovant Sciences Ltd.(4)(9) |
Drug Discovery & Development |
|
Senior Secured |
|
March 2021 |
Interest rate PRIME + 6.80% or Floor rate of 10.55% |
$ | 55,000 | 53,333 | 53,333 | ||||||||||||
| Bellicum Pharmaceuticals, Inc.(14A)(14B)(15) |
Drug Discovery & Development |
|
Senior Secured |
|
March 2020 |
Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 15,000 | 15,421 | 15,640 | ||||||||||||
| Drug Discovery & Development |
|
Senior Secured |
|
March 2020 |
Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 5,000 | 5,022 | 5,114 | |||||||||||||
| Drug Discovery & Development |
|
Senior Secured |
|
March 2020 |
Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 10,000 | 10,030 | 10,163 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Bellicum Pharmaceuticals, Inc. |
$ | 30,000 | 30,473 | 30,917 | ||||||||||||||||||
| Brickell Biotech, Inc.(11)(14B) |
Drug Discovery & Development |
|
Senior Secured |
|
September 2019 |
Interest rate PRIME + 5.70% or Floor rate of 9.20% |
$ | 7,262 | 7,426 | 7,458 | ||||||||||||
| Concert Pharmaceuticals, Inc.(14A)(15) |
Drug Discovery & Development |
|
Senior Secured |
|
June 2021 | Interest rate PRIME + 4.05% or Floor rate of 8.55% |
$ | 30,000 | 29,540 | 29,540 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(10)(14A) |
Drug Discovery & Development |
|
Senior Secured |
|
December 2018 |
Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 15,639 | 15,469 | 15,589 | ||||||||||||
| CytRx Corporation(10)(14B)(15) |
Drug Discovery & Development |
|
Senior Secured |
|
February 2020 |
Interest rate PRIME + 6.00% or Floor rate of 9.50% |
$ | 22,573 | 23,068 | 23,265 | ||||||||||||
| Genocea Biosciences, Inc.(10)(14A) |
Drug Discovery & Development |
|
Senior Secured |
|
January 2019 |
Interest rate PRIME + 2.25% or Floor rate of 7.25% |
$ | 17,000 | 17,475 | 17,532 | ||||||||||||
| Immune Pharmaceuticals(10)(14B) |
Drug Discovery & Development |
|
Senior Secured |
|
September 2018 |
Interest rate PRIME + 4.75% or Floor rate of 10.00% |
$ | 2,398 | 2,551 | 2,551 | ||||||||||||
| Insmed, Incorporated(10)(14A) |
Drug Discovery & Development |
|
Senior Secured |
|
October 2020 |
Interest rate PRIME + 4.75% or Floor rate of 9.25% |
$ | 55,000 | 55,065 | 55,082 | ||||||||||||
| Metuchen Pharmaceuticals LLC(13)(14A) |
Drug Discovery & Development |
|
Senior Secured |
|
October 2020 |
Interest rate PRIME + 7.25% or Floor rate of 10.75%, PIK Interest 1.35% |
$ | 35,322 | 35,030 | 35,221 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(14A)(15) |
Drug Discovery & Development |
|
Senior Secured |
|
September 2020 |
Interest rate PRIME + 2.75% or Floor rate of 8.50% |
$ | 40,000 | 39,721 | 39,744 | ||||||||||||
| Drug Discovery & Development |
|
Senior Secured |
|
September 2020 |
Interest rate PRIME + 2.75% or Floor rate of 8.50% |
$ | 10,000 | 9,934 | 9,937 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.) |
$ | 50,000 | 49,655 | 49,681 | ||||||||||||||||||
See notes to consolidated financial statements.
F-91
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| PhaseRx,Inc.(14B)(15) |
Drug Discovery & Development | |
Senior Secured |
|
December 2019 |
Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 6,000 | $ | 6,034 | $ | 6,047 | ||||||||||
| Sorrento Therapeutics, Inc.(9)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
December 2020 |
Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 30,000 | 28,879 | 28,736 | ||||||||||||
| Stealth Bio Therapeutics Corp.(4)(9)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
January 2021 |
Interest rate PRIME + 5.50% or Floor rate of 9.50% |
$ | 12,500 | 12,260 | 12,260 | ||||||||||||
| uniQure B.V.(4)(9)(10)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
May 2020 | Interest rate PRIME + 3.00% or Floor rate of 8.25% |
$ | 20,000 | 20,359 | 20,342 | ||||||||||||
| Verastem, Inc.(14A)(17) |
Drug Discovery & Development | |
Senior Secured |
|
December 2020 |
Interest rate PRIME + 6.00% or Floor rate of 10.50% |
$ | 2,500 | 2,465 | 2,465 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
421,810 | 422,840 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Drug Discovery & Development (51.82%)* |
|
425,951 | 423,632 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||||
| 908 DEVICES INC.(14A)(15)(17) |
Electronics & Computer Hardware | |
Senior Secured |
|
September 2020 |
Interest rate PRIME + 4.00% or Floor rate of 8.25% |
$ | 7,500 | 7,470 | 7,470 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
7,470 | 7,470 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.91%)* |
|
7,470 | 7,470 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| PH Group Holdings |
Healthcare Services, Other | |
Senior Secured |
|
September 2020 |
Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 20,000 | 19,841 | 19,955 | ||||||||||||
| Healthcare Services, Other | |
Senior Secured |
|
September 2020 |
Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 10,000 | 9,899 | 9,899 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total PH Group Holdings |
$ | 30,000 | 29,740 | 29,854 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
29,740 | 29,854 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Healthcare Services, Other (3.65%)* |
|
29,740 | 29,854 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Information Services |
|
|||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| MDX Medical, Inc.(13)(15)(17) |
Information Services | |
Senior Secured |
|
December 2020 |
Interest rate PRIME + 4.00% or Floor rate of 8.25%, PIK Interest 1.70% |
$ | 7,502 | 7,264 | 7,264 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
7,264 | 7,264 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Information Services (0.89%)* |
|
7,264 | 7,264 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Aria Systems, Inc.(10)(13) |
Internet Consumer & Business Services | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 3.20% or Floor rate of 6.95%, PIK Interest 1.95% |
$ | 2,082 | 2,071 | 2,068 | ||||||||||||
| Internet Consumer & Business Services | |
Senior Secured |
|
June 2019 | Interest rate PRIME + 5.20% or Floor rate of 8.95%, PIK Interest 1.95% |
$ | 18,646 | 18,539 | 18,533 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Aria Systems, Inc. |
$ | 20,728 | 20,610 | 20,601 | ||||||||||||||||||
See notes to consolidated financial statements.
F-92
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Intent Media, Inc.(13)(14A)(15) |
Internet Consumer & Business Services |
Senior Secured |
May 2019 | Interest rate PRIME + 5.25% or Floor rate of 8.75%, PIK Interest 1.00% |
$ | 5,025 | $ | 4,929 | $ | 4,957 | ||||||||||
| Internet Consumer & Business Services |
Senior Secured |
May 2019 | Interest rate PRIME + 5.50% or Floor rate of 9.00%, PIK Interest 2.35% |
$ | 2,000 | 1,938 | 1,940 | |||||||||||||
| Internet Consumer & Business Services | Senior Secured |
May 2019 | Interest rate PRIME + 5.50% or Floor rate of 9.00%, PIK Interest 2.50% |
$ | 2,000 | 1,938 | 1,940 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Intent Media, Inc. |
$ | 9,025 | 8,805 | 8,837 | ||||||||||||||||
| LogicSource(14B)(15) |
Internet Consumer & Business Services |
Senior Secured |
October 2019 |
Interest rate PRIME + 6.25% or Floor rate of 9.75% |
$ | 8,001 | 8,147 | 8,241 | ||||||||||||
| Snagajob.com, Inc.(12)(13)(14A) |
Internet Consumer & Business Services | Senior Secured |
July 2020 | Interest rate PRIME + 5.15% or Floor rate of 9.15%, PIK Interest 1.95% |
$ | 35,642 | 35,125 | 35,788 | ||||||||||||
| Tectura Corporation(6)(7)(8)(13) |
Internet Consumer & Business Services | Senior Secured |
June 2021 | Interest rate FIXED 6.00%, PIK Interest 3.00% |
$ | 19,991 | 19,991 | 19,991 | ||||||||||||
| Internet Consumer & Business Services | Senior Secured |
June 2021 | PIK Interest 8.00% | $ | 11,015 | 240 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Tectura Corporation |
$ | 31,006 | 20,231 | 19,991 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
92,918 | 93,458 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (11.43%)* |
|
92,918 | 93,458 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Media/Content/Info |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Machine Zone, Inc.(13)(16) |
Media/Content/Info | Senior Secured |
May 2018 | Interest rate PRIME + 2.50% or Floor rate of 6.75%, PIK Interest 3.00% |
$ | 105,369 | 104,512 | 104,512 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
104,512 | 104,512 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| FanDuel, Inc.(14B) |
Media/Content/Info | Senior Secured |
November 2019 |
Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ | 20,000 | 19,871 | 19,851 | ||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9)(11)(14B) |
Media/Content/Info | Senior Secured |
April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ | 5,000 | 5,080 | 5,177 | ||||||||||||
| Media/Content/Info | Senior Secured |
April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ | 5,000 | 4,997 | 5,077 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total WP Technology, Inc. (Wattpad, Inc.) |
$ | 10,000 | 10,077 | 10,254 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
29,948 | 30,105 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (16.47%)* |
|
134,460 | 134,617 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Amedica Corporation(8)(14B)(15) |
Medical Devices & Equipment | Senior Secured |
January 2018 |
Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 4,098 | 5,678 | 5,678 | ||||||||||||
| Gamma Medica, Inc.(7)(10)(14B) |
Medical Devices & Equipment | Senior Secured |
January 2018 |
Interest rate PRIME + 6.50% or Floor rate of 9.75% |
$ | 161 | 366 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
6,044 | 5,678 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-93
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Aspire Bariatrics, Inc.(14B)(15) |
Medical Devices & Equipment | Senior Secured |
October 2018 |
Interest rate PRIME + 4.00% or Floor rate of 9.25% |
$ | 3,943 | $ | 4,173 | $ | 4,126 | ||||||||||
| IntegenX, Inc.(14B)(15) |
Medical Devices & Equipment | Senior Secured |
June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 15,000 | 15,370 | 15,362 | ||||||||||||
| Medical Devices & Equipment | Senior Secured |
June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 2,500 | 2,528 | 2,528 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total IntegenX, Inc. |
$ | 17,500 | 17,898 | 17,890 | ||||||||||||||||
| Micell Technologies, Inc.(11)(14B) |
Medical Devices & Equipment | Senior Secured |
August 2019 |
Interest rate PRIME + 7.25% or Floor rate of 10.50% |
$ | 6,909 | 7,006 | 7,070 | ||||||||||||
| Quanta Fluid Solutions(4)(9)(10)(14B) |
Medical Devices & Equipment | Senior Secured |
April 2020 | Interest rate PRIME + 8.05% or Floor rate of 11.55% |
$ | 11,625 | 11,811 | 11,847 | ||||||||||||
| Quanterix Corporation(10)(14A) |
Medical Devices & Equipment | Senior Secured |
March 2019 | Interest rate PRIME + 2.75% or Floor rate of 8.00% |
$ | 9,043 | 9,427 | 9,424 | ||||||||||||
| Sebacia (14B)(15) |
Medical Devices & Equipment | Senior Secured |
July 2020 | Interest rate PRIME + 4.35% or Floor rate of 8.85% |
$ | 8,000 | 7,805 | 7,805 | ||||||||||||
| Tela Bio, Inc.(14A)(15) |
Medical Devices & Equipment | Senior Secured |
September 2020 |
Interest rate PRIME + 4.95% or Floor rate of 9.45% |
$ | 5,000 | 4,945 | 4,945 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
63,065 | 63,107 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Medical Devices & Equipment (8.41%)* |
|
69,109 | 68,785 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Semiconductors |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation (15) |
Semiconductors | Senior Secured |
November 2017 |
Interest rate PRIME + 7.00% or Floor rate of 10.50% |
$ | 4,025 | 4,025 | 4,025 | ||||||||||||
| Aquantia Corp.(17) |
Semiconductors | Senior Secured |
February 2018 |
Interest rate PRIME + 3.95% or Floor rate of 7.20% |
$ | 5,000 | 5,000 | 5,000 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
9,025 | 9,025 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(14B)(15) |
Semiconductors | Senior Secured |
July 2018 | Interest rate PRIME + 8.25% or Floor rate of 11.50% |
$ | 2,356 | 2,623 | 2,607 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
2,623 | 2,607 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Semiconductors (1.42%)* |
|
11,648 | 11,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Software |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Clickfox, Inc.(12)(14C) |
Software | Senior Secured |
May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.50% |
$ | 9,672 | 10,437 | 10,437 | ||||||||||||
| Cloud Technology Partners, Inc. |
Software | Senior Secured |
June 2018 | Interest rate PRIME + 3.05% or Floor rate of 7.05% |
$ | 3,400 | 3,400 | 3,400 | ||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(7)(13)(14A)(14C)(15)(18) |
Software | Senior Secured |
March 2018 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 13,000 | 12,747 | 3,220 | ||||||||||||
| Software | Senior Secured |
February 2017 |
Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 1,566 | 1,698 | 429 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.) |
$ | 14,566 | 14,445 | 3,649 | ||||||||||||||||
| RedSeal Inc.(14A)(15)(17) |
Software | Senior Secured |
August 2017 |
Interest rate PRIME + 3.25% or Floor rate of 6.50% |
$ | 1,205 | 1,205 | 1,205 | ||||||||||||
| Software | Senior Secured |
June 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 3,431 | 3,581 | 3,581 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total RedSeal Inc. |
$ | 4,636 | 4,786 | 4,786 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
33,068 | 22,272 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-94
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Clarabridge, Inc.(13) |
Software | Senior Secured |
April 2021 | Interest rate PRIME + 4.80% or Floor rate of 8.55%, PIK Interest 3.25% |
$ | 40,224 | $40,196 | $40,196 | ||||||||||||
| Cloud Technology Partners, Inc.(14A) |
Software | Senior Secured |
December 2019 |
Interest rate PRIME + 5.75% or Floor rate of 9.75% |
$ | 10,000 | 9,982 | 9,914 | ||||||||||||
| Evernote Corporation(13)(15)(17) |
Software | Senior Secured |
October 2020 |
Interest rate PRIME + 5.45% or Floor rate of 8.95% |
$ | 6,000 | 5,967 | 6,134 | ||||||||||||
| Software | Senior Secured |
July 2021 | Interest rate PRIME + 6.00% or Floor rate of 9.50%, PIK Interest 1.25% |
$ | 4,000 | 3,972 | 3,972 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Evernote Corporation |
$ | 10,000 | 9,939 | 10,106 | ||||||||||||||||
| Fuze, Inc.(13)(14A)(15) |
Software | Senior Secured |
July 2021 | Interest rate PRIME + 3.70% or Floor rate of 7.95%, PIK Interest 1.55% |
$ | 50,000 | 49,901 | 49,901 | ||||||||||||
| Impact Radius Holdings, Inc.(13)(14A) |
Software | Senior Secured |
December 2020 |
Interest rate PRIME + 4.25% or Floor rate of 8.75%, PIK Interest 1.55% |
$ | 5,000 | 4,990 | 4,990 | ||||||||||||
| Lithium Technologies, Inc.(13)(14A)(15)(19) |
Software | Senior Secured |
June 2020 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 1.80% |
$ | 25,247 | 25,351 | 25,351 | ||||||||||||
| OneLogin, Inc.(13)(15) |
Software | Senior Secured |
August 2019 |
Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 3.25% |
$ | 15,623 | 15,526 | 15,838 | ||||||||||||
| Quid, Inc.(13)(14A)(15) |
Software | Senior Secured |
October 2019 |
Interest rate PRIME + 4.75% or Floor rate of 8.25%, PIK Interest 2.25% |
$ | 8,208 | 8,278 | 8,399 | ||||||||||||
| RedSeal Inc.(14A)(15)(17) |
Software | Senior Secured |
January 2020 |
Interest rate PRIME + 7.75% or Floor rate of 11.25% |
$ | 5,000 | 4,952 | 4,952 | ||||||||||||
| Signpost, Inc.(13)(14A)(15) |
Software | Senior Secured |
February 2020 |
Interest rate PRIME + 4.15% or Floor rate of 8.15%, PIK Interest 1.75% |
$ | 15,373 | 15,306 | 15,447 | ||||||||||||
| Vela Trading Technologies (17) |
Software | Senior Secured |
July 2022 | Interest rate LIBOR + 9.50% or Floor rate of 10.50% |
$ | 15,200 | 14,782 | 14,782 | ||||||||||||
| Wrike, Inc.(13)(14A)(17) |
Software | Senior Secured |
February 2021 |
Interest rate PRIME + 6.00% or Floor rate of 9.50%, PIK Interest 2.00% |
$ | 10,062 | 9,790 | 9,790 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
208,993 | 209,666 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (28.37%)* |
|
242,061 | 231,938 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Alimera Sciences, Inc.(10)(13)(14A) |
Specialty Pharmaceuticals | Senior Secured |
November 2020 |
Interest rate PRIME + 7.50% or Floor rate of 11.00%, PIK Interest 1.00% |
$ | 35,218 | 35,049 | 35,398 | ||||||||||||
| Jaguar Animal Health, Inc.(10)(14B) |
Specialty Pharmaceuticals | Senior Secured |
August 2018 |
Interest rate PRIME + 5.65% or Floor rate of 9.90% |
$ | 2,520 | 2,876 | 2,821 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
37,925 | 38,219 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (4.68%)* |
|
37,925 | 38,219 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Surgical Devices |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Transmedics, Inc.(12)(14B) |
Surgical Devices | Senior Secured |
February 2020 |
Interest rate PRIME + 5.30% or Floor rate of 9.55% |
$ | 8,500 | 8,621 | 8,632 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
8,621 | 8,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (1.06%)* |
|
8,621 | 8,632 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-95
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity Date |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| FuelCell Energy, Inc.(11)(14B) |
Sustainable and Renewable Technology | Senior Secured |
October 2018 |
Interest rate PRIME + 5.50% or Floor rate of 9.50% |
$ | 20,000 | $ | 20,925 | $ | 21,034 | ||||||||||
| Proterra, Inc.(10)(14A)(14B) |
Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate PRIME + 6.95% or Floor rate of 10.20% |
$ | 5,000 | 5,109 | 5,137 | ||||||||||||
| Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate PRIME + 6.95% or Floor rate of 10.20% |
$ | 25,000 | 25,872 | 25,814 | |||||||||||||
| Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 10,000 | 10,089 | 10,115 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Proterra, Inc. |
$ | 40,000 | 41,070 | 41,066 | ||||||||||||||||
| Rive Technology, Inc.(14A)(15) |
Sustainable and Renewable Technology | Senior Secured |
January 2019 |
Interest rate PRIME + 6.20% or Floor rate of 9.45% |
$ | 6,061 | 6,234 | 6,283 | ||||||||||||
| Tendril Networks(11)(14B) |
Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate FIXED 9.25% | $ | 13,156 | 13,765 | 13,735 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
81,994 | 82,118 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (10.05%)* |
|
81,994 | 82,118 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total: Debt Investments (157.52%)* |
|
1,324,039 | 1,287,623 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-96
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Equity Investments |
||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||
| NuGEN Technologies, Inc.(15) |
Biotechnology Tools | Equity | Common Stock | 55,780 | $ | 500 | $ | — | ||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Biotechnology Tools (0.00%)* |
|
500 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Communications & Networking |
|
|||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(15) |
Communications & Networking | Equity | Common Stock | 100 | 4,000 | 1,188 | ||||||||||||
| GlowPoint, Inc.(3) |
Communications & Networking | Equity | Common Stock | 114,192 | 101 | 32 | ||||||||||||
| Peerless Network Holdings, Inc. |
Communications & Networking | Equity | Preferred Series A | 1,000,000 | 1,000 | 4,585 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Communications & Networking (0.71%)* |
|
5,101 | 5,805 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Consumer & Business Products |
||||||||||||||||||
| Market Force Information, Inc. |
Consumer & Business Products | Equity | Common Stock | 480,261 | — | 433 | ||||||||||||
| Consumer & Business Products | Equity | Preferred Series B-1 | 187,970 | 500 | 280 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Market Force Information, Inc. |
668,231 | 500 | 713 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Consumer & Business Products (0.09%)* |
|
500 | 713 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Diagnostic |
||||||||||||||||||
| Singulex, Inc. |
Diagnostic | Equity | Common Stock | 937,998 | 750 | 655 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Diagnostic (0.08%)* |
|
750 | 655 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Delivery |
||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9) |
Drug Delivery | Equity | Common Stock | 54,240 | 108 | 117 | ||||||||||||
| BioQ Pharma Incorporated(15) |
Drug Delivery | Equity | Preferred Series D | 165,000 | 500 | 599 | ||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 53,165 | 329 | 545 | ||||||||||||
| Merrion Pharmaceuticals, Plc(4)(9) |
Drug Delivery | Equity | Common Stock | 20,000 | 9 | — | ||||||||||||
| Neos Therapeutics, Inc.(3)(15) |
Drug Delivery | Equity | Common Stock | 125,000 | 1,500 | 913 | ||||||||||||
| Revance Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 22,765 | 557 | 601 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Delivery (0.34%)* |
|
3,003 | 2,775 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9)(15) |
Drug Discovery & Development | Equity | Common Stock | 426,931 | 1,060 | 950 | ||||||||||||
| Cerecor, Inc.(3) |
Drug Discovery & Development | Equity | Common Stock | 119,087 | 1,000 | 68 | ||||||||||||
| Cerulean Pharma, Inc.(3) |
Drug Discovery & Development | Equity | Common Stock | 135,501 | 1,000 | 60 | ||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Equity | Common Stock | 142,858 | 1,000 | 453 | ||||||||||||
| Dynavax Technologies(3)(9) |
Drug Discovery & Development | Equity | Common Stock | 20,000 | 550 | 193 | ||||||||||||
| Epirus Biopharmaceuticals, Inc. |
Drug Discovery & Development | Equity | Common Stock | 200,000 | 1,000 | — | ||||||||||||
| Genocea Biosciences, Inc.(3) |
Drug Discovery & Development | Equity | Common Stock | 223,463 | 2,000 | 1,166 | ||||||||||||
| Inotek Pharmaceuticals Corporation(3) |
Drug Discovery & Development | Equity | Common Stock | 3,778 | 1,500 | 7 | ||||||||||||
| Insmed, Incorporated(3) |
Drug Discovery & Development | Equity | Common Stock | 70,771 | 1,000 | 1,214 | ||||||||||||
| Melinta Therapeutics |
Drug Discovery & Development | Equity | Preferred Series 4 | 1,914,448 | 2,000 | 2,598 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3) |
Drug Discovery & Development | Equity | Common Stock | 76,362 | 2,743 | 1,840 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Discovery & Development (1.05%)* |
|
14,853 | 8,549 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-97
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| Identiv, Inc.(3) |
Electronics & Computer Hardware | Equity | Common Stock | 6,700 | $ | 34 | $ | 35 | ||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.00%)* |
|
34 | 35 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Information Services |
||||||||||||||||||
| DocuSign, Inc. |
Information Services | Equity | Common Stock | 385,000 | 6,081 | 7,201 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Information Services (0.88%)* |
|
6,081 | 7,201 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||
| Blurb, Inc.(15) |
Internet Consumer & Business Services | Equity | Preferred Series B | 220,653 | 175 | 170 | ||||||||||||
| Brigade Group, Inc. (p.k.a. Philotic, Inc.) |
Internet Consumer & Business Services | Equity | Common Stock | 9,023 | 93 | — | ||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services | Equity | Preferred Series C | 230,030 | 250 | 245 | ||||||||||||
| Internet Consumer & Business Services | Equity | Preferred Series D | 198,677 | 250 | 241 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Lightspeed POS, Inc. |
428,707 | 500 | 486 | |||||||||||||||
| OfferUp, Inc. |
Internet Consumer & Business Services | Equity | Preferred Series A | 286,080 | 1,663 | 1,917 | ||||||||||||
| Internet Consumer & Business Services | Equity | Preferred Series A-1 | 108,710 | 632 | 728 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total OfferUp, Inc. |
394,790 | 2,295 | 2,645 | |||||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services | Equity | Preferred Series G | 218,351 | 250 | 430 | ||||||||||||
| Internet Consumer & Business Services | Equity | Preferred Series H | 87,802 | 250 | 254 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Oportun (p.k.a. Progress Financial) |
306,153 | 500 | 684 | |||||||||||||||
| RazorGator Interactive Group, Inc. |
Internet Consumer & Business Services | Equity | Preferred Series AA | 34,783 | 15 | 46 | ||||||||||||
| Tectura Corporation(6) |
Internet Consumer & Business Services | Equity | Preferred Series BB | 1,000,000 | — | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.49%)* |
|
3,578 | 4,031 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Media/Content/Info |
||||||||||||||||||
| Pinterest, Inc. |
Media/Content/Info | Equity | Preferred Series Seed | 620,000 | 4,085 | 4,452 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Media/Content/Info (0.54%)* |
|
4,085 | 4,452 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||
| AtriCure, Inc.(3)(15) |
Medical Devices & Equipment | Equity | Common Stock | 7,536 | 266 | 168 | ||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment | Equity | Preferred Series AA | 221,893 | 1,500 | — | ||||||||||||
| Gelesis, Inc.(15) |
Medical Devices & Equipment | Equity | Common Stock | 198,202 | — | 888 | ||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series A-1 | 191,210 | 425 | 954 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series A-2 | 191,626 | 500 | 905 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gelesis, Inc. |
581,038 | 925 | 2,747 | |||||||||||||||
See notes to consolidated financial statements.
F-98
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| HercGamma, Inc.(6) |
Medical Devices & Equipment | Equity | Common Stock | 100 | $ | 1,169 | $ | 1,169 | ||||||||||
| Medrobotics Corporation(15) |
Medical Devices & Equipment | Equity | Preferred Series E | 136,798 | 250 | 236 | ||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series F | 73,971 | 155 | 185 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series G | 163,934 | 500 | 486 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Medrobotics Corporation |
374,703 | 905 | 907 | |||||||||||||||
| Optiscan Biomedical, Corp.(5)(15) |
Medical Devices & Equipment | Equity | Preferred Series B | 6,185,567 | 3,000 | 383 | ||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series C | 1,927,309 | 655 | 110 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series D | 55,103,923 | 5,257 | 3,826 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series E | 15,638,888 | 1,308 | 1,492 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Optiscan Biomedical, Corp. |
78,855,687 | 10,220 | 5,811 | |||||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment | Equity | Preferred Series B | 232,061 | 527 | 566 | ||||||||||||
| Quanterix Corporation |
Medical Devices & Equipment | Equity | Preferred Series D | 272,479 | 1,000 | 1,111 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (1.53%)* |
|
16,512 | 12,479 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| CapLinked, Inc. |
Software | Equity | Preferred Series A-3 | 53,614 | 51 | 96 | ||||||||||||
| Druva, Inc. |
Software | Equity | Preferred Series 2 | 458,841 | 1,000 | 1,584 | ||||||||||||
| ForeScout Technologies, Inc. |
Software | Equity | Preferred Series D | 319,099 | 398 | 1,937 | ||||||||||||
| Software | Equity | Preferred Series E | 80,587 | 131 | 493 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total ForeScout Technologies, Inc. |
399,686 | 529 | 2,430 | |||||||||||||||
| HighRoads, Inc. |
Software | Equity | Common Stock | 190 | 307 | — | ||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Equity | Preferred Series E | 669,173 | 963 | 1,343 | ||||||||||||
| Palantir Technologies |
Software | Equity | Preferred Series E | 727,696 | 5,431 | 5,774 | ||||||||||||
| Sprinklr, Inc. |
Software | Equity | Common Stock | 700,000 | 3,749 | 3,749 | ||||||||||||
| WildTangent, Inc.(15) |
Software | Equity | Preferred Series 3 | 100,000 | 402 | 175 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (1.85%)* |
|
12,432 | 15,151 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
||||||||||||||||||
| Gynesonics, Inc.(15) |
Surgical Devices | Equity | Preferred Series B | 219,298 | 250 | 41 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 656,538 | 282 | 57 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 1,991,157 | 712 | 772 | |||||||||||||
| Surgical Devices | Equity | Preferred Series E | 2,786,367 | 429 | 507 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
5,653,360 | 1,673 | 1,377 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Equity | Preferred Series B | 88,961 | 1,100 | 507 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 119,999 | 300 | 388 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 260,000 | 650 | 1,243 | |||||||||||||
| Surgical Devices | Equity | Preferred Series F | 100,200 | 500 | 600 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
569,160 | 2,550 | 2,738 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.50%)* |
|
4,223 | 4,115 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
Sustainable and Renewable Technology | Equity | Common Stock | 19,250 | 761 | — | ||||||||||||
| Glori Energy, Inc.(3) |
Sustainable and Renewable Technology | Equity | Common Stock | 18,208 | 165 | — | ||||||||||||
See notes to consolidated financial statements.
F-99
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Modumetal, Inc. |
Sustainable and Renewable Technology | Equity | Preferred Series C | 3,107,520 | $ | 500 | $ | 551 | ||||||||||
| Proterra, Inc. |
Sustainable and Renewable Technology | Equity | Preferred Series 5 | 99,280 | 500 | 516 | ||||||||||||
| Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.)(6) |
Sustainable and Renewable Technology | Equity | Common Stock | 333 | 61,502 | 8,288 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (1.14%)* |
|
63,428 | 9,355 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Equity Investments (9.21%)* |
|
135,080 | 75,316 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Warrant Investments |
||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||
| Exicure, Inc. |
Biotechnology Tools | Warrant | Preferred Series C | 104,348 | 107 | 202 | ||||||||||||
| Labcyte, Inc.(15) |
Biotechnology Tools | Warrant | Preferred Series C | 1,127,624 | 323 | 397 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
430 | 599 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Communications & Networking |
||||||||||||||||||
| PeerApp, Inc. |
Communications & Networking | Warrant | Preferred Series B | 298,779 | 61 | 27 | ||||||||||||
| Peerless Network Holdings, Inc. |
Communications & Networking | Warrant | Preferred Series A | 135,000 | 95 | 345 | ||||||||||||
| Spring Mobile Solutions, Inc. |
Communications & Networking | Warrant | Common Stock | 2,834,375 | 418 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Communications & Networking (0.05%)* |
|
574 | 372 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Consumer & Business Products |
||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(15) |
Consumer & Business Products | Warrant | Common Stock | 1,662,441 | 228 | — | ||||||||||||
| Intelligent Beauty, Inc.(15) |
Consumer & Business Products | Warrant | Preferred Series B | 190,234 | 230 | 288 | ||||||||||||
| The Neat Company(15) |
Consumer & Business Products | Warrant | Preferred Series C-1 | 540,540 | 365 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Consumer & Business Products (0.04%)* |
|
823 | 288 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Delivery |
||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9)(15) |
Drug Delivery | Warrant | Common Stock | 176,730 | 785 | 36 | ||||||||||||
| Agile Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 180,274 | 730 | 136 | ||||||||||||
| Aprecia Pharmaceuticals Company |
Drug Delivery | Warrant | Preferred Series A-1 | 735,981 | 366 | 31 | ||||||||||||
| BIND Therapeutics, Inc.(15) |
Drug Delivery | Warrant | Common Stock | 152,586 | 488 | — | ||||||||||||
| BioQ Pharma Incorporated |
Drug Delivery | Warrant | Common Stock | 459,183 | 1 | 379 | ||||||||||||
| Celsion Corporation(3) |
Drug Delivery | Warrant | Common Stock | 13,927 | 428 | — | ||||||||||||
| Dance Biopharm, Inc.(15) |
Drug Delivery | Warrant | Common Stock | 110,882 | 74 | — | ||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 78,595 | 390 | 266 | ||||||||||||
| Kaleo, Inc. (p.k.a. Intelliject, Inc.) |
Drug Delivery | Warrant | Preferred Series B | 82,500 | 594 | 306 | ||||||||||||
| Neos Therapeutics, Inc.(3)(15) |
Drug Delivery | Warrant | Common Stock | 70,833 | 285 | 25 | ||||||||||||
| Pulmatrix Inc.(3) |
Drug Delivery | Warrant | Common Stock | 25,150 | 116 | 14 | ||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(3) |
Drug Delivery | Warrant | Common Stock | 72,379 | 266 | 5 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Delivery (0.15%)* |
|
4,523 | 1,198 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||
| ADMA Biologics, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 89,750 | 295 | 15 | ||||||||||||
| Anthera Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 5,022 | 984 | — | ||||||||||||
| Audentes Therapeutics, Inc(3)(9)(15) |
Drug Discovery & Development | Warrant | Common Stock | 9,914 | 62 | 82 | ||||||||||||
| Auris Medical Holding, AG(3)(4)(9) |
Drug Discovery & Development | Warrant | Common Stock | 156,726 | 249 | 21 | ||||||||||||
See notes to consolidated financial statements.
F-100
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9) |
Drug Discovery & Development | Warrant | Common Stock | 2,069,880 | $ | 396 | $ | 2,089 | ||||||||||
| Axovant Sciences Ltd.(3)(4)(9) |
Drug Discovery & Development | Warrant | Common Stock | 274,086 | 1,269 | 3,237 | ||||||||||||
| Brickell Biotech, Inc. |
Drug Discovery & Development | Warrant | Preferred Series C | 26,086 | 119 | 124 | ||||||||||||
| Cerecor, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 22,328 | 70 | — | ||||||||||||
| Cerulean Pharma, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 171,901 | 369 | 17 | ||||||||||||
| Chroma Therapeutics, Ltd.(4)(9) |
Drug Discovery & Development | Warrant | Preferred Series D | 325,261 | 490 | — | ||||||||||||
| Cleveland BioLabs, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 7,813 | 105 | 1 | ||||||||||||
| Concert Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 132,069 | 545 | 369 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(3) |
Drug Discovery & Development | Warrant | Common Stock | 29,239 | 165 | 4 | ||||||||||||
| CytRx Corporation(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 634,146 | 416 | 150 | ||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 200 | 28 | — | ||||||||||||
| Epirus Biopharmaceuticals, Inc. |
Drug Discovery & Development | Warrant | Common Stock | 64,194 | 276 | — | ||||||||||||
| Fortress Biotech, Inc. (p.k.a. Coronado Biosciences, Inc.)(3) |
Drug Discovery & Development | Warrant | Common Stock | 73,009 | 142 | 54 | ||||||||||||
| Genocea Biosciences, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 73,725 | 266 | 86 | ||||||||||||
| Immune Pharmaceuticals(3) |
Drug Discovery & Development | Warrant | Common Stock | 10,742 | 164 | — | ||||||||||||
| Melinta Therapeutics |
Drug Discovery & Development | Warrant | Preferred Series 3 | 1,382,323 | 626 | 564 | ||||||||||||
| Nanotherapeutics, Inc.(15) |
Drug Discovery & Development | Warrant | Common Stock | 171,389 | 838 | 245 | ||||||||||||
| Neothetics, Inc. (p.k.a. Lithera, Inc)(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 46,838 | 266 | 11 | ||||||||||||
| Neuralstem, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 5,783 | 77 | 2 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 75,214 | 178 | 477 | ||||||||||||
| PhaseRx,Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 63,000 | 125 | 4 | ||||||||||||
| Savara Inc. (p.k.a. Mast Therapeutics, Inc.) |
Drug Discovery & Development | Warrant | Common Stock | 32,467 | 203 | 50 | ||||||||||||
| Sorrento Therapeutics, Inc. |
Drug Discovery & Development | Warrant | Common Stock | 306,748 | 890 | 180 | ||||||||||||
| Stealth Bio Therapeutics Corp. |
Drug Discovery & Development | Warrant | Preferred Series A | 487,500 | 116 | 116 | ||||||||||||
| uniQure B.V. |
Drug Discovery & Development | Warrant | Common Stock | 37,174 | 218 | 10 | ||||||||||||
| XOMA Corporation |
Drug Discovery & Development | Warrant | Common Stock | 9,063 | 279 | 10 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Discovery & Development (0.97%)* |
|
10,226 | 7,918 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| 908 DEVICES INC.(15) |
Electronics & Computer Hardware | Warrant | Preferred Series D | 79,856 | 100 | 114 | ||||||||||||
| Clustrix, Inc. |
Electronics & Computer Hardware | Warrant | Common Stock | 50,000 | 12 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.01%)* |
|
112 | 114 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-101
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Healthcare Services, Other |
||||||||||||||||||
| Chromadex Corporation(3)(15) |
Healthcare Services, Other | Warrant | Common Stock | 139,673 | $ | 157 | $ | 155 | ||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Healthcare Services, Other (0.02%)* |
|
157 | 155 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Information Services |
||||||||||||||||||
| INMOBI Inc.(4)(9) |
Information Services | Warrant | Common Stock | 46,874 | 82 | — | ||||||||||||
| InXpo, Inc.(15) |
Information Services | Warrant | Preferred Series C | 648,400 | 98 | 15 | ||||||||||||
| Information Services | Warrant | Preferred Series C-1 | 1,165,183 | 74 | 27 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total InXpo, Inc. |
1,813,583 | 172 | 42 | |||||||||||||||
| MDX Medical, Inc.(15) |
Information Services | Warrant | Common Stock | 2,250,000 | 246 | 215 | ||||||||||||
| RichRelevance, Inc. |
Information Services | Warrant | Preferred Series E | 112,612 | 98 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Information Services (0.03%)* |
|
598 | 257 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||
| Aria Systems, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series E | 239,692 | 73 | — | ||||||||||||
| Blurb, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 234,280 | 636 | 61 | ||||||||||||
| CashStar, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C-2 | 727,272 | 130 | 30 | ||||||||||||
| ClearObject, Inc. (p.k.a. CloudOne, Inc.) |
Internet Consumer & Business Services | Warrant | Preferred Series E | 968,992 | 19 | 112 | ||||||||||||
| Intent Media, Inc.(15) |
Internet Consumer & Business Services | Warrant | Common Stock | 140,077 | 168 | 148 | ||||||||||||
| Just Fabulous, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series B | 206,184 | 1,102 | 1,850 | ||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 245,610 | 20 | 25 | ||||||||||||
| LogicSource(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 79,625 | 30 | 32 | ||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services | Warrant | Preferred Series G | 174,562 | 78 | 175 | ||||||||||||
| ShareThis, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 493,502 | 547 | — | ||||||||||||
| Snagajob.com, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series A | 1,575,000 | 640 | 782 | ||||||||||||
| Tapjoy, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series D | 748,670 | 316 | 1 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.39%)* |
|
3,759 | 3,216 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Media/Content/Info |
||||||||||||||||||
| FanDuel, Inc. |
Media/Content/Info | Warrant | Preferred Series E-1 | 4,648 | 730 | 851 | ||||||||||||
| Machine Zone, Inc.(16) |
Media/Content/Info | Warrant | Common Stock | 1,552,710 | 1,958 | 4,484 | ||||||||||||
| Rhapsody International, Inc.(15) |
Media/Content/Info | Warrant | Common Stock | 715,755 | 385 | 17 | ||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9) |
Media/Content/Info | Warrant | Common Stock | 255,818 | 4 | 8 | ||||||||||||
| Zoom Media Group, Inc. |
Media/Content/Info | Warrant | Preferred Series A | 1,204 | 348 | 21 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Media/Content/Info (0.66%)* |
|
3,425 | 5,381 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||
| Amedica Corporation(3)(15) |
Medical Devices & Equipment | Warrant | Common Stock | 103,225 | 459 | 5 | ||||||||||||
| Aspire Bariatrics, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series B-1 | 112,858 | 455 | 303 | ||||||||||||
| Avedro, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series AA | 300,000 | 401 | 316 | ||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment | Warrant | Preferred Series AA | 155,325 | 362 | — | ||||||||||||
| Gelesis, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series A-1 | 74,784 | 78 | 215 | ||||||||||||
See notes to consolidated financial statements.
F-102
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| InspireMD, Inc.(3)(4)(9) |
Medical Devices & Equipment | Warrant | Common Stock | 39,364 | $ | 242 | $ | 1 | ||||||||||
| IntegenX, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series C | 547,752 | 15 | 33 | ||||||||||||
| Medrobotics Corporation(15) |
Medical Devices & Equipment | Warrant | Preferred Series E | 455,539 | 370 | 360 | ||||||||||||
| Micell Technologies, Inc. |
Medical Devices & Equipment | Warrant | Preferred Series D-2 | 84,955 | 262 | 277 | ||||||||||||
| NetBio, Inc. |
Medical Devices & Equipment | Warrant | Preferred Series A | 7,841 | 408 | 123 | ||||||||||||
| NinePoint Medical, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series A-1 | 587,840 | 170 | 73 | ||||||||||||
| Optiscan Biomedical, Corp.(5)(15) |
Medical Devices & Equipment | Warrant | Preferred Series D | 10,535,275 | 1,252 | 180 | ||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment | Warrant | Preferred Series A | 500,000 | 402 | 410 | ||||||||||||
| Quanterix Corporation |
Medical Devices & Equipment | Warrant | Preferred Series C | 173,428 | 180 | 82 | ||||||||||||
| Medical Devices & Equipment | Warrant | Preferred Series D | 38,828 | 25 | 16 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Quanterix Corporation |
212,256 | 205 | 98 | |||||||||||||||
| Sebacia (15) |
Medical Devices & Equipment | Warrant | Preferred Series D | 778,301 | 133 | 133 | ||||||||||||
| SonaCare Medical, LLC (p.k.a. US HIFU, LLC) |
Medical Devices & Equipment | Warrant | Preferred Series A | 6,464 | 188 | — | ||||||||||||
| Strata Skin Sciences, Inc. (p.k.a. MELA Sciences, Inc.)(3) |
Medical Devices & Equipment | Warrant | Common Stock | 13,864 | 402 | — | ||||||||||||
| Tela Bio, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series B | 129,310 | 20 | 12 | ||||||||||||
| ViewRay, Inc.(3)(15) |
Medical Devices & Equipment | Warrant | Common Stock | 128,231 | 333 | 130 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (0.33%)* |
|
6,157 | 2,669 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Semiconductors |
||||||||||||||||||
| Achronix Semiconductor Corporation(15) |
Semiconductors | Warrant | Preferred Series C | 360,000 | 160 | 15 | ||||||||||||
| Semiconductors | Warrant | Preferred Series D-2 | 750,000 | 99 | 307 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Achronix Semiconductor Corporation |
1,110,000 | 259 | 322 | |||||||||||||||
| Aquantia Corp. |
Semiconductors | Warrant | Preferred Series G | 196,831 | 4 | 168 | ||||||||||||
| Avnera Corporation |
Semiconductors | Warrant | Preferred Series E | 141,567 | 46 | 114 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Semiconductors (0.07%)* |
|
309 | 604 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| Actifio, Inc. |
Software | Warrant | Common Stock | 73,584 | 249 | 79 | ||||||||||||
| Software | Warrant | Preferred Series F | 31,673 | 343 | 60 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Actifio, Inc. |
105,257 | 592 | 139 | |||||||||||||||
| Braxton Technologies, LLC |
Software | Warrant | Preferred Series A | 168,750 | 188 | — | ||||||||||||
| CareCloud Corporation(15) |
Software | Warrant | Preferred Series B | 413,433 | 258 | 634 | ||||||||||||
| Clickfox, Inc.(15) |
Software | Warrant | Preferred Series B | 1,038,563 | 330 | 152 | ||||||||||||
| Software | Warrant | Preferred Series C | 592,019 | 730 | 183 | |||||||||||||
| Software | Warrant | Preferred Series C-A | 2,218,214 | 230 | 3,673 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Clickfox, Inc. |
3,848,796 | 1,290 | 4,008 | |||||||||||||||
| Cloud Technology Partners, Inc. |
Software | Warrant | Preferred Series C | 113,960 | 34 | 4 | ||||||||||||
| Evernote Corporation(15) |
Software | Warrant | Common Stock | 62,500 | 106 | 131 | ||||||||||||
| Fuze, Inc.(15) |
Software | Warrant | Preferred Series F | 256,158 | 89 | 89 | ||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(15) |
Software | Warrant | Preferred Series E | 614,333 | 16 | — | ||||||||||||
| Mattersight Corporation(3) |
Software | Warrant | Common Stock | 357,143 | 538 | 173 | ||||||||||||
| Message Systems, Inc.(15) |
Software | Warrant | Preferred Series C | 503,718 | 334 | 306 | ||||||||||||
| Mobile Posse, Inc.(15) |
Software | Warrant | Preferred Series C | 396,430 | 130 | 130 | ||||||||||||
See notes to consolidated financial statements.
F-103
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| Neos, Inc.(15) |
Software | Warrant | Common Stock | 221,150 | $ | 22 | $ | 18 | ||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Warrant | Preferred Series E | 225,586 | 33 | 125 | ||||||||||||
| OneLogin, Inc.(15) |
Software | Warrant | Common Stock | 228,972 | 150 | 348 | ||||||||||||
| Poplicus, Inc.(15) |
Software | Warrant | Preferred Series C | 2,595,230 | — | 5 | ||||||||||||
| Quid, Inc.(15) |
Software | Warrant | Preferred Series D | 71,576 | 1 | 5 | ||||||||||||
| RedSeal Inc.(15) |
Software | Warrant | Preferred Series C-Prime |
640,603 | 66 | 81 | ||||||||||||
| Signpost, Inc.(15) |
Software | Warrant | Preferred Series C | 324,005 | 314 | 130 | ||||||||||||
| Sonian, Inc.(15) |
Software | Warrant | Preferred Series C | 185,949 | 106 | 109 | ||||||||||||
| Wrike, Inc. |
Software | Warrant | Common Stock | 139,751 | 462 | 691 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (0.87%)* |
|
4,729 | 7,126 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||
| Alimera Sciences, Inc.(3) |
Specialty Pharmaceuticals |
Warrant | Common Stock | 1,717,709 | 861 | 584 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.07%)* |
|
861 | 584 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
||||||||||||||||||
| Gynesonics, Inc.(15) |
Surgical Devices | Warrant | Preferred Series C | 180,480 | 75 | 14 | ||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 1,575,965 | 320 | 278 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
1,756,445 | 395 | 292 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Warrant | Preferred Series B | 40,436 | 225 | 12 | ||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 175,000 | 100 | 543 | |||||||||||||
| Surgical Devices | Warrant | Preferred Series F | 50,544 | 38 | 66 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
265,980 | 363 | 621 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.11%)* |
|
758 | 913 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||
| Agrivida, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series D | 471,327 | 120 | 110 | ||||||||||||
| Alphabet Energy, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series 1B | 13,667 | 82 | — | ||||||||||||
| American Superconductor Corporation(3) |
Sustainable and Renewable Technology |
Warrant | Common Stock | 58,823 | 39 | 29 | ||||||||||||
| Brightsource Energy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series 1 | 116,666 | 104 | — | ||||||||||||
| Calera, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 44,529 | 513 | — | ||||||||||||
| EcoMotors, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series B | 437,500 | 308 | 51 | ||||||||||||
| Fluidic, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series D | 61,804 | 102 | 4 | ||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
Sustainable and Renewable Technology |
Warrant | Common Stock | 530,811 | 181 | — | ||||||||||||
| Sustainable and Renewable Technology |
Warrant | Preferred Series 2-A | 6,229 | 50 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
537,040 | 231 | — | |||||||||||||||
| Fulcrum Bioenergy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series C-1 | 280,897 | 275 | 292 | ||||||||||||
| GreatPoint Energy, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series D-1 | 393,212 | 548 | — | ||||||||||||
| Polyera Corporation(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series C | 311,609 | 338 | — | ||||||||||||
| Proterra, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series 4 | 477,517 | 41 | 548 | ||||||||||||
| Rive Technology, Inc.(15) |
Sustainable and Renewable Technology |
Warrant | Preferred Series E | 234,477 | 12 | 4 | ||||||||||||
| Stion Corporation(5) |
Sustainable and Renewable Technology |
Warrant | Preferred Series Seed |
2,154 | 1,378 | — | ||||||||||||
See notes to consolidated financial statements.
F-104
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
June 30, 2017
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry | Type of Investment(1) | Series | Shares | Cost(2) | Value(3) | ||||||||||||
| TAS Energy, Inc. |
Sustainable and Renewable Technology |
Warrant | Preferred Series AA | 428,571 | $ | 299 | $ | — | ||||||||||
| Tendril Networks |
Sustainable and Renewable Technology |
Warrant | Preferred Series 3-A | 1,019,793 | 189 | 98 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.14%)* |
|
4,579 | 1,136 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Warrant Investments (3.98%)* |
|
42,020 | 32,530 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total Investments (170.71%)* |
|
$ | 1,501,139 | $ | 1,395,469 | |||||||||||||
|
|
|
|
|
|||||||||||||||
| * | Value as a percent of net assets |
| (1) | Preferred and common stock, warrants, and equity interests are generally non-income producing. |
| (2) | Gross unrealized appreciation, gross unrealized depreciation, and net unrealized depreciation for federal income tax purposes totaled $22.2 million, $135.8 million and $113.6 million respectively. The tax cost of investments is $1.5 billion. |
| (3) | Except for warrants in 39 publicly traded companies and common stock in 17 publicly traded companies, all investments are restricted at June 30, 2017 and were valued at fair value as determined in good faith by the Company’s board of directors (the “Board of Directors”). No unrestricted securities of the same issuer are outstanding. The Company uses the Standard Industrial Code for classifying the industry grouping of its portfolio companies. |
| (4) | Non-U.S. company or the company’s principal place of business is outside the United States. |
| (5) | Affiliate investment as defined under the Investment Company Act of 1940, as amended, (the “1940 Act”) in which Hercules owns at least 5% but generally less than 25% of the company’s voting securities. |
| (6) | Control investment as defined under the 1940 Act in which Hercules owns at least 25% of the company’s voting securities or has greater than 50% representation on its board. |
| (7) | Debt is on non-accrual status at June 30, 2017, and is therefore considered non-income producing. Note that at June 30, 2017, only the $11.0 million PIK, or payment-in-kind, loan is on non-accrual for the Company’s debt investment in Tectura Corporation. |
| (8) | Denotes that all or a portion of the debt investment is convertible debt. |
| (9) | Indicates assets that the Company deems not “qualifying assets” under section 55(a) of 1940 Act. Qualifying assets must represent at least 70% of the Company’s total assets at the time of acquisition of any additional non-qualifying assets. |
| (10) | Denotes that all or a portion of the debt investment secures the notes offered in the Debt Securitization (as defined in Note 4). |
| (11) | Denotes that all or a portion of the debt investment is pledged as collateral under the Wells Facility (as defined in Note 4). |
| (12) | Denotes that all or a portion of the debt investment is pledged as collateral under the Union Bank Facility (as defined in Note 4). |
| (13) | Denotes that all or a portion of the debt investment principal includes accumulated PIK interest and is net of repayments. |
| (14) | Denotes that all or a portion of the debt investment includes an exit fee receivable. |
| A. | This fee ranges from 1.0% to 5.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| B. | This fee ranges from 5.0% to 10.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| C. | This fee ranges from 10.0% to 15.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| (15) | Denotes that all or a portion of the investment in this portfolio company is held by Hercules Technology II, L.P., or HT II, or Hercules Technology III, L.P., or HT III, the Company’s wholly owned small business investment companies, or SBIC, subsidiaries. |
| (16) | Denotes that the fair value of the Company’s total investments in this portfolio company represent greater than 5% of the Company’s total assets at June 30, 2017. |
| (17) | Denotes that there is an unfunded contractual commitment available at the request of this portfolio company at June 30, 2017. Refer to Note 10. |
| (18) | Repayment of debt investment is delinquent of the contractual maturity date as of June 30, 2017. |
| (19) | The stated PIK interest rate may be reduced to 1.45% subject to achievement of a milestone by the portfolio company. |
See notes to consolidated financial statements.
F-105
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Debt Investments |
|
|||||||||||||||||||
| Biotechnology Tools |
|
|||||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Exicure, Inc.(11)(14A) |
Biotechnology Tools | Senior Secured |
September 2019 | Interest rate PRIME + 6.45% or Floor rate of 9.95% |
$ | 6,000 | $5,971 | $6,035 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
5,971 | 6,035 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Biotechnology Tools (0.77%)* |
|
5,971 | 6,035 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Communications & Networking |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(13)(14B) |
Communications & Networking | Senior Secured |
August 2017 | PIK Interest 10.50% | $ | 1,278 | 1,304 | 1,304 | ||||||||||||
| OpenPeak, Inc.(7) |
Communications & Networking | Senior Secured |
April 2017 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 12,211 | 8,975 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
10,279 | 1,304 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Avanti Communications Group(4)(9) |
Communications & Networking | Senior Secured |
October 2019 | Interest rate FIXED 10.00% | $ | 8,025 | 7,212 | 4,825 | ||||||||||||
| SkyCross, Inc.(6)(7)(13)(14B)(15) |
Communications & Networking | Senior Secured |
January 2018 | Interest rate FIXED 10.95%, PIK Interest 5.00% |
$ | 16,758 | 16,900 | — | ||||||||||||
| Spring Mobile Solutions, Inc.(12)(14B) |
Communications & Networking | Senior Secured |
January 2019 | Interest rate PRIME + 6.70% or Floor rate of 9.95% |
$ | 3,000 | 3,038 | 3,044 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
27,150 | 7,869 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Communications & Networking (1.16%)* |
|
37,429 | 9,173 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Consumer & Business Products |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(14A)(15) |
Consumer & Business Products | Senior Secured |
December 2019 | Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 20,000 | 19,837 | 19,837 | ||||||||||||
| Consumer & Business Products | Senior Secured |
December 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.50% |
$ | 1,000 | 965 | 965 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Antenna79 (p.k.a. Pong Research Corporation) |
$ | 21,000 | 20,802 | 20,802 | ||||||||||||||||
| Nasty Gal(14B)(15) |
Consumer & Business Products | Senior Secured |
May 2019 | Interest rate PRIME + 5.45% or Floor rate of 8.95% |
$ | 13,241 | 13,148 | 13,148 | ||||||||||||
| Second Time Around (Simplify Holdings, LLC)(14A)(15) |
Consumer & Business Products | Senior Secured |
February 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ | 2,280 | 2,302 | 2,283 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
36,252 | 36,233 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Consumer & Business Products (4.60%)* |
|
36,252 | 36,233 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Drug Delivery |
|
|||||||||||||||||||
| Under 1 Year Maturity |
|
|||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(9)(10)(14A)(15) |
Drug Delivery | Senior Secured |
October 2017 | Interest rate PRIME + 3.85% or Floor rate of 9.10% |
$ | 20,466 | 21,151 | 21,151 | ||||||||||||
| Celsion Corporation(10)(14A) |
Drug Delivery | Senior Secured |
June 2017 | Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 2,246 | 2,575 | 2,575 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
23,726 | 23,726 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| Agile Therapeutics, Inc.(10)(14A) |
Drug Delivery | Senior Secured |
December 2018 | Interest rate PRIME + 4.75% or Floor rate of 9.00% |
$ | 16,500 | 16,524 | 16,434 | ||||||||||||
| Aprecia Pharmaceuticals Company(11)(14A) |
Drug Delivery | Senior Secured |
January 2020 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 20,000 | 19,700 | 19,706 | ||||||||||||
See notes to consolidated financial statements.
F-106
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||||
| BioQ Pharma Incorporated(10)(14A)(14B) |
Drug Delivery | |
Senior Secured |
|
May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.25% |
$ | 8,231 | $ | 8,636 | $ | 8,577 | ||||||||||
| Drug Delivery | |
Senior Secured |
|
May 2018 | Interest rate PRIME + 7.00% or Floor rate of 10.25% |
$ | 2,464 | 2,511 | 2,509 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total BioQ Pharma Incorporated |
$ | 10,695 | 11,147 | 11,086 | ||||||||||||||||||
| Edge Therapeutics, Inc.(11)(14A)(17) |
Drug Delivery | |
Senior Secured |
|
February 2020 | Interest rate PRIME + 4.65% or Floor rate of 9.15% |
$ | 15,000 | 15,004 | 15,045 | ||||||||||||
| Pulmatrix Inc.(8)(10)(14A) |
Drug Delivery | |
Senior Secured |
|
July 2018 | Interest rate PRIME + 6.25% or Floor rate of 9.50% |
$ | 5,954 | 6,022 | 6,013 | ||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(10)(14A) |
Drug Delivery | |
Senior Secured |
|
December 2018 | Interest rate PRIME + 2.70% or Floor rate of 7.95% |
$ | 12,123 | 12,325 | 12,238 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
80,722 | 80,522 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Drug Delivery (13.23%)* |
|
104,448 | 104,248 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Drug Discovery & Development |
|
|||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||||
| Cerecor, Inc.(11)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
August 2017 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 2,374 | 2,499 | 2,499 | ||||||||||||
| Neuralstem, Inc.(14A)(15) |
Drug Discovery & Development | |
Senior Secured |
|
April 2017 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 3,766 | 3,996 | 3,996 | ||||||||||||
|
|
|
|
|
|||||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
6,495 | 6,495 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||||
| Auris Medical Holding, AG(4)(9)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
January 2020 | Interest rate PRIME + 6.05% or Floor rate of 9.55% |
$ | 12,500 | 12,317 | 12,326 | ||||||||||||
| Aveo
Pharmaceuticals, |
Drug Discovery & Development | |
Senior Secured |
|
December 2019 | Interest rate PRIME + 6.90% or Floor rate of 11.90% |
$ | 10,000 | 10,269 | 10,218 | ||||||||||||
| Drug Discovery & Development | |
Senior Secured |
|
December 2019 | Interest rate PRIME + 6.90% or Floor rate of 11.90% |
$ | 5,000 | 4,926 | 4,918 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Aveo Pharmaceuticals, Inc. |
$ | 15,000 | 15,195 | 15,136 | ||||||||||||||||||
| Bellicum
Pharmaceuticals, |
Drug Discovery & Development | |
Senior Secured |
|
March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 15,000 | 15,212 | 15,387 | ||||||||||||
| Drug Discovery & Development | |
Senior Secured |
|
March 2020 | Interest rate PRIME + 5.85% or Floor rate of 9.35% |
$ | 5,000 | 4,981 | 5,049 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
| Total Bellicum Pharmaceuticals, Inc. |
$ | 20,000 | 20,193 | 20,436 | ||||||||||||||||||
| Brickell Biotech, Inc.(11)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
September 2019 | Interest rate PRIME + 5.70% or Floor rate of 9.20% |
$ | 7,500 | 7,521 | 7,560 | ||||||||||||
| Cerulean Pharma, Inc.(12)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
July 2018 | Interest rate PRIME + 1.55% or Floor rate of 7.30% |
$ | 13,078 | 13,994 | 13,908 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(10)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
December 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 19,548 | 19,276 | 19,372 | ||||||||||||
| CytRx Corporation(10)(14B)(15) |
Drug Discovery & Development | |
Senior Secured |
|
February 2020 | Interest rate PRIME + 6.00% or Floor rate of 9.50% |
$ | 25,000 | 25,086 | 25,166 | ||||||||||||
| Epirus
Biopharmaceuticals, |
Drug Discovery & Development | |
Senior Secured |
|
April 2018 | Interest rate PRIME + 4.70% or Floor rate of 7.95% |
$ | 3,066 | 3,349 | — | ||||||||||||
| Genocea Biosciences, Inc.(10)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
January 2019 | Interest rate PRIME + 2.25% or Floor rate of 7.25% |
$ | 17,000 | 17,313 | 17,376 | ||||||||||||
| Immune Pharmaceuticals(10)(14B) |
Drug Discovery & Development | |
Senior Secured |
|
September 2018 | Interest rate PRIME + 4.75% or Floor rate of 10.00% |
$ | 3,271 | 3,350 | 2,693 | ||||||||||||
| Insmed, Incorporated(10)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
October 2020 | Interest rate PRIME + 4.75% or Floor rate of 9.25% |
$ | 55,000 | 54,695 | 54,559 | ||||||||||||
| Mast Therapeutics, Inc.(14A)(15) |
Drug Discovery & Development | |
Senior Secured |
|
January 2019 | Interest rate PRIME + 5.70% or Floor rate of 8.95% |
$ | 3,347 | 3,921 | 3,923 | ||||||||||||
| Melinta Therapeutics(12)(14A) |
Drug Discovery & Development | |
Senior Secured |
|
June 2018 | Interest rate PRIME + 3.75% or Floor rate of 8.25% |
$ | 24,502 | 25,001 | 24,945 | ||||||||||||
See notes to consolidated financial statements.
F-107
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Merrimack Pharmaceuticals, Inc.(9) |
Drug Discovery & Development | Senior Secured |
December 2022 | Interest rate FIXED 11.50% | $ | 25,000 | $ | 25,000 | $ | 25,000 | ||||||||||
| Metuchen
Pharmaceuticals |
Drug Discovery & Development | Senior Secured |
October 2020 | Interest rate PRIME + 7.25% or Floor rate of 10.75%, PIK Interest 1.35% |
$ | 35,081 | 34,541 | 34,541 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(14A)(15) |
Drug Discovery & Development | Senior Secured |
September 2020 | Interest rate PRIME + 2.75% or Floor rate of 8.50% |
$ | 40,000 | 39,388 | 39,504 | ||||||||||||
| PhaseRx,Inc.(14B)(15) |
Drug Discovery & Development | Senior Secured |
December 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 6,000 | 5,921 | 5,945 | ||||||||||||
| Sorrento Therapeutics, Inc.(9)(14B) |
Drug Discovery & Development | Senior Secured |
December 2020 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 50,000 | 48,069 | 48,069 | ||||||||||||
| uniQure B.V.(4)(9)(10)(14B) |
Drug Discovery & Development | Senior Secured |
May 2020 | Interest rate PRIME + 3.00% or Floor rate of 8.25% |
$ | 20,000 | 20,133 | 20,081 | ||||||||||||
| XOMA Corporation(9)(14B)(15) |
Drug Discovery & Development | Senior Secured |
September 2018 | Interest rate PRIME + 2.15% or Floor rate of 9.40% |
$ | 16,380 | 16,970 | 16,901 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
411,233 | 407,441 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Drug Discovery & Development (52.53%)* |
|
417,728 | 413,936 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Persimmon Technologies(11)(13)(14B) |
Electronics & Computer Hardware | Senior Secured |
June 2019 | Interest rate PRIME + 7.50% or Floor rate of 11.00%, PIK Interest 1.50% |
$ | 7,012 | 7,096 | 7,134 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
7,096 | 7,134 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.91%)* |
|
7,096 | 7,134 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| InstaMed
Communications, |
Healthcare Services, Other | Senior Secured |
February 2019 | Interest rate PRIME + 6.75% or Floor rate of 10.00% |
$ | 10,000 | 10,125 | 10,261 | ||||||||||||
| PH Group Holdings |
Healthcare Services, Other | Senior Secured |
September 2020 | Interest rate PRIME + 7.45% or Floor rate of 10.95% |
$ | 20,000 | 19,802 | 19,802 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
29,927 | 30,063 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Healthcare Services, Other (3.82%)* |
|
29,927 | 30,063 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Internet Consumer & Business Services |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Aria Systems, Inc.(10)(13) |
Internet Consumer & Business Services | Senior Secured |
June 2019 | Interest rate PRIME + 3.20% or Floor rate of 6.95%, PIK Interest 1.95% |
$ | 2,061 | 2,045 | 1,728 | ||||||||||||
| Internet Consumer & Business Services | Senior Secured |
June 2019 | Interest rate PRIME + 5.20% or Floor rate of 8.95%, PIK Interest 1.95% |
$ | 18,463 | 18,307 | 15,467 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Aria Systems, Inc. |
$ | 20,524 | 20,352 | 17,195 | ||||||||||||||||
| CloudOne, Inc.(10)(14B) |
Internet Consumer & Business Services | Senior Secured |
April 2019 | Interest rate PRIME + 6.35% or Floor rate of 9.85% |
$ | 5,000 | 5,091 | 5,138 | ||||||||||||
| Intent Media, Inc.(13)(14A)(15) |
Internet Consumer & Business Services | Senior Secured |
December 2018 | Interest rate PRIME + 5.25% or Floor rate of 8.75%, PIK Interest 1.00% |
$ | 5,000 | 4,851 | 4,851 | ||||||||||||
| LogicSource(14B)(15) |
Internet Consumer & Business Services | Senior Secured |
October 2019 | Interest rate PRIME + 6.25% or Floor rate of 9.75% |
$ | 8,500 | 8,533 | 8,649 | ||||||||||||
| Snagajob.com, Inc.(12)(13)(14A) |
Internet Consumer & Business Services | Senior Secured |
July 2020 | Interest rate PRIME + 5.15% or Floor rate of 9.15%, PIK Interest 1.95% |
$ | 35,293 | 34,517 | 35,067 | ||||||||||||
See notes to consolidated financial statements.
F-108
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Tectura Corporation(7)(8)(13) |
Internet Consumer & Business Services | Senior Secured |
June 2021 | Interest rate FIXED 6.00%, PIK Interest 3.00% |
$ | 19,691 | $ | 19,691 | $ | 19,691 | ||||||||||
| Internet Consumer & Business Services | Senior Secured |
June 2021 | PIK Interest 8.00% | $ | 11,015 | 240 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Tectura Corporation |
$ | 30,706 | 19,931 | 19,691 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
93,275 | 90,591 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Internet Consumer & Business Services (11.50%)* |
|
93,275 | 90,591 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Media/Content/Info |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| FanDuel, Inc.(14B) |
Media/Content/Info | Senior Secured |
November 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.75% |
$ | 20,000 | 19,352 | 19,352 | ||||||||||||
| Machine Zone, Inc.(13)(16) |
Media/Content/Info | Senior Secured |
May 2018 | Interest rate PRIME + 2.50% or Floor rate of 6.75%, PIK Interest 3.00% |
$ | 103,785 | 102,444 | 103,083 | ||||||||||||
| WP Technology, Inc.
(Wattpad, |
Media/Content/Info | Senior Secured |
April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ | 5,000 | 5,029 | 5,099 | ||||||||||||
| Media/Content/Info | Senior Secured |
April 2020 | Interest rate PRIME + 4.75% or Floor rate of 8.25% |
$ | 2,500 | 2,471 | 2,510 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total WP Technology, Inc. (Wattpad, Inc.) |
$ | 7,500 | 7,500 | 7,609 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
129,296 | 130,044 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Media/Content/Info (16.50%)* |
|
129,296 | 130,044 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| InspireMD, Inc.(4)(9)(14B) |
Medical Devices & Equipment | Senior Secured |
June 2017 | Interest rate PRIME + 5.00% or Floor rate of 10.50% |
$ | 2,237 | 2,743 | 2,743 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
2,743 | 2,743 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Amedica Corporation(8)(14B)(15) |
Medical Devices & Equipment | Senior Secured |
January 2018 | Interest rate PRIME + 7.70% or Floor rate of 10.95% |
$ | 7,417 | 8,816 | 8,715 | ||||||||||||
| Aspire Bariatrics, Inc.(14B)(15) |
Medical Devices & Equipment | Senior Secured |
October 2018 | Interest rate PRIME + 4.00% or Floor rate of 9.25% |
$ | 5,295 | 5,400 | 5,368 | ||||||||||||
| Avedro, Inc.(14A)(15) |
Medical Devices & Equipment | Senior Secured |
June 2018 | Interest rate PRIME + 6.00% or Floor rate of 9.25% |
$ | 9,777 | 9,975 | 9,982 | ||||||||||||
| Flowonix Medical Incorporated(12)(14B) |
Medical Devices & Equipment | Senior Secured |
May 2018 | Interest rate PRIME + 4.75% or Floor rate of 10.00% |
$ | 10,905 | 11,340 | 11,275 | ||||||||||||
| Medical Devices & Equipment | Senior Secured |
March 2019 | Interest rate PRIME + 6.50% or Floor rate of 10.00% |
$ | 4,255 | 4,243 | 4,214 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Flowonix Medical Incorporated |
$ | 15,160 | 15,583 | 15,489 | ||||||||||||||||
| Gamma Medica, Inc.(10)(14B) |
Medical Devices & Equipment | Senior Secured |
January 2018 | Interest rate PRIME + 6.50% or Floor rate of 9.75% |
$ | 2,500 | 2,650 | 2,645 | ||||||||||||
| IntegenX, Inc.(14B)(15) |
Medical Devices & Equipment | Senior Secured |
June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 15,000 | 15,068 | 15,168 | ||||||||||||
| Medical Devices & Equipment | Senior Secured |
June 2019 | Interest rate PRIME + 6.05% or Floor rate of 10.05% |
$ | 1,750 | 1,694 | 1,730 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total IntegenX, Inc. |
$ | 16,750 | 16,762 | 16,898 | ||||||||||||||||
| Micell Technologies, Inc.(11)(14B) |
Medical Devices & Equipment | Senior Secured |
August 2019 | Interest rate PRIME + 7.25% or Floor rate of 10.50% |
$ | 8,277 | 8,255 | 8,321 | ||||||||||||
| Quanta Fluid Solutions(4)(9)(10)(14B) |
Medical Devices & Equipment | Senior Secured |
April 2020 | Interest rate PRIME + 8.05% or Floor rate of 11.55% |
$ | 12,500 | 12,547 | 12,500 | ||||||||||||
| Quanterix Corporation(10)(14A) |
Medical Devices & Equipment | Senior Secured |
February 2018 | Interest rate PRIME + 2.75% or Floor rate of 8.00% |
$ | 9,964 | 10,276 | 10,316 | ||||||||||||
See notes to consolidated financial statements.
F-109
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| SynergEyes, Inc.(14B)(15) |
Medical Devices & Equipment | Senior Secured |
January 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 2,347 | $ | 2,762 | $ | 2,719 | ||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
93,026 | 92,953 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Medical Devices & Equipment (12.15%)* |
|
95,769 | 95,696 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Semiconductors |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(14B)(15)(17) |
Semiconductors | Senior Secured |
November 2017 | Interest rate PRIME + 7.00% or Floor rate of 10.50% |
$ | 1,682 | 1,682 | 1,682 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
1,682 | 1,682 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Achronix Semiconductor Corporation(14B)(15)(17) |
Semiconductors | Senior Secured |
July 2018 | Interest rate PRIME + 8.25% or Floor rate of 11.50% |
$ | 3,341 | 3,546 | 3,530 | ||||||||||||
| Avnera Corporation(10)(14A) |
Semiconductors | Senior Secured |
April 2018 | Interest rate PRIME + 5.25% or Floor rate of 8.50% |
$ | 5,577 | 5,699 | 5,816 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
9,245 | 9,346 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Semiconductors (1.40%)* |
|
10,927 | 11,028 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Software |
||||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(7)(13)(14C)(15)(18) |
Software | Senior Secured |
October 2016 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 1,566 | 1,698 | 730 | ||||||||||||
| RedSeal Inc.(15)(17) |
Software | Senior Secured |
June 2017 | Interest rate PRIME + 3.25% or Floor rate of 6.50% |
$ | 2,635 | 2,635 | 2,635 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
4,333 | 3,365 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Actifio, Inc.(13)(14A) |
Software | Senior Secured |
January 2019 | Interest rate PRIME + 4.25% or Floor rate of 8.25%, PIK Interest 2.25% |
$ | 30,961 | 30,830 | 30,918 | ||||||||||||
| Software | Senior Secured |
January 2019 | Interest rate PRIME + 4.75% or Floor rate of 8.75%, PIK Interest 2.50% |
$ | 10,171 | 9,929 | 10,036 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Actifio, Inc. |
$ | 41,132 | 40,759 | 40,954 | ||||||||||||||||
| Clickfox, Inc.(12)(14C) |
Software | Senior Secured |
May 2018 | Interest rate PRIME + 8.00% or Floor rate of 11.50% |
$ | 12,000 | 12,261 | 12,273 | ||||||||||||
| Cloud Technology Partners, Inc.(14A) |
Software | Senior Secured |
June 2018 | Interest rate PRIME + 3.05% or Floor rate of 7.05% |
$ | 3,000 | 2,966 | 2,966 | ||||||||||||
| Software | Senior Secured |
December 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.75% |
$ | 10,000 | 9,863 | 9,863 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Cloud Technology Partners, Inc. |
$ | 13,000 | 12,829 | 12,829 | ||||||||||||||||
| Druva, Inc.(10)(12)(14B)(17) |
Software | Senior Secured |
March 2018 | Interest rate PRIME + 4.60% or Floor rate of 7.85% |
$ | 9,157 | 9,604 | 9,613 | ||||||||||||
| Software | Senior Secured |
May 2018 | Interest rate PRIME + 4.60% or Floor rate of 7.85% |
$ | 10,000 | 10,066 | 10,141 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Druva, Inc. |
$ | 19,157 | 19,670 | 19,754 | ||||||||||||||||
| Evernote Corporation(15)(17) |
Software | Senior Secured |
October 2020 | Interest rate PRIME + 5.45% or Floor rate of 8.95% |
$ | 6,000 | 5,961 | 5,961 | ||||||||||||
| Lithium Technologies, Inc.(13)(14A)(15)(19) |
Software | Senior Secured |
June 2020 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 1.80% |
$ | 25,019 | 24,999 | 24,999 | ||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(7)(13)(14A)(15) |
Software | Senior Secured |
March 2018 | Interest rate FIXED 5.75%, PIK Interest 10.75% |
$ | 13,000 | 12,747 | 5,477 | ||||||||||||
See notes to consolidated financial statements.
F-110
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Mattersight Corporation(11)(13) |
Software | Senior Secured |
February 2020 | Interest rate PRIME + 6.25% or Floor rate of 9.75%, PIK Interest 2.15% |
$ | 22,664 | $ | 22,023 | $ | 22,280 | ||||||||||
| OneLogin, Inc.(13)(15) |
Software | Senior Secured |
August 2019 | Interest rate PRIME + 6.45% or Floor rate of 9.95%, PIK Interest 3.25% |
$ | 15,369 | 15,249 | 15,488 | ||||||||||||
| Quid, Inc.(13)(14A)(15) |
Software | Senior Secured |
October 2019 | Interest rate PRIME + 4.75% or Floor rate of 8.25%, PIK Interest 2.25% |
$ | 8,116 | 8,126 | 8,220 | ||||||||||||
| RedSeal Inc.(14A)(15)(17) |
Software | Senior Secured |
June 2018 | Interest rate PRIME + 7.75% or Floor rate of 11.00% |
$ | 5,000 | 5,120 | 5,107 | ||||||||||||
| Software | Senior Secured |
January 2020 | Interest rate PRIME + 7.75% or Floor rate of 11.25% |
$ | 5,000 | 4,880 | 4,880 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total RedSeal Inc. |
$ | 10,000 | 10,000 | 9,987 | ||||||||||||||||
| Signpost, Inc.(13)(14A)(15) |
Software | Senior Secured |
February 2020 | Interest rate PRIME + 4.15% or Floor rate of 8.15%, PIK Interest 1.75% |
$ | 15,237 | 15,022 | 15,190 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
199,646 | 193,412 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Software (24.97%)* |
|
203,979 | 196,777 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Alimera Sciences, Inc.(10)(13)(14A) |
Specialty Pharmaceuticals | Senior Secured |
November 2020 | Interest rate PRIME + 7.50% or Floor rate of 11.00%, PIK Interest 1.00% |
$ | 35,041 | 34,606 | 34,798 | ||||||||||||
| Jaguar Animal Health, Inc.(10)(14B) |
Specialty Pharmaceuticals | Senior Secured |
August 2018 | Interest rate PRIME + 5.65% or Floor rate of 9.90% |
$ | 3,511 | 3,803 | 3,725 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
38,409 | 38,523 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Specialty Pharmaceuticals (4.89%)* |
|
38,409 | 38,523 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Surgical Devices |
||||||||||||||||||||
| 1-5 Years Maturity |
||||||||||||||||||||
| Transmedics, Inc.(12)(14B) |
Surgical Devices | Senior Secured |
February 2020 | Interest rate PRIME + 5.30% or Floor rate of 9.55% |
$ | 8,500 | 8,497 | 8,529 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
8,497 | 8,529 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Surgical Devices (1.08%)* |
|
8,497 | 8,529 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Sustainable and Renewable Technology |
|
|||||||||||||||||||
| Under 1 Year Maturity |
||||||||||||||||||||
| American Superconductor Corporation(10)(14B) |
Sustainable and Renewable Technology | Senior Secured |
June 2017 | Interest rate PRIME + 7.25% or Floor rate of 11.00% |
$ | 1,500 | 1,550 | 1,550 | ||||||||||||
| Modumetal, Inc.(11)(14C)(14D) |
Sustainable and Renewable Technology | Senior Secured |
March 2017 | Interest rate PRIME + 8.70% or Floor rate of 11.95% |
$ | 376 | 882 | 882 | ||||||||||||
| Sustainable and Renewable Technology | Senior Secured |
October 2017 | Interest rate PRIME + 6.00% or Floor rate of 9.25% |
$ | 3,370 | 4,115 | 4,115 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Modumetal, Inc. |
$ | 3,746 | 4,997 | 4,997 | ||||||||||||||||
| Stion Corporation(5)(14A) |
Sustainable and Renewable Technology | Senior Secured |
February 2017 | Interest rate PRIME + 8.75% or Floor rate of 12.00% |
$ | 333 | 333 | 333 | ||||||||||||
See notes to consolidated financial statements.
F-111
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Maturity |
Interest Rate and Floor |
Principal Amount |
Cost(2) | Value(3) | |||||||||||||
| Sungevity, Inc.(12)(14D) |
Sustainable and Renewable Technology | Senior Secured |
October 2017 | Interest rate PRIME + 3.70% or Floor rate of 6.95% |
$ | 35,000 | $ | 39,834 | $ | 29,709 | ||||||||||
| Sustainable and Renewable Technology | Senior Secured |
October 2017 | Interest rate PRIME + 3.70% or Floor rate of 6.95% |
$ | 20,000 | 20,000 | 14,917 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Sungevity, Inc. |
$ | 55,000 | 59,834 | 44,626 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Under 1 Year Maturity |
|
66,714 | 51,506 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 1-5 Years Maturity |
|
|||||||||||||||||||
| FuelCell Energy, Inc.(11)(14B) |
Sustainable and Renewable Technology | Senior Secured |
October 2018 | Interest rate PRIME + 5.50% or Floor rate of 9.50% |
$ | 20,000 | 20,488 | 20,707 | ||||||||||||
| Proterra, Inc.(10)(14A)(14B) |
Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate PRIME + 6.95% or Floor rate of 10.20% |
$ | 30,000 | 30,670 | 30,592 | ||||||||||||
| Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate PRIME + 5.75% or Floor rate of 9.25% |
$ | 10,000 | 9,921 | 9,916 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total Proterra, Inc. |
$ | 40,000 | 40,591 | 40,508 | ||||||||||||||||
| Rive Technology, Inc.(14A)(15) |
Sustainable and Renewable Technology | Senior Secured |
January 2019 | Interest rate PRIME + 6.20% or Floor rate of 9.45% |
$ | 7,500 | 7,586 | 7,650 | ||||||||||||
| Tendril Networks(11)(14B) |
Sustainable and Renewable Technology | Senior Secured |
June 2019 | Interest rate FIXED 7.25% | $ | 15,000 | 15,405 | 15,324 | ||||||||||||
| Verdezyne, Inc.(14B)(15) |
Sustainable and Renewable Technology | Senior Secured |
April 2019 | Interest rate PRIME + 8.25% or Floor rate of 11.75% |
$ | 15,000 | 15,084 | 15,098 | ||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: 1-5 Years Maturity |
|
99,154 | 99,287 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Subtotal: Sustainable and Renewable Technology (19.14%)* |
|
165,868 | 150,793 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total: Debt Investments (168.64%)* |
|
1,384,871 | 1,328,803 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See notes to consolidated financial statements.
F-112
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Equity Investments |
||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||
| NuGEN Technologies, Inc.(15) |
Biotechnology Tools | Equity | Preferred Series C | 189,394 | $ | 500 | $ | 575 | ||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
500 | 575 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Communications & Networking |
|
|||||||||||||||||
| Achilles Technology Management Co II, Inc.(6)(15) |
Communications & Networking | Equity | Common Stock | 100 | 4,000 | 3,396 | ||||||||||||
| GlowPoint, Inc.(3) |
Communications & Networking | Equity | Common Stock | 114,192 | 101 | 31 | ||||||||||||
| Peerless Network Holdings, Inc. |
Communications & Networking | Equity | Preferred Series A | 1,000,000 | 1,000 | 4,990 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Communications & Networking (1.07%)* |
|
5,101 | 8,417 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Consumer & Business Products |
||||||||||||||||||
| Market Force Information, Inc. |
Consumer & Business Products | Equity | Common Stock | 480,261 | — | 279 | ||||||||||||
| Consumer & Business Products | Equity | Preferred Series B-1 | 187,970 | 500 | 273 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Market Force Information, Inc. |
668,231 | 500 | 552 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Consumer & Business Products (0.07%)* |
|
500 | 552 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Diagnostic |
||||||||||||||||||
| Singulex, Inc. |
Diagnostic | Equity | Common Stock | 937,998 | 750 | 574 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Diagnostic (0.07%)* |
|
750 | 574 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Delivery |
||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9) |
Drug Delivery | Equity | Common Stock | 54,240 | 108 | 141 | ||||||||||||
| BioQ Pharma Incorporated(15) |
Drug Delivery | Equity | Preferred Series D | 165,000 | 500 | 542 | ||||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 161,856 | 1,000 | 2,023 | ||||||||||||
| Merrion Pharmaceuticals, Plc(4)(9) |
Drug Delivery | Equity | Common Stock | 20,000 | 9 | — | ||||||||||||
| Neos Therapeutics, Inc.(3)(15) |
Drug Delivery | Equity | Common Stock | 125,000 | 1,500 | 731 | ||||||||||||
| Revance Therapeutics, Inc.(3) |
Drug Delivery | Equity | Common Stock | 22,765 | 557 | 472 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Delivery (0.50%)* |
|
3,674 | 3,909 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9)(15) |
Drug Discovery & Development | Equity | Common Stock | 426,931 | 1,060 | 231 | ||||||||||||
| Cerecor, Inc.(3) |
Drug Discovery & Development | Equity | Common Stock | 119,087 | 1,000 | 105 | ||||||||||||
| Cerulean Pharma, Inc.(3) |
Drug Discovery & Development | Equity | Common Stock | 135,501 | 1,000 | 96 | ||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Equity | Common Stock | 142,858 | 1,000 | 411 | ||||||||||||
| Dynavax Technologies(3)(9) |
Drug Discovery & Development | Equity | Common Stock | 20,000 | 550 | 79 | ||||||||||||
| Epirus Biopharmaceuticals, Inc. |
Drug Discovery & Development | Equity | Common Stock | 200,000 | 1,000 | — | ||||||||||||
| Genocea Biosciences, Inc.(3) |
Drug Discovery & Development | Equity | Common Stock | 223,463 | 2,000 | 921 | ||||||||||||
| Inotek Pharmaceuticals Corporation(3) |
Drug Discovery & Development | Equity | Common Stock | 3,778 | 1,500 | 23 | ||||||||||||
| Insmed, Incorporated(3) |
Drug Discovery & Development | Equity | Common Stock | 70,771 | 1,000 | 936 | ||||||||||||
| Melinta Therapeutics |
Drug Discovery & Development | Equity | Preferred Series 4 | 1,914,448 | 2,000 | 2,042 | ||||||||||||
See notes to consolidated financial statements.
F-113
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3) |
Drug Discovery & Development | Equity | Common Stock | 76,362 | $ | 2,743 | $ | 1,175 | ||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Discovery & Development (0.76%)* |
|
14,853 | 6,019 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| Identiv, Inc.(3) |
Electronics & Computer Hardware | Equity | Common Stock | 6,700 | 34 | 21 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.00%)* |
|
34 | 21 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Information Services |
||||||||||||||||||
| DocuSign, Inc.(15) |
Information Services | Equity | Common Stock | 385,000 | 6,081 | 6,081 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Information Services (0.77%)* |
|
6,081 | 6,081 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
|
|||||||||||||||||
| Blurb, Inc.(15) |
Internet Consumer & Business Services | Equity | Preferred Series B | 220,653 | 175 | 197 | ||||||||||||
| Brigade Group, Inc. (p.k.a. Philotic, Inc.) |
Internet Consumer & Business Services | Equity | Common Stock | 9,023 | 93 | — | ||||||||||||
| Lightspeed POS, Inc.(4) (9) |
Internet Consumer & Business Services | Equity | Preferred Series C | 230,030 | 250 | 228 | ||||||||||||
| Internet Consumer & Business Services | Equity | Preferred Series D | 198,677 | 250 | 221 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Lightspeed POS, Inc. |
428,707 | 500 | 449 | |||||||||||||||
| OfferUp, Inc.(15) |
Internet Consumer & Business Services | Equity | Preferred Series A | 286,080 | 1,663 | 1,663 | ||||||||||||
| Internet Consumer & Business Services | Equity | Preferred Series A-1 | 108,710 | 632 | 632 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total OfferUp, Inc. |
394,790 | 2,295 | 2,295 | |||||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services | Equity | Preferred Series G | 218,351 | 250 | 431 | ||||||||||||
| Internet Consumer & Business Services | Equity | Preferred Series H | 87,802 | 250 | 249 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Oportun (p.k.a. Progress Financial) |
306,153 | 500 | 680 | |||||||||||||||
| RazorGator Interactive Group, Inc. |
Internet Consumer & Business Services | Equity | Preferred Series AA | 34,783 | 15 | 34 | ||||||||||||
| Tectura Corporation |
Internet Consumer & Business Services | Equity | Preferred Series BB | 1,000,000 | — | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.46%)* |
|
3,578 | 3,655 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Media/Content/Info |
|
|||||||||||||||||
| Pinterest, Inc. |
Media/Content/Info | Equity | Preferred Series Seed | 620,000 | 4,085 | 4,085 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Media/Content/Info (0.52%)* |
|
4,085 | 4,085 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||
| AtriCure, Inc.(3) (15) |
Medical Devices & Equipment | Equity | Common Stock | 7,536 | 266 | 147 | ||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment | Equity | Preferred Series AA | 221,893 | 1,500 | 359 | ||||||||||||
| Gelesis, Inc.(15) |
Medical Devices & Equipment | Equity | Common Stock | 198,202 | — | 634 | ||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series A-1 | 191,210 | 425 | 687 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series A-2 | 191,626 | 500 | 650 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gelesis, Inc. |
581,038 | 925 | 1,971 | |||||||||||||||
See notes to consolidated financial statements.
F-114
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Medrobotics Corporation(15) |
Medical Devices & Equipment | Equity | Preferred Series E | 136,798 | $ | 250 | $ | 216 | ||||||||||
| Medical Devices & Equipment | Equity | Preferred Series F | 73,971 | 155 | 188 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series G | 163,934 | 500 | 514 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Medrobotics Corporation |
374,703 | 905 | 918 | |||||||||||||||
| Optiscan Biomedical, Corp.(5)(15) |
Medical Devices & Equipment | Equity | Preferred Series B | 6,185,567 | 3,000 | 292 | ||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series C | 1,927,309 | 655 | 85 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series D | 55,103,923 | 5,257 | 3,014 | |||||||||||||
| Medical Devices & Equipment | Equity | Preferred Series E | 13,573,546 | 1,136 | 1,138 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Optiscan Biomedical, Corp. |
76,790,345 | 10,048 | 4,529 | |||||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment | Equity | Preferred Series B | 232,061 | 527 | 548 | ||||||||||||
| Quanterix Corporation |
Medical Devices & Equipment | Equity | Preferred Series D | 272,479 | 1,000 | 1,086 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (1.21%)* |
|
15,171 | 9,558 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| Box, Inc.(3) |
Software | Equity | Common Stock | 611,442 | 4,709 | 8,475 | ||||||||||||
| CapLinked, Inc. |
Software | Equity | Preferred Series A-3 | 53,614 | 51 | 86 | ||||||||||||
| Druva, Inc. |
Software | Equity | Preferred Series 2 | 458,841 | 1,000 | 1,288 | ||||||||||||
| ForeScout Technologies, Inc. |
Software | Equity | Preferred Series D | 319,099 | 398 | 1,725 | ||||||||||||
| Software | Equity | Preferred Series E | 80,587 | 131 | 440 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total ForeScout Technologies, Inc. |
399,686 | 529 | 2,165 | |||||||||||||||
| HighRoads, Inc. |
Software | Equity | Common Stock | 190 | 307 | — | ||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Equity | Preferred Series E | 669,173 | 963 | 1,025 | ||||||||||||
| Palantir Technologies |
Software | Equity | Preferred Series E | 727,696 | 5,431 | 5,431 | ||||||||||||
| WildTangent, Inc.(15) |
Software | Equity | Preferred Series 3 | 100,000 | 402 | 148 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (2.36%)* |
|
13,392 | 18,618 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Specialty Pharmaceuticals |
|
|||||||||||||||||
| QuatRx Pharmaceuticals Company |
Specialty Pharmaceuticals | Equity | Preferred Series E | 241,829 | 750 | — | ||||||||||||
| Specialty Pharmaceuticals | Equity | Preferred Series E-1 | 26,955 | — | — | |||||||||||||
| Specialty Pharmaceuticals | Equity | Preferred Series G | 4,667,636 | — | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total QuatRx Pharmaceuticals Company |
4,936,420 | 750 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.00%)* |
|
750 | — | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
|
|||||||||||||||||
| Gynesonics, Inc.(15) |
Surgical Devices | Equity | Preferred Series B | 219,298 | 250 | 37 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 656,538 | 282 | 52 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 1,991,157 | 712 | 671 | |||||||||||||
| Surgical Devices | Equity | Preferred Series E | 2,786,367 | 429 | 450 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
5,653,360 | 1,673 | 1,210 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Equity | Preferred Series B | 88,961 | 1,100 | 357 | ||||||||||||
| Surgical Devices | Equity | Preferred Series C | 119,999 | 300 | 291 | |||||||||||||
| Surgical Devices | Equity | Preferred Series D | 260,000 | 650 | 912 | |||||||||||||
| Surgical Devices | Equity | Preferred Series F | 100,200 | 500 | 523 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
569,160 | 2,550 | 2,083 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.42%)* |
|
4,223 | 3,293 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-115
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Sustainable and Renewable Technology |
|
|||||||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) |
Sustainable and Renewable Technology | Equity | Common Stock | 19,250 | $ | 761 | $ | — | ||||||||||
| Glori Energy, Inc.(3) |
Sustainable and Renewable Technology | Equity | Common Stock | 18,208 | 165 | 1 | ||||||||||||
| Modumetal, Inc. |
Sustainable and Renewable Technology | Equity | Preferred Series C | 3,107,520 | 500 | 533 | ||||||||||||
| Proterra, Inc. |
Sustainable and Renewable Technology | Equity | Preferred Series 5 | 99,280 | 500 | 512 | ||||||||||||
| Sungevity, Inc.(15) |
Sustainable and Renewable Technology | Equity | Preferred Series D | 68,807,339 | 6,750 | — | ||||||||||||
| TPI Composites, Inc.(3) |
Sustainable and Renewable Technology | Equity | Common Stock | 78,018 | 273 | 1,251 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.29%)* |
|
8,949 | 2,297 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Equity Investments (8.59%)* |
|
81,641 | 67,654 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Warrant Investments |
||||||||||||||||||
| Biotechnology Tools |
||||||||||||||||||
| Exicure, Inc. |
Biotechnology Tools | Warrant | Preferred Series C | 104,348 | 107 | 181 | ||||||||||||
| Labcyte, Inc.(15) |
Biotechnology Tools | Warrant | Preferred Series C | 1,127,624 | 323 | 409 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Biotechnology Tools (0.07%)* |
|
430 | 590 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Communications & Networking |
||||||||||||||||||
| Intelepeer, Inc.(15) |
Communications & Networking | Warrant | Common Stock | 117,958 | 102 | — | ||||||||||||
| OpenPeak, Inc. |
Communications & Networking | Warrant | Common Stock | 108,982 | 149 | — | ||||||||||||
| PeerApp, Inc. |
Communications & Networking | Warrant | Preferred Series B | 298,779 | 61 | 14 | ||||||||||||
| Peerless Network Holdings, Inc. |
Communications & Networking | Warrant | Preferred Series A | 135,000 | 95 | 415 | ||||||||||||
| SkyCross, Inc.(6)(15) |
Communications & Networking | Warrant | Preferred Series F | 9,762,777 | 394 | — | ||||||||||||
| Spring Mobile Solutions, Inc. |
Communications & Networking | Warrant | Common Stock | 2,834,375 | 418 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Communications & Networking (0.05%)* |
|
1,219 | 429 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Consumer & Business Products |
||||||||||||||||||
| Antenna79 (p.k.a. Pong Research Corporation)(15) |
Consumer & Business Products | Warrant | Common Stock | 1,662,441 | 228 | — | ||||||||||||
| Intelligent Beauty, Inc.(15) |
Consumer & Business Products | Warrant | Preferred Series B | 190,234 | 230 | 354 | ||||||||||||
| IronPlanet, Inc. |
Consumer & Business Products | Warrant | Preferred Series D | 1,155,821 | 1,076 | 5,574 | ||||||||||||
| Nasty Gal(15) |
Consumer & Business Products | Warrant | Preferred Series C | 845,194 | 23 | — | ||||||||||||
| The Neat Company(15) |
Consumer & Business Products | Warrant | Preferred Series C-1 | 540,540 | 365 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Consumer & Business Products (0.75%)* |
|
1,922 | 5,928 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Delivery |
||||||||||||||||||
| AcelRx Pharmaceuticals, Inc.(3)(9)(15) |
Drug Delivery | Warrant | Common Stock | 176,730 | 785 | 92 | ||||||||||||
| Agile Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 180,274 | 730 | 269 | ||||||||||||
| Aprecia Pharmaceuticals Company |
Drug Delivery | Warrant | Preferred Series A-1 | 735,981 | 366 | 242 | ||||||||||||
| BIND Therapeutics, Inc.(15) |
Drug Delivery | Warrant | Common Stock | 152,586 | 488 | — | ||||||||||||
| BioQ Pharma Incorporated |
Drug Delivery | Warrant | Common Stock | 459,183 | 1 | 264 | ||||||||||||
| Celsion Corporation(3) |
Drug Delivery | Warrant | Common Stock | 194,986 | 428 | — | ||||||||||||
See notes to consolidated financial statements.
F-116
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Dance Biopharm, Inc.(15) |
Drug Delivery | Warrant | Common Stock | 110,882 | $ | 74 | $ | — | ||||||||||
| Edge Therapeutics, Inc.(3) |
Drug Delivery | Warrant | Common Stock | 78,595 | 390 | 402 | ||||||||||||
| Kaleo, Inc. (p.k.a. Intelliject, Inc.) |
Drug Delivery | Warrant | Preferred Series B | 82,500 | 594 | 391 | ||||||||||||
| Neos Therapeutics, Inc.(3)(15) |
Drug Delivery | Warrant | Common Stock | 70,833 | 285 | 17 | ||||||||||||
| Pulmatrix Inc.(3) |
Drug Delivery | Warrant | Common Stock | 25,150 | 116 | — | ||||||||||||
| ZP Opco, Inc (p.k.a. Zosano Pharma)(3) |
Drug Delivery | Warrant | Common Stock | 72,379 | 266 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Delivery (0.21%)* |
|
4,523 | 1,677 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Drug Discovery & Development |
||||||||||||||||||
| ADMA Biologics, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 89,750 | 295 | 43 | ||||||||||||
| Anthera Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 40,178 | 984 | — | ||||||||||||
| Auris Medical Holding, AG(3)(4)(9) |
Drug Discovery & Development | Warrant | Common Stock | 156,726 | 249 | 51 | ||||||||||||
| Aveo Pharmaceuticals, Inc.(3)(9) |
Drug Discovery & Development | Warrant | Common Stock | 2,069,880 | 396 | 123 | ||||||||||||
| Brickell Biotech, Inc. |
Drug Discovery & Development | Warrant | Preferred Series C | 26,086 | 119 | 139 | ||||||||||||
| Cerecor, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 22,328 | 70 | — | ||||||||||||
| Cerulean Pharma, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 171,901 | 369 | 14 | ||||||||||||
| Chroma Therapeutics, Ltd.(4)(9) |
Drug Discovery & Development | Warrant | Preferred Series D | 325,261 | 490 | — | ||||||||||||
| Cleveland BioLabs, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 7,813 | 105 | — | ||||||||||||
| Concert Pharmaceuticals, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 70,796 | 367 | 56 | ||||||||||||
| CTI BioPharma Corp. (p.k.a. Cell Therapeutics, Inc.)(3) |
Drug Discovery & Development | Warrant | Common Stock | 292,398 | 165 | 8 | ||||||||||||
| CytRx Corporation(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 634,146 | 416 | 78 | ||||||||||||
| Dicerna Pharmaceuticals, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 200 | 28 | — | ||||||||||||
| Epirus Biopharmaceuticals, Inc. |
Drug Discovery & Development | Warrant | Common Stock | 64,194 | 276 | — | ||||||||||||
| Fortress Biotech, Inc. (p.k.a. Coronado Biosciences, Inc.)(3) |
Drug Discovery & Development | Warrant | Common Stock | 73,009 | 142 | 13 | ||||||||||||
| Genocea Biosciences, Inc.(3) |
Drug Discovery & Development | Warrant | Common Stock | 73,725 | 266 | 75 | ||||||||||||
| Immune Pharmaceuticals(3) |
Drug Discovery & Development | Warrant | Common Stock | 214,853 | 164 | — | ||||||||||||
| Mast Therapeutics, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 2,272,724 | 203 | 85 | ||||||||||||
| Melinta Therapeutics |
Drug Discovery & Development | Warrant | Preferred Series 3 | 1,382,323 | 626 | 295 | ||||||||||||
| Nanotherapeutics, Inc.(15) |
Drug Discovery & Development | Warrant | Common Stock | 171,389 | 838 | 767 | ||||||||||||
| Neothetics, Inc. (p.k.a. Lithera, Inc)(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 46,838 | 266 | 29 | ||||||||||||
| Neuralstem, Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 75,187 | 77 | 1 | ||||||||||||
| Paratek Pharmaceuticals, Inc. (p.k.a. Transcept Pharmaceuticals, Inc.)(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 69,840 | 152 | 157 | ||||||||||||
| PhaseRx,Inc.(3)(15) |
Drug Discovery & Development | Warrant | Common Stock | 63,000 | 125 | 15 | ||||||||||||
| Sorrento Therapeutics, Inc.(3)(9) |
Drug Discovery & Development | Warrant | Common Stock | 306,748 | 890 | 632 | ||||||||||||
See notes to consolidated financial statements.
F-117
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| uniQure B.V.(3)(4)(9) |
Drug Discovery & Development | Warrant | Common Stock | 37,174 | $ | 218 | $ | 8 | ||||||||||
| XOMA Corporation(3)(9)(15) |
Drug Discovery & Development | Warrant | Common Stock | 9,063 | 279 | 6 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Drug Discovery & Development (0.33%)* |
|
8,575 | 2,595 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Electronics & Computer Hardware |
||||||||||||||||||
| Clustrix, Inc. |
Electronics & Computer Hardware | Warrant | Common Stock | 50,000 | 12 | — | ||||||||||||
| Persimmon Technologies |
Electronics & Computer Hardware | Warrant | Preferred Series D | 63,348 | 40 | 509 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Electronics & Computer Hardware (0.06%)* |
|
52 | 509 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Healthcare Services, Other |
||||||||||||||||||
| Chromadex Corporation(3)(15) |
Healthcare Services, Other | Warrant | Common Stock | 139,673 | 157 | 137 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Healthcare Services, Other (0.02%)* |
|
157 | 137 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Information Services |
||||||||||||||||||
| INMOBI Inc.(4)(9) |
Information Services | Warrant | Common Stock | 46,874 | 82 | — | ||||||||||||
| InXpo, Inc.(15) |
Information Services | Warrant | Preferred Series C | 648,400 | 98 | 4 | ||||||||||||
| Information Services | Warrant | Preferred Series C-1 | 1,165,183 | 74 | 6 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total InXpo, Inc. |
1,813,583 | 172 | 10 | |||||||||||||||
| RichRelevance, Inc.(15) |
Information Services | Warrant | Preferred Series E | 112,612 | 98 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Information Services (0.00%)* |
|
352 | 10 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Internet Consumer & Business Services |
|
|||||||||||||||||
| Aria Systems, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series E | 239,692 | 73 | — | ||||||||||||
| Blurb, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 234,280 | 636 | 96 | ||||||||||||
| CashStar, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C-2 | 727,272 | 130 | 24 | ||||||||||||
| CloudOne, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series E | 968,992 | 19 | 46 | ||||||||||||
| Intent Media, Inc.(15) |
Internet Consumer & Business Services | Warrant | Common Stock | 140,077 | 168 | 167 | ||||||||||||
| Just Fabulous, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series B | 206,184 | 1,102 | 1,093 | ||||||||||||
| Lightspeed POS, Inc.(4)(9) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 245,610 | 20 | 31 | ||||||||||||
| LogicSource(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 79,625 | 30 | 59 | ||||||||||||
| Oportun (p.k.a. Progress Financial) |
Internet Consumer & Business Services | Warrant | Preferred Series G | 174,562 | 78 | 190 | ||||||||||||
| Prism Education Group, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series B | 200,000 | 43 | — | ||||||||||||
| ShareThis, Inc.(15) |
Internet Consumer & Business Services | Warrant | Preferred Series C | 493,502 | 547 | 1 | ||||||||||||
| Snagajob.com, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series A | 1,575,000 | 640 | 1,075 | ||||||||||||
| Tapjoy, Inc. |
Internet Consumer & Business Services | Warrant | Preferred Series D | 748,670 | 316 | 19 | ||||||||||||
| Tectura Corporation |
Internet Consumer & Business Services | Warrant | Preferred Series B-1 | 253,378 | 51 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Internet Consumer & Business Services (0.36%)* |
|
3,853 | 2,801 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
See notes to consolidated financial statements.
F-118
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Media/Content/Info |
||||||||||||||||||
| FanDuel, Inc. |
Media/Content/Info | Warrant | Preferred Series E-1 | 4,648 | $ | 730 | $ | 682 | ||||||||||
| Machine Zone, Inc.(16) |
Media/Content/Info | Warrant | Common Stock | 1,552,710 | 1,958 | 2,729 | ||||||||||||
| Rhapsody International, Inc.(15) |
Media/Content/Info | Warrant | Common Stock | 715,755 | 385 | 7 | ||||||||||||
| WP Technology, Inc. (Wattpad, Inc.)(4)(9) |
Media/Content/Info | Warrant | Common Stock | 127,909 | 1 | 6 | ||||||||||||
| Zoom Media Group, Inc. |
Media/Content/Info | Warrant | Preferred Series A | 1,204 | 348 | 14 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Media/Content/Info (0.44%)* |
|
3,422 | 3,438 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Medical Devices & Equipment |
||||||||||||||||||
| Amedica Corporation(3)(15) |
Medical Devices & Equipment | Warrant | Common Stock | 103,225 | 459 | 14 | ||||||||||||
| Aspire Bariatrics, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series D | 395,000 | 455 | 217 | ||||||||||||
| Avedro, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series AA | 300,000 | 401 | 254 | ||||||||||||
| Flowonix Medical Incorporated |
Medical Devices & Equipment | Warrant | Preferred Series AA | 155,325 | 362 | 21 | ||||||||||||
| Gamma Medica, Inc. |
Medical Devices & Equipment | Warrant | Preferred Series A | 450,956 | 170 | 234 | ||||||||||||
| Gelesis, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series A-1 | 74,784 | 78 | 153 | ||||||||||||
| InspireMD, Inc.(3)(4)(9) |
Medical Devices & Equipment | Warrant | Common Stock | 39,364 | 242 | 20 | ||||||||||||
| IntegenX, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series C | 547,752 | 15 | 35 | ||||||||||||
| Medrobotics Corporation(15) |
Medical Devices & Equipment | Warrant | Preferred Series E | 455,539 | 370 | 292 | ||||||||||||
| Micell Technologies, Inc. |
Medical Devices & Equipment | Warrant | Preferred Series D-2 | 84,955 | 262 | 347 | ||||||||||||
| NetBio, Inc. |
Medical Devices & Equipment | Warrant | Preferred Series A | 7,841 | 408 | 158 | ||||||||||||
| NinePoint Medical, Inc.(15) |
Medical Devices & Equipment | Warrant | Preferred Series A-1 | 587,840 | 170 | 65 | ||||||||||||
| Optiscan Biomedical, Corp.(5)(15) |
Medical Devices & Equipment | Warrant | Preferred Series D | 10,535,275 | 1,252 | 170 | ||||||||||||
| Outset Medical, Inc. (p.k.a. Home Dialysis Plus, Inc.) |
Medical Devices & Equipment | Warrant | Preferred Series A | 500,000 | 402 | 355 | ||||||||||||
| Quanterix Corporation |
Medical Devices & Equipment | Warrant | Preferred Series C | 173,428 | 180 | 104 | ||||||||||||
| SonaCare Medical, LLC (p.k.a. US HIFU, LLC) |
Medical Devices & Equipment | Warrant | Preferred Series A | 6,464 | 188 | — | ||||||||||||
| Strata Skin Sciences, Inc. (p.k.a. MELA Sciences, Inc.)(3) |
Medical Devices & Equipment | Warrant | Common Stock | 69,320 | 402 | — | ||||||||||||
| ViewRay, Inc.(3)(15) |
Medical Devices & Equipment | Warrant | Common Stock | 128,231 | 333 | 2 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Medical Devices & Equipment (0.31%)* |
|
6,149 | 2,441 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Semiconductors |
||||||||||||||||||
| Achronix Semiconductor Corporation(15) |
Semiconductors | Warrant | Preferred Series C | 360,000 | 160 | 71 | ||||||||||||
| Semiconductors | Warrant | Preferred Series D-1 | 500,000 | 7 | 25 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Achronix Semiconductor Corporation |
860,000 | 167 | 96 | |||||||||||||||
| Aquantia Corp. |
Semiconductors | Warrant | Preferred Series G | 196,831 | 4 | 88 | ||||||||||||
| Avnera Corporation |
Semiconductors | Warrant | Preferred Series E | 141,567 | 46 | 114 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Semiconductors (0.04%)* |
|
217 | 298 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Software |
||||||||||||||||||
| Actifio, Inc. |
Software | Warrant | Common Stock | 73,584 | 249 | 83 | ||||||||||||
| Software | Warrant | Preferred Series F | 31,673 | 343 | 54 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Actifio, Inc. |
105,257 | 592 | 137 | |||||||||||||||
See notes to consolidated financial statements.
F-119
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| Braxton Technologies, LLC |
Software | Warrant | Preferred Series A | 168,750 | $ | 188 | $ | — | ||||||||||
| CareCloud Corporation(15) |
Software | Warrant | Preferred Series B | 413,433 | 258 | 488 | ||||||||||||
| Clickfox, Inc.(15) |
Software | Warrant | Preferred Series B | 1,038,563 | 330 | 63 | ||||||||||||
| Software | Warrant | Preferred Series C | 592,019 | 730 | 76 | |||||||||||||
| Software | Warrant | Preferred Series C-A | 2,218,214 | 230 | 1,604 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Clickfox, Inc. |
3,848,796 | 1,290 | 1,743 | |||||||||||||||
| Cloud Technology Partners, Inc. |
Software | Warrant | Preferred Series C | 113,960 | 34 | 35 | ||||||||||||
| Evernote Corporation(15) |
Software | Warrant | Common Stock | 62,500 | 106 | 110 | ||||||||||||
| JumpStart Games, Inc. (p.k.a Knowledge Holdings, Inc.)(15) |
Software | Warrant | Preferred Series E | 614,333 | 16 | — | ||||||||||||
| Mattersight Corporation(3) |
Software | Warrant | Common Stock | 357,143 | 538 | 386 | ||||||||||||
| Message Systems, Inc.(15) |
Software | Warrant | Preferred Series C | 503,718 | 334 | 325 | ||||||||||||
| Mobile Posse, Inc.(15) |
Software | Warrant | Preferred Series C | 396,430 | 130 | 102 | ||||||||||||
| Neos, Inc.(15) |
Software | Warrant | Common Stock | 221,150 | 22 | 64 | ||||||||||||
| NewVoiceMedia Limited(4)(9) |
Software | Warrant | Preferred Series E | 225,586 | 33 | 45 | ||||||||||||
| OneLogin, Inc.(15) |
Software | Warrant | Common Stock | 228,972 | 150 | 188 | ||||||||||||
| Poplicus, Inc.(15) |
Software | Warrant | Preferred Series C | 2,595,230 | — | 6 | ||||||||||||
| Quid, Inc.(15) |
Software | Warrant | Preferred Series D | 71,576 | 1 | 8 | ||||||||||||
| RedSeal Inc.(15) |
Software | Warrant | Preferred Series C-Prime | 640,603 | 66 | 65 | ||||||||||||
| Signpost, Inc.(15) |
Software | Warrant | Preferred Series C | 324,005 | 314 | 167 | ||||||||||||
| Soasta, Inc.(15) |
Software | Warrant | Preferred Series E | 410,800 | 691 | 190 | ||||||||||||
| Sonian, Inc.(15) |
Software | Warrant | Preferred Series C | 185,949 | 106 | 105 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Software (0.53%)* |
|
4,869 | 4,164 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Specialty Pharmaceuticals |
||||||||||||||||||
| Alimera Sciences, Inc.(3) |
Specialty Pharmaceuticals | Warrant | Common Stock | 1,717,709 | 860 | 421 | ||||||||||||
| QuatRx Pharmaceuticals Company | Specialty Pharmaceuticals | Warrant | Preferred Series E | 155,324 | 308 | — | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Specialty Pharmaceuticals (0.05%)* |
|
1,168 | 421 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Surgical Devices |
||||||||||||||||||
| Gynesonics, Inc.(15) |
Surgical Devices | Warrant | Preferred Series C | 180,480 | 75 | 14 | ||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 1,575,965 | 320 | 240 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Gynesonics, Inc. |
1,756,445 | 395 | 254 | |||||||||||||||
| Transmedics, Inc. |
Surgical Devices | Warrant | Preferred Series B | 40,436 | 225 | 16 | ||||||||||||
| Surgical Devices | Warrant | Preferred Series D | 175,000 | 100 | 405 | |||||||||||||
| Surgical Devices | Warrant | Preferred Series F | 50,544 | 38 | 56 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Transmedics, Inc. |
265,980 | 363 | 477 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Surgical Devices (0.09%)* |
|
758 | 731 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Sustainable and Renewable Technology |
||||||||||||||||||
| Agrivida, Inc. (15) | Sustainable and Renewable Technology | Warrant | Preferred Series D | 471,327 | 120 | 99 | ||||||||||||
| Alphabet Energy, Inc.(15) | Sustainable and Renewable Technology | Warrant | Preferred Series A | 86,329 | 82 | — | ||||||||||||
| American Superconductor Corporation(3) | Sustainable and Renewable Technology | Warrant | Common Stock | 58,823 | 39 | 85 | ||||||||||||
| Beamreach Solar (p.k.a. Solexel, Inc.)(15) | Sustainable and Renewable Technology | Warrant | Preferred Series C | 1,171,625 | 1,162 | — | ||||||||||||
| Brightsource Energy, Inc. | Sustainable and Renewable Technology | Warrant | Preferred Series 1 | 116,666 | 104 | — | ||||||||||||
| Calera, Inc.(15) | Sustainable and Renewable Technology | Warrant | Preferred Series C | 44,529 | 513 | — | ||||||||||||
See notes to consolidated financial statements.
F-120
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| Portfolio Company |
Sub-Industry |
Type of Investment(1) |
Series |
Shares | Cost(2) | Value(3) | ||||||||||||
| EcoMotors, Inc.(15) | Sustainable and Renewable Technology | Warrant | Preferred Series B | 437,500 | $ | 308 | $ | 30 | ||||||||||
| Fluidic, Inc. | Sustainable and Renewable Technology | Warrant | Preferred Series D | 61,804 | 102 | 20 | ||||||||||||
| Flywheel Building Intelligence, Inc. (p.k.a. SCIEnergy, Inc.) | Sustainable and Renewable Technology | Warrant | Common Stock | 530,811 | 181 | — | ||||||||||||
| Sustainable and Renewable Technology | Warrant | Preferred Series 2-A | 6,229 | 50 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Flywheel Building Intelligence, Inc. (p.k.a. |
537,040 | 231 | — | |||||||||||||||
| Fulcrum Bioenergy, Inc. | Sustainable and Renewable Technology | Warrant | Preferred Series C-1 | 280,897 | 275 | 201 | ||||||||||||
| GreatPoint Energy, Inc.(15) | Sustainable and Renewable Technology | Warrant | Preferred Series D-1 | 393,212 | 548 | — | ||||||||||||
| Polyera Corporation(15) | Sustainable and Renewable Technology | Warrant | Preferred Series C | 311,609 | 338 | — | ||||||||||||
| Proterra, Inc. | Sustainable and Renewable Technology | Warrant | Preferred Series 4 | 477,517 | 41 | 457 | ||||||||||||
| Rive Technology, Inc.(15) | Sustainable and Renewable Technology | Warrant | Preferred Series E | 234,477 | 12 | 3 | ||||||||||||
| Stion Corporation(5) | Sustainable and Renewable Technology | Warrant | Preferred Series Seed | 2,154 | 1,378 | — | ||||||||||||
| Sungevity, Inc. | Sustainable and Renewable Technology | Warrant | Common Stock | 20,000,000 | 543 | — | ||||||||||||
| Sustainable and Renewable Technology | Warrant | Preferred Series C | 32,472,222 | 902 | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||
| Total Sungevity, Inc. |
52,472,222 | 1,445 | — | |||||||||||||||
| TAS Energy, Inc. | Sustainable and Renewable Technology | Warrant | Preferred Series AA | 428,571 | 299 | — | ||||||||||||
| Tendril Networks | Sustainable and Renewable Technology | Warrant | Preferred Series 3-A | 1,019,793 | 189 | 219 | ||||||||||||
| Trilliant, Inc.(15) | Sustainable and Renewable Technology | Warrant | Preferred Series A | 320,000 | 162 | 202 | ||||||||||||
|
|
|
|
|
|||||||||||||||
| Subtotal: Sustainable and Renewable Technology (0.17%)* |
|
7,348 | 1,316 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total: Warrant Investments (3.49%)* |
|
45,014 | 27,485 | |||||||||||||||
|
|
|
|
|
|||||||||||||||
| Total Investments (180.72%)* |
|
$ | 1,511,526 | $ | 1,423,942 | |||||||||||||
|
|
|
|
|
|||||||||||||||
| * | Value as a percent of net assets |
| (1) | Preferred and common stock, warrants, and equity interests are generally non-income producing. |
| (2) | Gross unrealized appreciation, gross unrealized depreciation, and net depreciation for federal income tax purposes totaled $24.7 million, $114.5 million and $89.8 million respectively. The tax cost of investments is $1.5 billion. |
| (3) | Except for warrants in 37 publicly traded companies and common stock in 19 publicly traded companies, all investments are restricted at December 31, 2016 and were valued at fair value as determined in good faith by the Company’s board of directors (the “Board of Directors”). No unrestricted securities of the same issuer are outstanding. The Company uses the Standard Industrial Code for classifying the industry grouping of its portfolio companies. |
| (4) | Non-U.S. company or the company’s principal place of business is outside the United States. |
| (5) | Affiliate investment as defined under the Investment Company Act of 1940, as amended, (the “1940 Act”) in which Hercules owns at least 5% but generally less than 25% of the company’s voting securities. |
| (6) | Control investment as defined under the 1940 Act in which Hercules owns at least 25% of the company’s voting securities or has greater than 50% representation on its board. |
See notes to consolidated financial statements.
F-121
HERCULES CAPITAL, INC.
CONSOLIDATED SCHEDULE OF INVESTMENTS
December 31, 2016
(unaudited)
(dollars in thousands)
| (7) | Debt is on non-accrual status at December 31, 2016, and is therefore considered non-income producing. Note that at December 31, 2016, only the $11.0 million PIK, or payment-in-kind, loan is on non-accrual for the Company’s debt investment in Tectura Corporation. |
| (8) | Denotes that all or a portion of the debt investment is convertible debt. |
| (9) | Indicates assets that the Company deems not “qualifying assets” under section 55(a) of 1940 Act. Qualifying assets must represent at least 70% of the Company’s total assets at the time of acquisition of any additional non-qualifying assets. |
| (10) | Denotes that all or a portion of the debt investment secures the notes offered in the Debt Securitization (as defined in Note 4). |
| (11) | Denotes that all or a portion of the debt investment is pledged as collateral under the Wells Facility (as defined in Note 4). |
| (12) | Denotes that all or a portion of the debt investment is pledged as collateral under the Union Bank Facility (as defined in Note 4). |
| (13) | Denotes that all or a portion of the debt investment principal includes accumulated PIK interest and is net of repayments. |
| (14) | Denotes that all or a portion of the debt investment includes an exit fee receivable. |
| A. | This fee ranges from 1.0% to 5.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| B. | This fee ranges from 5.0% to 10.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| C. | This fee ranges from 10.0% to 15.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| D. | This fee is greater than 15.0% of the total debt commitment based on the contractual terms of our loan servicing agreements. |
| (15) | Denotes that all or a portion of the investment in this portfolio company is held by Hercules Technology II, L.P., or HT II, or Hercules Technology III, L.P., or HT III, the Company’s wholly owned small business investment companies, or SBIC, subsidiaries. |
| (16) | Denotes that the fair value of the Company’s total investments in this portfolio company represent greater than 5% of the Company’s total assets at December 31, 2016. |
| (17) | Denotes that there is an unfunded contractual commitment available at the request of this portfolio company at December 31, 2016. Refer to Note 10. |
| (18) | Repayment of debt investment is delinquent of the contractual maturity date as of December 31, 2016. |
| (19) | The stated PIK interest rate may be reduced to 1.45% subject to achievement of a milestone by the portfolio company. |
See notes to consolidated financial statements.
F-122
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(unaudited)
1. Description of Business and Basis of Presentation
Hercules Capital, Inc. (the “Company”) is a specialty finance company focused on providing senior secured loans to high-growth, innovative venture capital-backed companies in a variety of technology, life sciences, and sustainable and renewable technology industries. The Company sources its investments through its principal office located in Palo Alto, CA, as well as through its additional offices in Boston, MA, New York, NY, Washington, DC, Santa Monica, CA, Hartford, CT, and San Diego, CA. The Company was incorporated under the General Corporation Law of the State of Maryland in December 2003.
The Company is an internally managed, non-diversified closed-end investment company that has elected to be regulated as a business development company (“BDC”) under the Investment Company Act of 1940, as amended (the “1940 Act”). From incorporation through December 31, 2005, the Company was subject to tax as a corporation under Subchapter C of the Internal Revenue Code of 1986, as amended (the “Code”). Effective January 1, 2006, the Company elected to be treated for tax purposes as a regulated investment company, or RIC, under Subchapter M of the Code (see Note 5). As an investment company, the Company follows accounting and reporting guidance as set forth in Topic 946 (“Financial Services – Investment Companies”) of the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification, as amended (“ASC”).
Hercules Technology II, L.P. (“HT II”), Hercules Technology III, L.P. (“HT III”), and Hercules Technology IV, L.P. (“HT IV”), are Delaware limited partnerships that were formed in January 2005, September 2009 and December 2010, respectively. HT II and HT III were licensed to operate as small business investment companies (“SBICs”) under the authority of the Small Business Administration (“SBA”) on September 27, 2006 and May 26, 2010, respectively. As SBICs, HT II and HT III are subject to a variety of regulations concerning, among other things, the size and nature of the companies in which they may invest and the structure of those investments. HT IV was formed in anticipation of receiving an additional SBIC license; however, the Company has not received such license, and HT IV currently has no material assets or liabilities. The Company also formed Hercules Technology SBIC Management, LLC, or (“HTM”), a limited liability company in November 2003. HTM is a wholly owned subsidiary of the Company and serves as the limited partner and general partner of HT II and HT III (see Note 4 to the Company’s consolidated financial statements).
HT II and HT III hold approximately $104.8 million and $271.5 million in assets, respectively, and they accounted for approximately 5.8% and 14.9% of the Company’s total assets, respectively, prior to consolidation at June 30, 2017.
The Company also established wholly owned subsidiaries, all of which are structured as Delaware corporations and limited liability companies, to hold portfolio companies organized as limited liability companies, or LLCs (or other forms of pass-through entities). By investing through these wholly owned subsidiaries, the Company is able to benefit from the tax treatment of these entities and create a tax structure that is more advantageous with respect to the Company’s RIC status. These taxable subsidiaries are consolidated for financial reporting purposes and in accordance with U.S. generally accepted accounting principles (“GAAP”), and the portfolio investments held by these taxable subsidiaries are included in the Company’s consolidated financial statements and recorded at fair value. These taxable subsidiaries are not consolidated with Hercules for income tax purposes and may generate income tax expense, or benefit, and tax assets and liabilities as a result of their ownership of certain portfolio investments.
The consolidated financial statements include the accounts of the Company, its subsidiaries and its consolidated securitization VIE. All significant inter-company accounts and transactions have been eliminated in consolidation. In accordance with Article 10 of Regulation S-X, the Company does not consolidate portfolio company investments. It is not appropriate for an investment company to consolidate a portfolio company that is
F-123
not an investment company or that provides services to the Company. Rather, an investment company’s interest in portfolio companies that are not investment companies should be measured at fair value in accordance with ASC Topic 946.
The accompanying consolidated interim financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information, and pursuant to the requirements for reporting on Form 10-Q and Articles 6 and 10 of Regulation S-X. Accordingly, certain disclosures accompanying annual consolidated financial statements prepared in accordance with U.S. GAAP are omitted. In the opinion of management, all adjustments consisting solely of normal recurring accruals considered necessary for the fair presentation of consolidated financial statements for the interim periods have been included. The current period’s results of operations are not necessarily indicative of results that ultimately may be achieved for the full fiscal year. Therefore, the interim unaudited consolidated financial statements and notes should be read in conjunction with the audited consolidated financial statements and notes thereto for the period ended December 31, 2016. The year-end Consolidated Statement of Assets and Liabilities data was derived from audited financial statements, but does not include all disclosures required by U.S. GAAP.
Financial statements prepared on a GAAP basis require management to make estimates and assumptions that affect the amounts and disclosures reported in the consolidated financial statements and accompanying notes. Such estimates and assumptions could change in the future as more information becomes known, which could impact the amounts reported and disclosed herein.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The Consolidated Financial Statements include the accounts of the Company and its subsidiaries and all VIEs of which the Company is the primary beneficiary. All intercompany accounts and transactions have been eliminated in consolidation.
A VIE is an entity that either (i) has insufficient equity to permit the entity to finance its activities without additional subordinated financial support or (ii) has equity investors who lack the characteristics of a controlling financial interest. The primary beneficiary of a VIE is the party with both the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and the obligation to absorb the losses or the right to receive benefits that could be significant to the VIE.
To assess whether the Company has the power to direct the activities of a VIE that most significantly impact its economic performance, the Company considers all the facts and circumstances including its role in establishing the VIE and its ongoing rights and responsibilities. This assessment includes identifying the activities that most significantly impact the VIE’s economic performance and identifying which party, if any, has power over those activities. In general, the party that makes the most significant decisions affecting the VIE is determined to have the power to direct the activities of a VIE. To assess whether the Company has the obligation to absorb the losses or the right to receive benefits that could potentially be significant to the VIE, the Company considers all of its economic interests, including debt and equity interests, servicing rights and fee arrangements, and any other variable interests in the VIE. If the Company determines that it is the party with the power to make the most significant decisions affecting the VIE, and the Company has a potentially significant interest in the VIE, then it consolidates the VIE.
The Company performs periodic reassessments, usually quarterly, of whether it is the primary beneficiary of a VIE. The reassessment process considers whether the Company has acquired or divested the power to direct the activities of the VIE through changes in governing documents or other circumstances. The Company also reconsiders whether entities previously determined not to be VIEs have become VIEs, based on certain events, and therefore are subject to the VIE consolidation framework.
F-124
As of the date of this report, the only VIE consolidated by the Company is its securitization VIE formed in conjunction with the issuance of the 2021 Asset-Backed Notes (as defined herein). See “Note 4—Borrowings”.
Reclassification
Certain balances from prior years have been reclassified in order to conform to the current year presentation.
Valuation of Investments
The most significant estimate inherent in the preparation of the Company’s consolidated financial statements is the valuation of investments and the related amounts of unrealized appreciation and depreciation of investments recorded.
At June 30, 2017, approximately 87.8% of the Company’s total assets represented investments in portfolio companies whose fair value is determined in good faith by the Board of Directors. Value, as defined in Section 2(a)(41) of the 1940 Act, is (i) the market price for those securities for which a market quotation is readily available and (ii) for all other securities and assets, fair value is as determined in good faith by the Board of Directors. The Company’s investments are carried at fair value in accordance with the 1940 Act and ASC Topic 946 and measured in accordance with ASC Topic 820 (“Fair Value Measurements”). The Company’s debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of the Company’s investments in these portfolio companies are considered Level 3 assets under ASC Topic 820 because there is no known or accessible market or market indexes for these investment securities to be traded or exchanged. As such, the Company values substantially all of its investments at fair value as determined in good faith pursuant to a consistent valuation policy by the Company’s Board of Directors in accordance with the provisions of ASC Topic 820 and the 1940 Act. Due to the inherent uncertainty in determining the fair value of investments that do not have a readily available market value, the fair value of the Company’s investments determined in good faith by its Board of Directors may differ significantly from the value that would have been used had a readily available market existed for such investments, and the differences could be material.
The Company may from time to time engage an independent valuation firm to provide the Company with valuation assistance with respect to certain portfolio investments. The Company engages independent valuation firms on a discretionary basis. Specifically, on a quarterly basis, the Company will identify portfolio investments with respect to which an independent valuation firm will assist in valuing. The Company selects these portfolio investments based on a number of factors, including, but not limited to, the potential for material fluctuations in valuation results, credit quality and the time lapse since the last valuation of the portfolio investment by an independent valuation firm.
The Company intends to continue to engage an independent valuation firm to provide management with assistance regarding the Company’s determination of the fair value of selected portfolio investments each quarter unless directed by the Board of Directors to cancel such valuation services. The scope of services rendered by an independent valuation firm is at the discretion of the Board of Directors. The Company’s Board of Directors is ultimately, and solely, responsible for determining the fair value of the Company’s investments in good faith.
With respect to investments for which market quotations are not readily available or when such market quotations are deemed not to represent fair value, the Company’s Board of Directors has approved a multi-step valuation process each quarter, as described below:
(1) the Company’s quarterly valuation process begins with each portfolio company being initially valued by the investment professionals responsible for the portfolio investment;
F-125
(2) preliminary valuation conclusions are then documented and business based assumptions are discussed with the Company’s investment committee;
(3) the Audit Committee of the Board of Directors reviews the preliminary valuation of the investments in the portfolio as provided by the investment committee, which incorporates the results of the independent valuation firm as appropriate; and
(4) the Board of Directors, upon the recommendation of the Audit Committee, discusses valuations and determines the fair value of each investment in the Company’s portfolio in good faith based on the input of, where applicable, the respective independent valuation firm and the investment committee.
ASC Topic 820 establishes a framework for measuring the fair value of assets and liabilities and outlines a fair value hierarchy which prioritizes the inputs used to measure fair value and the effect of fair value measures on earnings. ASC Topic 820 also requires disclosure for fair value measurements based on the level within the hierarchy of the information used in the valuation. ASC Topic 820 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value. ASC Topic 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The Company has categorized all investments recorded at fair value in accordance with ASC Topic 820 based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels, defined by ASC Topic 820 and directly related to the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities, are as follows:
Level 1—Inputs are unadjusted, quoted prices in active markets for identical assets at the measurement date. The types of assets carried at Level 1 fair value generally are equities listed in active markets.
Level 2—Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset in connection with market data at the measurement date and for the extent of the instrument’s anticipated life. Fair valued assets that are generally included in this category are publicly held debt investments and warrants held in a public company.
Level 3—Inputs reflect management’s best estimate of what market participants would use in pricing the asset at the measurement date. It includes prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. Generally, assets carried at fair value and included in this category are the debt investments and warrants and equities held in a private company.
Investments measured at fair value on a recurring basis are categorized in the tables below based upon the lowest level of significant input to the valuations as of June 30, 2017 and as of December 31, 2016. The Company transfers investments in and out of Level 1, 2 and 3 as of the beginning balance sheet date, based on changes in the use of observable and unobservable inputs utilized to perform the valuation for the period. During the six months ended June 30, 2017, there were no transfers between Levels 1 or 2.
| (in thousands) Description |
Balance June 30, 2017 |
Quoted Prices In Active Markets For Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Senior Secured Debt |
$ | 1,287,623 | $ | — | $ | — | $ | 1,287,623 | ||||||||
| Preferred Stock |
43,385 | — | — | 43,385 | ||||||||||||
| Common Stock |
31,931 | 8,361 | — | 23,570 | ||||||||||||
| Warrants |
32,530 | — | 8,426 | 24,104 | ||||||||||||
| Escrow Receivable |
2,171 | — | — | 2,171 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,397,640 | $ | 8,361 | $ | 8,426 | $ | 1,380,853 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-126
| (in thousands) Description |
Balance December 31, 2016 |
Quoted Prices In Active Markets For Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Senior Secured Debt |
$ | 1,328,803 | $ | — | $ | 4,825 | $ | 1,323,978 | ||||||||
| Preferred Stock |
39,418 | — | — | 39,418 | ||||||||||||
| Common Stock |
28,236 | 17,271 | — | 10,965 | ||||||||||||
| Warrants |
27,485 | — | 3,239 | 24,246 | ||||||||||||
| Escrow Receivable |
1,382 | — | — | 1,382 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,425,324 | $ | 17,271 | $ | 8,064 | $ | 1,399,989 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The table below presents a reconciliation for all financial assets and liabilities measured at fair value on a recurring basis, excluding accrued interest components, using significant unobservable inputs (Level 3) for the six months ended June 30, 2017 and the year ended December 31, 2016.
| (in thousands) |
Balance January 1, 2017 |
Net Realized Gains (Losses)(1) |
Net Change in Unrealized Appreciation (Depreciation)(2) |
Purchases(5) | Sales | Repayments(6) | Gross Transfers into Level 3(3) |
Gross Transfers out of Level 3(3) |
Balance June 30, 2017 |
|||||||||||||||||||||||||||
| Senior Debt |
$ | 1,323,978 | $ | — | $ | 17,265 | $ | 347,275 | $ | — | $ | (338,224 | ) | $ | — | $ | (62,671 | ) | $ | 1,287,623 | ||||||||||||||||
| Preferred Stock |
39,418 | (7,263 | ) | 11,293 | 173 | (236 | ) | — | — | — | 43,385 | |||||||||||||||||||||||||
| Common Stock |
10,965 | — | (53,814 | ) | 3,748 | — | — | 62,671 | — | 23,570 | ||||||||||||||||||||||||||
| Warrants |
24,246 | 1,173 | 4,389 | 1,286 | (6,990 | ) | — | — | — | 24,104 | ||||||||||||||||||||||||||
| Escrow Receivable |
1,382 | 20 | — | 2,737 | (1,968 | ) | — | — | — | 2,171 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 1,399,989 | $ | (6,070 | ) | $ | (20,867 | ) | $ | 355,219 | $ | (9,194 | ) | $ | (338,224 | ) | $ | 62,671 | $ | (62,671 | ) | $ | 1,380,853 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| (in thousands) |
Balance January 1, 2016 |
Net Realized Gains (Losses)(1) |
Net Change in Unrealized Appreciation (Depreciation)(2) |
Purchases(5) | Sales | Repayments(6) | Gross Transfers into Level 3(4) |
Gross Transfers out of Level 3(4) |
Balance December 31, 2016 |
|||||||||||||||||||||||||||
| Senior Debt |
$ | 1,102,396 | $ | (6,968 | ) | $ | (12,675 | ) | $ | 687,353 | $ | — | $ | (441,567 | ) | $ | — | $ | (4,561 | ) | $ | 1,323,978 | ||||||||||||||
| Preferred Stock |
35,245 | (334 | ) | (7,864 | ) | 13,873 | (1,367 | ) | — | 626 | (761 | ) | 39,418 | |||||||||||||||||||||||
| Common Stock |
1,527 | — | (1,404 | ) | 6,081 | — | — | 4,761 | — | 10,965 | ||||||||||||||||||||||||||
| Warrants |
18,565 | (116 | ) | 3,465 | 4,082 | (1,186 | ) | — | — | (564 | ) | 24,246 | ||||||||||||||||||||||||
| Escrow Receivable |
2,967 | (6 | ) | — | 2,009 | (3,588 | ) | — | — | — | 1,382 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 1,160,700 | $ | (7,424 | ) | $ | (18,478 | ) | $ | 713,398 | $ | (6,141 | ) | $ | (441,567 | ) | $ | 5,387 | $ | (5,886 | ) | $ | 1,399,989 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| (1) | Included in net realized gains or losses in the accompanying Consolidated Statement of Operations. |
| (2) | Included in net change in unrealized appreciation (depreciation) in the accompanying Consolidated Statement of Operations. |
| (3) | Transfers into Level 3 during the six months ended June 30, 2017 relate to the conversion of the Company’s debt investment in Sungevity, Inc. and a portion of the Company’s debt investment in Gamma Medica, Inc. to common stock through bankruptcy transactions. Transfers out of Level 3 during the six months ended June 30, 2017 relate to the conversion of the Company’s debt investment in Sungevity, Inc. and a portion of the Company’s debt investment in Gamma Medica, Inc. to common stock through bankruptcy transactions. |
| (4) | Transfers into Level 3 during the year ended December 31, 2016 relate to the acquisition of preferred stock as a result of the exercise of warrants in Ping Identity Corporation, the conversion of debt to equity in Optiscan Biomedical Corp and Achilles Technology Management Co II, Inc. and the conversion of the Company’s preferred shares to common shares in SCIEnergy, Inc. Transfers out of Level 3 during the year ended December 31, 2016 relate to the exercise of warrants in TPI Composites, Inc. and Touchcommerce, Inc. to common stock in an initial public offering, or IPO, and acquisition, respectively; the exercise of warrants in Ping Identity Corporation to preferred stock; the conversion of debt to equity in Optiscan Biomedical Corp and Achilles Technology Management Co II, Inc. and the conversion of the Company’s preferred shares to common shares in SCIEnergy, Inc. |
| (5) | Amounts listed above are inclusive of loan origination fees received at the inception of the loan which are deferred and amortized into fee income as well as the accretion of existing loan discounts and fees during the period. Escrow receivable purchases may include additions due to proceeds held in escrow from the liquidation of level 3 investments. |
| (6) | Amounts listed above include the acceleration and payment of loan discounts and loan fees due to early payoffs or restructures. |
For the six months ended June 30, 2017, approximately $3.8 million in net unrealized appreciation and $53.8 million in net unrealized depreciation was recorded for preferred stock and common stock Level 3 investments, respectively, relating to assets still held at the reporting date. The depreciation on common stock during the period reflects the conversion of the Company’s debt investment in Sungevity, Inc. to common stock
F-127
at cost through a bankruptcy transaction and subsequent depreciation to fair value. For the same period, approximately $2.6 million and $5.3 million in net unrealized appreciation was recorded for debt and warrant Level 3 investments, respectively, relating to assets still held at the reporting date.
For the year ended December 31, 2016, approximately $9.1 million and $1.4 million in net unrealized depreciation was recorded for preferred stock and common stock Level 3 investments, respectively, relating to assets still held at the reporting date. For the same period, approximately $25.7 million in net unrealized depreciation and $2.8 million in net unrealized appreciation was recorded for debt and warrant Level 3 investments, respectively, relating to assets still held at the reporting date.
The following tables provide quantitative information about the Company’s Level 3 fair value measurements as of June 30, 2017 and December 31, 2016. In addition to the techniques and inputs noted in the tables below, according to the Company’s valuation policy the Company may also use other valuation techniques and methodologies when determining the Company’s fair value measurements. The tables below are not intended to be all-inclusive, but rather provide information on the significant Level 3 inputs as they relate to the Company’s fair value measurements.
The significant unobservable input used in the fair value measurement of the Company’s escrow receivables is the amount recoverable at the contractual maturity date of the escrow receivable.
| Investment Type - Level Three Debt Investments |
Fair Value at June 30, 2017 (in thousands) |
Valuation Techniques/ |
Unobservable Input(a) |
Range | Weighted Average(b) |
|||||||||
| Pharmaceuticals |
$ 107,497 | Originated Within 6 Months | Origination Yield | 11.25% - 13.41% | 12.60% | |||||||||
| 484,125 | Market Comparable Companies | Hypothetical Market Yield | 8.93% - 15.48% | 12.88% | ||||||||||
| Premium/(Discount) | (0.25%) - 0.75% | |||||||||||||
| — | Liquidation(c) | Probability weighting of alternative outcomes | 100.00% | |||||||||||
| Technology |
130,894 | Originated Within 6 Months | Origination Yield | 10.98% - 14.88% | 12.17% | |||||||||
| 183,058 | Market Comparable Companies | Hypothetical Market Yield | 9.13% - 18.50% | 13.48% | ||||||||||
| Premium/(Discount) | (0.25%) - 1.00% | |||||||||||||
| 4,576 | Liquidation(c) | Probability weighting of alternative outcomes | 100.00% | |||||||||||
| Sustainable and Renewable |
82,118 | Market Comparable Companies | Hypothetical Market Yield | 11.91% - 14.63% | 13.37% | |||||||||
| Premium/(Discount) | 0.00% - 0.25% | |||||||||||||
| Medical Devices |
45,082 | Originated Within 6 Months | Origination Yield | 10.38% - 13.18% | 11.06% | |||||||||
| 47,142 | Market Comparable Companies | Hypothetical Market Yield | 9.49% - 18.53% | 13.57% | ||||||||||
| Premium/(Discount) | 0.00% - 0.75% | |||||||||||||
| — | Liquidation(c) | Probability weighting of alternative outcomes | 50.00% | |||||||||||
| Lower Middle Market |
19,991 | Market Comparable Companies | Hypothetical Market Yield | 9.03% | 9.03% | |||||||||
| Premium/(Discount) | 0.50% | |||||||||||||
| — | Liquidation(c) | Probability weighting of alternative outcomes | 100.00% | |||||||||||
| Debt Investments Where Fair Value Approximates Cost |
| |||||||||||||
| 40,466 | Imminent Payoffs(d) | |||||||||||||
| 142,674 | Debt Investments Maturing in Less than One Year | |||||||||||||
|
|
|
|||||||||||||
| $1,287,623 | Total Level Three Debt Investments | |||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s debt securities are hypothetical market yields and premiums/(discounts). The hypothetical market yield is defined as the exit price of an investment in a hypothetical market to hypothetical market participants where buyers and sellers are willing participants. The premiums (discounts) relate to company specific characteristics such as underlying investment performance, security liens, and other characteristics of the investment. Significant increases (decreases) in the inputs in isolation may result in a significantly lower (higher) fair value measurement, depending on the materiality of the investment. Debt investments in the industries noted in the Company’s Consolidated Schedule of Investments are included in the industries noted above as follows: |
| • | Pharmaceuticals, above, is comprised of debt investments in the Specialty Pharmaceuticals, Drug Discovery and Development, Drug Delivery and Biotechnology Tools industries in the Consolidated Schedule of Investments. |
F-128
| • | Technology, above, is comprised of debt investments in the Software, Semiconductors, Internet Consumer and Business Services, Consumer and Business Products, Information Services, and Communications and Networking industries in the Consolidated Schedule of Investments. |
| • | Sustainable and Renewable Technology, above, aligns with the Sustainable and Renewable Technology Industry in the Consolidated Schedule of Investments. |
| • | Medical Devices, above, is comprised of debt investments in the Surgical Devices and Medical Devices and Equipment industries in the Consolidated Schedule of Investments. |
| • | Lower Middle Market, above, is comprised of debt investments in the Communications and Networking, Electronics and Computer Hardware, Healthcare Services - Other, Information Services, Internet Consumer and Business Services, Media/Content/Info, and Specialty Pharmaceuticals industries in the Consolidated Schedule of Investments. |
| (b) | The weighted averages are calculated based on the fair market value of each investment. |
| (c) | The significant unobservable input used in the fair value measurement of impaired debt securities is the probability weighting of alternative outcomes. |
| (d) | Imminent payoffs represent debt investments that the Company expects to be fully repaid within the next three months, prior to their scheduled maturity date. |
| Investment Type - Level Three Debt Investments |
Fair Value
at December 31, 2016 (in thousands) |
Valuation Techniques/ Methodologies |
Unobservable Input(a) |
Range | Weighted Average(b) |
|||||||||
| Pharmaceuticals |
$ 102,412 | Originated Within 6 Months | Origination Yield | 12.24% - 14.59% | 13.64% | |||||||||
| 434,718 | Market Comparable Companies | Hypothetical Market Yield | 9.07% - 15.62% | 12.44% | ||||||||||
| Premium/(Discount) | (0.25%) - 0.75% | |||||||||||||
| 2,693 | Liquidation(c) | Probability weighting of alternative outcomes | 25.00% - 100.00% | |||||||||||
| Technology |
93,674 | Originated Within 6 Months | Origination Yield | 7.29% - 16.53% | 13.69% | |||||||||
| 325,553 | Market Comparable Companies | Hypothetical Market Yield | 10.14% - 21.66% | 12.69% | ||||||||||
| Premium/(Discount) | (0.50%) - 0.50% | |||||||||||||
| 24,706 | Liquidation(c) | Probability weighting of alternative outcomes | 20.00% - 100.00% | |||||||||||
| Sustainable and Renewable |
99,286 | Market Comparable Companies | Hypothetical Market Yield | 11.77% - 16.84% | 13.45% | |||||||||
| Technology |
Premium/(Discount) | 0.00% - 0.25% | ||||||||||||
| 44,626 | Liquidation(c) | Probability weighting of alternative outcomes | 10.00% - 40.00% | |||||||||||
| Medical Devices |
88,983 | Market Comparable Companies | Hypothetical Market Yield | 10.25% - 18.60% | 14.01% | |||||||||
| Premium/(Discount) | (0.25%) - 0.75% | |||||||||||||
| Lower Middle Market |
25,017 | Market Comparable Companies | Hypothetical Market Yield | 8.85% - 15.79% | 10.10% | |||||||||
| Premium/(Discount) | 0.00% - 0.25% | |||||||||||||
| 13,148 | Liquidation(c) | Probability weighting of alternative outcomes | 100.00% | |||||||||||
| Debt Investments Where Fair Value Approximates Cost |
| |||||||||||||
| 25,000 | Imminent Payoffs(d) | |||||||||||||
| 44,162 | Debt Investments Maturing in Less than One Year | |||||||||||||
|
|
|
|||||||||||||
| $1,323,978 | Total Level Three Debt Investments | |||||||||||||
|
|
|
|||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s debt securities are hypothetical market yields and premiums/(discounts). The hypothetical market yield is defined as the exit price of an investment in a hypothetical market to hypothetical market participants where buyers and sellers are willing participants. The premiums (discounts) relate to company specific characteristics such as underlying investment performance, security liens, and other characteristics of the investment. Significant increases (decreases) in the inputs in isolation may result in a significantly lower (higher) fair value measurement, depending on the materiality of the investment. Debt investments in the industries noted in the Company’s Consolidated Schedule of Investments are included in the industries noted above as follows: |
| • | Pharmaceuticals, above, is comprised of debt investments in the Specialty Pharmaceuticals, Drug Discovery and Development and Drug Delivery industries in the Consolidated Schedule of Investments. |
| • | Technology, above, is comprised of debt investments in the Software, Semiconductors, Internet Consumer and Business Services, Consumer and Business Products, Information Services, and Communications and Networking industries in the Consolidated Schedule of Investments. |
| • | Sustainable and Renewable Technology, above, aligns with the Sustainable and Renewable Technology Industry in the Consolidated Schedule of Investments. |
| • | Medical Devices, above, is comprised of debt investments in the Surgical Devices and Medical Devices and Equipment industries in the Consolidated Schedule of Investments. |
F-129
| • | Lower Middle Market, above, is comprised of debt investments in the Communications and Networking, Electronics and Computer Hardware, Healthcare Services - Other, Information Services, Internet Consumer and Business Services, Media/Content/Info, and Specialty Pharmaceuticals industries in the Consolidated Schedule of Investments. |
| (b) | The weighted averages are calculated based on the fair market value of each investment. |
| (c) | The significant unobservable input used in the fair value measurement of impaired debt securities is the probability weighting of alternative outcomes. |
| (d) | Imminent payoffs represent debt investments that the Company expects to be fully repaid within the next three months, prior to their scheduled maturity date. |
| Investment Type - Level Three Equity and Warrant Investments |
Fair Value at June 30, 2017 (in thousands) |
Valuation Techniques/ Methodologies |
Unobservable Input(a) |
Range | Weighted Average(f) | |||||||
| Equity Investments |
$ 9,102 | Market Comparable Companies | EBITDA Multiple(b) | 5.2x - 57.5x | 16.8x | |||||||
| Revenue Multiple(b) | 0.8x - 11.3x | 3.9x | ||||||||||
| Discount for Lack of Marketability(c) | 11.18% - 22.41% | 13.91% | ||||||||||
| Average Industry Volatility(d) | 39.43% - 65.04% | 50.92% | ||||||||||
| Risk-Free Interest Rate | 1.20% - 1.49% | 1.25% | ||||||||||
| Estimated Time to Exit (in months) | 9 - 32 | 14 | ||||||||||
| 23,388 | Market Adjusted OPM Backsolve | Market Equity Adjustment(e) | (20.33%) - 42.06% | 16.12% | ||||||||
| Average Industry Volatility(d) | 28.99% - 109.39% | 78.43% | ||||||||||
| Risk-Free Interest Rate | 0.70% - 1.44% | 1.10% | ||||||||||
| Estimated Time to Exit (in months) | 5 - 29 | 11 | ||||||||||
| 34,465 | Other(g) | |||||||||||
| Warrant Investments |
16,570 | Market Comparable Companies | EBITDA Multiple(b) | 5.0x - 57.5x | 15.6x | |||||||
| Revenue Multiple(b) | 0.3x - 7.7x | 2.9x | ||||||||||
| Discount for Lack of Marketability(c) | 10.15% - 33.15% | 16.25% | ||||||||||
| Average Industry Volatility(d) | 33.86% - 105.93% | 52.46% | ||||||||||
| Risk-Free Interest Rate | 1.14% - 1.70% | 1.31% | ||||||||||
| Estimated Time to Exit (in months) | 6 - 47 | 18 | ||||||||||
| 7,534 | Market Adjusted OPM Backsolve | Market Equity Adjustment(e) | (83.25%) - 149.47% | 12.71% | ||||||||
| Average Industry Volatility(d) | 28.99% - 109.88% | 76.99% | ||||||||||
| Risk-Free Interest Rate | 0.73% - 1.82% | 1.19% | ||||||||||
| Estimated Time to Exit (in months) | 10 - 48 | 17 | ||||||||||
|
|
|
|||||||||||
| Total Level Three Warrant and Equity Investments |
$91,059 | |||||||||||
|
|
|
|||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s warrant and equity-related securities are revenue and/or EBITDA multiples, market equity adjustment factors, and discounts for lack of marketability. Additional inputs used in the Black Scholes option pricing model include industry volatility, risk free interest rate and estimated time to exit. Significant increases (decreases) in the inputs in isolation would result in a significantly higher (lower) fair value measurement, depending on the materiality of the investment. For some investments, additional consideration may be given to data from the last round of financing or merger/acquisition events near the measurement date. |
| (b) | Represents amounts used when the Company has determined that market participants would use such multiples when pricing the investments. |
| (c) | Represents amounts used when the Company has determined market participants would take into account these discounts when pricing the investments. |
| (d) | Represents the range of industry volatility used by market participants when pricing the investment. |
| (e) | Represents the range of changes in industry valuations since the portfolio company’s last external valuation event. |
| (f) | Weighted averages are calculated based on the fair market value of each investment. |
| (g) | The fair market value of these investments is derived based on recent private market and merger and acquisition transaction prices. |
F-130
| Investment Type - Level Three Equity and
Warrant |
Fair Value
at December 31, 2016 (in thousands) |
Valuation Techniques/ Methodologies |
Unobservable Input(a) | Range | Weighted Average(e) |
|||||||||||||
| Equity Investments |
$9,258 | Market Comparable Companies | EBITDA Multiple(b) | 0.0x - 38.7x | 12.3x | |||||||||||||
| Revenue Multiple(b) | 0.9x - 8.7x | 3.1x | ||||||||||||||||
| Discount for Lack of Marketability(c) | 13.75% - 25.97% | 16.73% | ||||||||||||||||
| Average Industry Volatility(d) | 45.54% - 113.16% | 61.06% | ||||||||||||||||
| Risk-Free Interest Rate | 0.79% - 1.50% | 0.91% | ||||||||||||||||
| Estimated Time to Exit (in months) | 10 - 38 | 15 | ||||||||||||||||
| 19,836 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) | 29.93% - 109.95% | 73.49% | ||||||||||||||
| Risk-Free Interest Rate | 0.65% - 1.44% | 0.92% | ||||||||||||||||
| Estimated Time to Exit (in months) | 10 - 34 | 15 | ||||||||||||||||
| 21,289 | Other(f) | |||||||||||||||||
| Warrant Investments |
8,959 | Market Comparable Companies | EBITDA Multiple(b) | 2.6x - 51.4x | 13.8x | |||||||||||||
| Revenue Multiple(b) | 0.4x - 6.1x | 2.5x | ||||||||||||||||
| Discount for Lack of Marketability(c) | 11.74% - 27.25% | 19.02% | ||||||||||||||||
| Average Industry Volatility(d) | 38.58% - 111.15% | 62.03% | ||||||||||||||||
| Risk-Free Interest Rate | 0.68% - 1.68% | 1.04% | ||||||||||||||||
| Estimated Time to Exit (in months) | 7 - 47 | 20 | ||||||||||||||||
| 9,713 | Market Adjusted OPM Backsolve | Average Industry Volatility(d) | 29.93% - 116.29% | 67.20% | ||||||||||||||
| Risk-Free Interest Rate | 0.45% - 1.84% | 0.99% | ||||||||||||||||
| Estimated Time to Exit (in months) | 3 - 47 | 20 | ||||||||||||||||
| 5,574 | Other(f) | |||||||||||||||||
|
|
|
|||||||||||||||||
| Total Level Three Warrant and Equity Investments | $74,629 | |||||||||||||||||
|
|
|
|||||||||||||||||
| (a) | The significant unobservable inputs used in the fair value measurement of the Company’s warrant and equity-related securities are revenue and/or EBITDA multiples and discounts for lack of marketability. Additional inputs used in the Black Scholes OPM include industry volatility, risk free interest rate and estimated time to exit. Significant increases (decreases) in the inputs in isolation may result in a significantly higher (lower) fair value measurement, depending on the materiality of the investment. For some investments, additional consideration may be given to data from the last round of financing or merger/acquisition events near the measurement date. |
| (b) | Represents amounts used when the Company has determined that market participants would use such multiples when pricing the investments. |
| (c) | Represents amounts used when the Company has determined market participants would take into account these discounts when pricing the investments. |
| (d) | Represents the range of industry volatility used by market participants when pricing the investment. |
| (e) | Weighted averages are calculated based on the fair market value of each investment. |
| (f) | The fair market value of these investments is derived based on recent private market and merger and acquisition transaction prices. |
Debt Investments
The Company follows the guidance set forth in ASC Topic 820 which establishes a framework for measuring the fair value of assets and liabilities and outlines a fair value hierarchy, which prioritizes the inputs used to measure fair value and the effect of fair value measures on earnings. The Company’s debt securities are primarily invested in venture capital-backed companies in technology-related industries including technology, drug discovery and development, biotechnology, life sciences, healthcare, and sustainable and renewable technology at all stages of development. Given the nature of lending to these types of businesses, substantially all of the Company’s investments in these portfolio companies are considered Level 3 assets under ASC Topic 820 because there is no known or accessible market or market indexes for debt instruments for these investment securities to be traded or exchanged. In addition, the Company may, from time to time, invest in public debt of companies that meet the Company’s investment objectives. These investments are considered Level 2 assets.
In making a good faith determination of the value of the Company’s investments, the Company generally starts with the cost basis of the investment, which includes the value attributed to the original issue discount (“OID”), if any, and payment-in-kind (“PIK”) interest or other receivables which have been accrued as earned. The Company then applies the valuation methods as set forth below.
The Company applies a procedure for debt investments that assumes the sale of each investment in a hypothetical market to a hypothetical market participant where buyers and sellers are willing participants. The hypothetical market does not include scenarios where the underlying security was simply repaid or extinguished, but includes an exit concept. The Company determines the yield at inception for each debt investment. The Company then uses senior secured, leveraged loan
F-131
yields provided by third party providers to determine the change in market yields between inception of the debt security and the measurement date. Industry specific indices and other relevant market data are used to benchmark/assess market based movements.
Under this process, the Company also evaluates the collateral for recoverability of the debt investments. The Company considers each portfolio company’s credit rating, security liens and other characteristics of the investment to adjust the baseline yield to derive a credit adjusted hypothetical yield for each investment as of the measurement date. The anticipated future cash flows from each investment are then discounted at the hypothetical yield to estimate each investment’s fair value as of the measurement date.
The Company’s process includes an analysis of, among other things, the underlying investment performance, the current portfolio company’s financial condition and market changing events that impact valuation, estimated remaining life, current market yield and interest rate spreads of similar securities as of the measurement date. The Company values its syndicated debt investments using broker quotes and bond indices amongst other factors. If there is a significant deterioration of the credit quality of a debt investment, the Company may consider other factors to estimate fair value, including the proceeds that would be received in a liquidation analysis.
The Company records unrealized depreciation on investments when it believes that an investment has decreased in value, including where collection of a debt investment is doubtful or, if under the in-exchange premise, when the value of a debt security is less than amortized cost of the investment. Conversely, where appropriate, the Company records unrealized appreciation if it believes that the underlying portfolio company has appreciated in value and, therefore, that its investment has also appreciated in value or, if under the in-exchange premise, the value of a debt security is greater than amortized cost.
When originating a debt instrument, the Company generally receives warrants or other equity-related securities from the borrower. The Company determines the cost basis of the warrants or other equity-related securities received based upon their respective fair values on the date of receipt in proportion to the total fair value of the debt and warrants or other equity-related securities received. Any resulting discount on the debt investments from recordation of the warrant or other equity instruments is accreted into interest income over the life of the debt investment.
Debt investments that are traded on a public exchange are valued at the prevailing market price as of the valuation date.
Equity-Related Securities and Warrants
Securities that are traded in the over-the-counter markets or on a stock exchange will be valued at the prevailing bid price at period end. The Company has a limited amount of equity securities in public companies. In accordance with the 1940 Act, unrestricted publicly traded securities for which market quotations are readily available are valued at the closing market quote on the measurement date.
The Company estimates the fair value of warrants using a Black Scholes OPM. At each reporting date, privately held warrant and equity-related securities are valued based on an analysis of various factors including, but not limited to, the portfolio company’s operating performance and financial condition and general market conditions, price to enterprise value or price to equity ratios, discounted cash flow, valuation comparisons to comparable public companies or other industry benchmarks. When an external event occurs, such as a purchase transaction, public offering, or subsequent equity sale, the pricing indicated by that external event is utilized to corroborate the Company’s valuation of the warrant and equity-related securities. The Company periodically reviews the valuation of its portfolio companies that have not been involved in a qualifying external event to determine if the enterprise value of the portfolio company may have increased or decreased since the last valuation measurement date.
F-132
Escrow Receivables
Escrow receivables are collected in accordance with the terms and conditions of the escrow agreement. Escrow balances are typically distributed over a period greater than one year and may accrue interest during the escrow period. Escrow balances are measured for collectability on at least a quarterly basis and fair value is determined based on the amount of the estimated recoverable balances and the contractual maturity date. As of June 30, 2017 there were no material past due escrow receivables.
Portfolio Composition
As required by the 1940 Act, the Company classifies its investments by level of control. “Control investments” are defined in the 1940 Act as investments in those companies that the Company is deemed to “control.” Under the 1940 Act, the Company is generally deemed to “control” a company in which it has invested if it owns 25% or more of the voting securities of such company or has greater than 50% representation on its board. “Affiliate investments” are investments in those companies that are “affiliated companies” of the Company, as defined in the 1940 Act, which are not control investments. The Company is deemed to be an “affiliate” of a company in which it has invested if it owns 5% or more, but generally less than 25%, of the voting securities of such company. “Non-control/non-affiliate investments” are investments that are neither control investments nor affiliate investments.
The following table summarizes the Company’s realized gains and losses and changes in our unrealized appreciation and depreciation on control and affiliate investments for the three and six months ended June 30, 2017 and 2016.
| (in thousands) | For the Three Months Ended June 30, 2017 | For the Six Months Ended June 30, 2017 | ||||||||||||||||||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at June 30, 2017 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/(Loss) |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/(Loss) |
||||||||||||||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||||||||||||||||||
| SkyCross, Inc. |
Control | $ | — | $ | — | $ | (261 | ) | $ | 394 | $ | (394 | ) | $ | — | $ | 1,842 | $ | 394 | $ | (394 | ) | ||||||||||||||||||
| Achilles Technology Management Co II, Inc. |
Control | 2,116 | 78 | (267 | ) | — | — | 152 | (2,208 | ) | — | — | ||||||||||||||||||||||||||||
| HercGamma, Inc. |
Control | 1,169 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Tectura Corporation |
Control | 19,991 | 454 | — | — | — | 899 | — | 51 | (51 | ) | |||||||||||||||||||||||||||||
| Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.) |
Control | 8,288 | — | (53,215 | ) | — | — | — | (53,214 | ) | — | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control Investments |
$ | 31,564 | $ | 532 | $ | (53,743 | ) | $ | 394 | $ | (394 | ) | $ | 1,051 | $ | (53,580 | ) | $ | 445 | $ | (445 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | $ | 5,991 | $ | — | $ | 681 | $ | — | $ | — | $ | — | $ | 1,119 | $ | — | $ | — | |||||||||||||||||||||
| Stion Corporation |
Affiliate | — | — | — | — | — | 2 | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Affiliate Investments |
$ | 5,991 | $ | — | $ | 681 | $ | — | $ | — | $ | 2 | $ | 1,119 | $ | — | $ | — | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control & Affiliate Investments |
$ | 37,555 | $ | 532 | $ | (53,062 | ) | $ | 394 | $ | (394 | ) | $ | 1,053 | $ | (52,461 | ) | $ | 445 | $ | (445 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
F-133
| (in thousands) | For the Three Months Ended June 30, 2016 | For the Six Months Ended June 30, 2016 | ||||||||||||||||||||||||||||||||||||||
| Portfolio Company |
Type | Fair Value at June 30, 2016 |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/(Loss) |
Investment Income |
Net Change in Unrealized Appreciation/ (Depreciation) |
Reversal of Unrealized Appreciation / (Depreciation)(1) |
Realized Gain/(Loss) |
||||||||||||||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||||||||||||||||||
| SkyCross, Inc. |
Control | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | |||||||||||||||||||
| Achilles Technology Management Co II, Inc. |
Control | 4,000 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control Investments |
$ | 4,000 | $ | — | $ | (3,421 | ) | $ | — | $ | — | $ | — | $ | (3,421 | ) | $ | — | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Affiliate | $ | 4,549 | $ | 6 | $ | (2,972 | ) | $ | — | $ | — | $ | 12 | $ | (3,386 | ) | $ | — | $ | — | |||||||||||||||||||
| Stion Corporation |
Affiliate | 1,295 | 44 | — | 648 | — | 103 | 539 | 648 | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Affiliate Investments |
$ | 5,844 | $ | 50 | $ | (2,972 | ) | $ | 648 | $ | — | $ | 115 | $ | (2,847 | ) | $ | 648 | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total Control & Affiliate Investments |
$ | 9,844 | $ | 50 | $ | (6,393 | ) | $ | 648 | $ | — | $ | 115 | $ | (6,268 | ) | $ | 648 | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| (1) | Represents reversals of prior period net unrealized depreciation upon being realized as a loss due to write off. |
In June 2017, the Company acquired 100% ownership of the equity in HercGamma, Inc. and classified it as a control investment in accordance with the requirements of the 1940 Act. In June 2017, HercGamma, Inc. acquired the assets of a medical device company that develops advanced digital imaging to detect breast cancer, as part of an article 9 consensual foreclosure and public auction for consideration with an estimated fair value of $1.2 million. The Company’s investment in HercGamma, Inc. is carried on the consolidated statement of assets and liabilities at fair value.
In April 2017, the Company’s investment in Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.) became classified as a control investment as a result of obtaining more than 25% of the portfolio company’s voting securities. In April 2017, under Section 363 of the Bankruptcy Code, Sungevity, Inc. entered into a $50.0 million asset purchase agreement and DIP financing facility with a group of investors, led by Northern Pacific Group and including the Company. On April 7, 2017, the U.S. Bankruptcy Court approved the DIP financing facility and on April 17, the U.S. Bankruptcy Court approved the asset purchase agreement. On April 26, 2017, Solar Spectrum Holdings LLC, a new company backed by the investment group, announced that it had acquired certain assets of Sungevity, Inc. as part of the bankruptcy court-approved sale. As a result, the cost basis of the Company’s debt investment in Sungevity, Inc. was converted to an equity position in Solar Spectrum Holdings LLC and the Company’s warrant and equity positions in Sungevity, Inc. were written off.
In January 2017, the Company’s investment in Tectura Corporation became classified as a control investment as a result of obtaining more than 50% representation on the portfolio company’s board. In March 2017, the Company’s warrants in Tectura Corporation expired and were written off for a realized loss.
In June 2016, the Company’s investments in SkyCross, Inc. became classified as a control investment as a result of obtaining more than 50% representation on the portfolio company’s board. In June 2016, the Company also acquired 100% ownership of the equity of Achilles Technology Management Co II, Inc. and classified it as a control investment in accordance with the requirements of the 1940 Act. In June 2016, Achilles Technology Management Co II, Inc. acquired the assets of a global antenna company that produces radio frequency system solutions as part of an article 9 consensual foreclosure and public auction for total consideration in the amount of $4.0 million. In September and November 2016, the Company made a $1.0 million and $250,000 debt investment, respectively, in Achilles Technology Management II to provide working capital under the terms of a loan servicing agreement. The Company’s investments in Achilles Technology Management Co II, Inc. are carried on the consolidated statement of assets and liabilities at fair value.
F-134
The following table shows the fair value of the Company’s portfolio of investments by asset class as of June 30, 2017 and December 31, 2016:
| June 30, 2017 | December 31, 2016 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| Senior Secured Debt with Warrants |
$ | 999,813 | 71.6 | % | $ | 1,078,779 | 75.7 | % | ||||||||
| Senior Secured Debt |
320,340 | 23.0 | % | 277,509 | 19.5 | % | ||||||||||
| Preferred Stock |
43,385 | 3.1 | % | 39,418 | 2.8 | % | ||||||||||
| Common Stock |
31,931 | 2.3 | % | 28,236 | 2.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,395,469 | 100.0 | % | $ | 1,423,942 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
A summary of the Company’s investment portfolio, at value, by geographic location as of June 30, 2017 and December 31, 2016 is shown as follows:
| June 30, 2017 | December 31, 2016 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| United States |
$ | 1,269,476 | 91.0 | % | $ | 1,362,223 | 95.6 | % | ||||||||
| England |
69,884 | 5.0 | % | 18,395 | 1.3 | % | ||||||||||
| Netherlands |
20,352 | 1.4 | % | 20,089 | 1.4 | % | ||||||||||
| Switzerland |
12,607 | 0.9 | % | 12,377 | 0.9 | % | ||||||||||
| Cayman Islands |
12,376 | 0.9 | % | — | 0.0 | % | ||||||||||
| Canada |
10,773 | 0.8 | % | 8,095 | 0.6 | % | ||||||||||
| Israel |
1 | 0.0 | % | 2,763 | 0.2 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,395,469 | 100.0 | % | $ | 1,423,942 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table shows the fair value of the Company’s portfolio by industry sector at June 30, 2017 and December 31, 2016:
| June 30, 2017 | December 31, 2016 | |||||||||||||||
| (in thousands) |
Investments at Fair Value |
Percentage of Total Portfolio |
Investments at Fair Value |
Percentage of Total Portfolio |
||||||||||||
| Drug Discovery & Development |
$ | 440,099 | 31.5 | % | $ | 422,550 | 29.7 | % | ||||||||
| Software |
254,215 | 18.2 | % | 219,559 | 15.4 | % | ||||||||||
| Media/Content/Info |
144,450 | 10.4 | % | 137,567 | 9.7 | % | ||||||||||
| Drug Delivery |
122,952 | 8.8 | % | 109,834 | 7.7 | % | ||||||||||
| Internet Consumer & Business Services |
100,705 | 7.2 | % | 97,047 | 6.8 | % | ||||||||||
| Sustainable and Renewable Technology |
92,609 | 6.6 | % | 154,406 | 10.9 | % | ||||||||||
| Medical Devices & Equipment |
83,933 | 6.0 | % | 107,695 | 7.6 | % | ||||||||||
| Specialty Pharmaceuticals |
38,803 | 2.8 | % | 38,944 | 2.7 | % | ||||||||||
| Healthcare Services, Other |
30,009 | 2.2 | % | 30,200 | 2.1 | % | ||||||||||
| Consumer & Business Products |
22,147 | 1.6 | % | 42,713 | 3.0 | % | ||||||||||
| Information Services |
14,722 | 1.1 | % | 6,091 | 0.4 | % | ||||||||||
| Surgical Devices |
13,660 | 1.0 | % | 12,553 | 0.9 | % | ||||||||||
| Semiconductors |
12,236 | 0.9 | % | 11,326 | 0.8 | % | ||||||||||
| Communications & Networking |
9,932 | 0.7 | % | 18,019 | 1.3 | % | ||||||||||
| Electronics & Computer Hardware |
7,619 | 0.5 | % | 7,664 | 0.5 | % | ||||||||||
| Biotechnology Tools |
6,723 | 0.5 | % | 7,200 | 0.5 | % | ||||||||||
| Diagnostic |
655 | 0.0 | % | 574 | 0.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 1,395,469 | 100.0 | % | $ | 1,423,942 | 100.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
No single portfolio investment represents more than 10% of the fair value of the investments as of June 30, 2017 and December 31, 2016.
F-135
Portfolio Activity
During the three and six months ended June 30, 2017, the Company funded and or restructured investments in debt securities totaling approximately $187.4 million, and $336.7 million, respectively. During the six months ended June 30, 2017, the Company funded equity investments totaling approximately $3.9 million. During the three and six months ended June 30, 2017, the Company converted approximately $62.7 million of debt to equity in two portfolio companies.
During the three and six months ended June 30, 2016, the Company funded and or restructured investments in debt securities totaling approximately $153.7 million and $7.0 million, respectively. During the three and six months ended June 30, 2016, the Company funded equity investments totaling approximately $6.1 million and $7.0 million, respectively. During the three and six months ended June 30, 2016, the Company converted approximately $4.6 million of debt to equity in two portfolio companies.
During the three and six months ended June 30, 2017, the Company recognized net realized losses of $5.7 million and $2.5 million, respectively. During the three months ended June 30, 2017, the Company recorded gross realized gains of $5.1 million primarily from the acquisition of the Company’s holdings in one portfolio company, IronPlanet, Inc. ($5.1 million). These gains were offset by gross realized losses of $10.8 million primarily from the liquidation or write off of the Company’s warrant and equity investments in ten portfolio companies.
During the six months ended June 30, 2017, the Company recorded gross realized gains of $11.5 million primarily from the sale or acquisition of the Company’s holdings in four portfolio companies, including IronPlanet, Inc. ($5.1 million), Box, Inc. ($4.0 million) TPI Composites, Inc. ($1.2 million) and Edge Therapeutics, Inc. ($708,000). These gains were offset by gross realized losses of $14.0 million primarily from the liquidation or write off of the Company’s warrant and equity investments in twelve portfolio companies and the Company’s debt investment in one portfolio company.
During the three and six months ended June 30, 2016, the Company recognized net realized gains of $25,000 and net realized losses of $4.4 million, respectively. During the three months ended June 30, 2016, the Company recorded gross realized gains of $1.4 million primarily from the acquisition of the Company’s holdings in one portfolio company, Ping Identity Corporation. These gains were offset by gross realized losses of $1.4 million primarily from the liquidation or write off of the Company’s warrant and equity investments in two portfolio companies.
During the six months ended June 30, 2016, the Company recorded gross realized gains of $4.2 million primarily from the sale or acquisition of the Company’s holdings in three portfolio companies, including Celator Pharmaceuticals, Inc. ($1.5 million), Ping Identity Corporation ($1.3 million) and the sale of options on Box, Inc. ($1.1 million). These gains were partially offset by gross realized losses of $8.6 million primarily from the liquidation or write off of the Company’s warrant and equity investments in five portfolio companies and the Company’s debt investment in three portfolio companies, including the settlement of the Company’s outstanding debt investment in the Neat Company ($6.2 million).
Investment Collateral
In the majority of cases, the Company collateralizes its investments by obtaining a first priority security interest in a portfolio company’s assets, which may include its intellectual property. In other cases, the Company may obtain a negative pledge covering a company’s intellectual property. At June 30, 2017, approximately 85.9% of the Company’s debt investments were in a senior secured first lien position, with 40.2% secured by a first priority security in all of the assets of the portfolio company, including its intellectual property and 45.7% secured by a first priority security in all of the assets of the portfolio company and the portfolio company was prohibited from pledging or encumbering its intellectual property. The remaining 14.1% of the Company’s debt
F-136
investments were secured by a second priority security interest in all of the portfolio company’s assets, other than intellectual property. At June 30, 2017 the Company had no equipment only liens on material investments.
Income Recognition
The Company records interest income on an accrual basis and recognizes it as earned in accordance with the contractual terms of the loan agreement, to the extent that such amounts are expected to be collected. OID initially represents the value of detachable equity warrants obtained in conjunction with the acquisition of debt securities and is accreted into interest income over the term of the loan as a yield enhancement. When a loan becomes 90 days or more past due, or if management otherwise does not expect that principal, interest and other obligations due will be collected in full, the Company will generally place the loan on non-accrual status and cease recognizing interest income on that loan until all principal and interest due has been paid or the Company believes the portfolio company has demonstrated the ability to repay the Company’s current and future contractual obligations. Any uncollected interest related to prior periods is reversed from income in the period that collection of the interest receivable is determined to be doubtful. However, the Company may make exceptions to this policy if the investment has sufficient collateral value and is in the process of collection.
At June 30, 2017, the Company had seven debt investments on non-accrual with a cumulative investment cost and approximate fair value of $43.6 million and $3.6 million, respectively. At December 31, 2016, the Company had five debt investments on non-accrual with cumulative investment cost and fair value of approximately $43.9 million and $6.2 million, respectively.
Fee income, generally collected in advance, includes loan commitment and facility fees for due diligence and structuring, as well as fees for transaction services and management services rendered by us to portfolio companies and other third parties. Loan and commitment fees are amortized into income over the contractual life of the loan. Management fees are generally recognized as income when the services are rendered. Loan origination fees are capitalized and then amortized into interest income using the effective interest rate method. In certain loan arrangements, warrants or other equity interests are received from the borrower as additional origination fees. The Company had approximately $35.3 million of unamortized fees at June 30, 2017, of which approximately $31.8 million was included as an offset to the cost basis of the Company’s current debt investments and approximately $3.5 million was deferred contingent upon the occurrence of a funding or milestone. At December 31, 2016 the Company had approximately $38.2 million of unamortized fees, of which approximately $35.8 million was included as an offset to the cost basis of the Company’s current debt investments and approximately $2.4 million was deferred contingent upon the occurrence of a funding or milestone.
The Company recognizes nonrecurring fees amortized over the remaining term of the loan commencing in the quarter relating to specific loan modifications. Certain fees may still be recognized as one-time fee income, including prepayment penalties, fees related to select covenant default, waiver fees and acceleration of previously deferred loan fees and OID related to early loan pay-off or material modification of the specific debt outstanding. The Company recorded approximately $5.5 million and $791,000 in one-time fee income during the three months ended June 30, 2017 and 2016, respectively. The Company recorded approximately $6.1 million and $956,000 in one-time fee income during the six months ended June 30, 2017 and 2016, respectively.
In addition, the Company may also be entitled to an exit fee that is amortized into income over the life of the loan. Loan exit fees to be paid at the termination of the loan are accreted into interest income over the contractual life of the loan. At June 30, 2017 the Company had approximately $25.5 million in exit fees receivable, of which approximately $22.9 million was included as a component of the cost basis of the Company’s current debt investments and approximately $2.6 million was a deferred receivable related to expired commitments. At December 31, 2016 the Company had approximately $32.8 million in exit fees receivable, of which approximately $30.3 million was included as an offset to the cost basis of the Company’s current debt investments and approximately $2.5 million was deferred related to expired commitments.
F-137
The Company has debt investments in its portfolio that contain a PIK provision. Contractual PIK interest, which represents contractually deferred interest added to the loan balance that is generally due at the end of the loan term, is generally recorded on the accrual basis to the extent such amounts are expected to be collected. The Company will generally cease accruing PIK interest if there is insufficient value to support the accrual or management does not expect the portfolio company to be able to pay all principal and interest due. The Company recorded approximately $2.5 million and $1.8 million in PIK income during the three months ended June 30, 2017 and 2016, respectively. The Company recorded approximately $4.7 million and $3.5 million in PIK income during the six months ended June 30, 2017 and 2016, respectively.
To maintain the Company’s status as a RIC, PIK and exit fee income must be paid out to stockholders in the form of distributions even though the cash has not yet been collected. Amounts necessary to pay these distributions may come from available cash or the liquidation of certain investments.
In certain investment transactions, the Company may provide advisory services. For services that are separately identifiable and external evidence exists to substantiate fair value, income is recognized as earned, which is generally when the investment transaction closes. The Company had no income from advisory services in the three and six months ended June 30, 2017 and 2016.
3. Fair Value of Financial Instruments
Fair value estimates are made at discrete points in time based on relevant information. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and, therefore, cannot be determined with precision. The Company believes that the carrying amounts of its financial instruments, consisting of cash and cash equivalents, receivables including escrow receivables, accounts payable and accrued liabilities, approximate the fair values of such items due to the short maturity of such instruments. The borrowings of the Company are recorded at amortized cost and not at fair value on the Consolidated Statement of Assets and Liabilities. The fair value of the Company’s outstanding borrowings is based on observable market trading prices or quotations and unobservable market rates as applicable for each instrument.
Based on market quotations on or around June 30, 2017, the 2021 Asset-Backed Notes and 2022 Convertible Notes were quoted for 1.002 and 1.028 per dollar at par value, respectively. At June 30, 2017, the 2024 Notes were trading on the NYSE for $25.47 per share at par value. The par value at underwriting for the 2024 Notes was $25.00 per share. Calculated based on the net present value of payments over the term of the notes using estimated market rates for similar notes and remaining terms, the fair value of the SBA debentures is approximately $195.7 million, compared to the carrying amount of $190.2 million as of June 30, 2017.
See the accompanying Consolidated Schedule of Investments for the fair value of the Company’s investments. The methodology for the determination of the fair value of the Company’s investments is discussed in Note 2.
The following tables provide additional information about the fair value and level in the fair value hierarchy of the Company’s outstanding borrowings at June 30, 2017 and December 31, 2016:
| (in thousands) Description(1)(2) |
June 30, 2017 | Identical Assets (Level 1) |
Observable Inputs (Level 2) |
Unobservable Inputs (Level 3) |
||||||||||||
| 2021 Asset-Backed Notes |
$ | 87,869 | $ | — | $ | 87,869 | $ | — | ||||||||
| 2022 Convertible Notes |
236,491 | — | 236,491 | — | ||||||||||||
| 2024 Notes |
263,370 | — | 263,370 | — | ||||||||||||
| SBA Debentures |
195,749 | — | — | 195,749 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 783,479 | $ | — | $ | 587,730 | $ | 195,749 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-138
| (in thousands) Description |
December 31, 2016 |
Identical Assets (Level 1) |
Observable Inputs (Level 2) |
Unobservable Inputs (Level 3) |
||||||||||||
| Wells Facility(1) |
$ | 5,016 | $ | — | $ | — | $ | 5,016 | ||||||||
| 2021 Asset-Backed Notes |
109,376 | — | 109,376 | — | ||||||||||||
| April 2019 Notes(2) |
65,909 | — | 65,909 | — | ||||||||||||
| September 2019 Notes(2) |
46,920 | — | 46,920 | — | ||||||||||||
| 2024 Notes |
256,919 | — | 256,919 | — | ||||||||||||
| SBA Debentures |
202,364 | — | — | 202,364 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 686,504 | $ | — | $ | 479,124 | $ | 207,380 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | As of June 30, 2017 there were no borrowings outstanding on both the Wells Facility and Union Facility. |
| (2) | The 2019 Notes were redeemed in full on February 24, 2017. |
4. Borrowings
Outstanding Borrowings
At June 30, 2017 and December 31, 2016, the Company had the following available and outstanding borrowings:
| June 30, 2017 | December 31, 2016 | |||||||||||||||||||||||
| (in thousands) |
Total Available | Principal | Carrying Value(1) | Total Available | Principal | Carrying Value(1) | ||||||||||||||||||
| SBA Debentures(2) |
$ | 190,200 | $ | 190,200 | $ | 187,824 | $ | 190,200 | $ | 190,200 | $ | 187,501 | ||||||||||||
| 2019 Notes(3) |
— | — | — | 110,364 | 110,364 | 108,818 | ||||||||||||||||||
| 2024 Notes |
258,510 | 258,510 | 251,478 | 252,873 | 252,873 | 245,490 | ||||||||||||||||||
| 2021 Asset-Backed Notes |
87,678 | 87,678 | 86,865 | 109,205 | 109,205 | 107,972 | ||||||||||||||||||
| 2022 Convertible Notes |
230,000 | 230,000 | 222,898 | — | — | — | ||||||||||||||||||
| Wells Facility(4) |
120,000 | — | — | 120,000 | 5,016 | 5,016 | ||||||||||||||||||
| Union Bank Facility(4) |
75,000 | — | — | 75,000 | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 961,388 | $ | 766,388 | $ | 749,065 | $ | 857,642 | $ | 667,658 | $ | 654,797 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | Except for the Wells Facility and Union Bank Facility, all carrying values represent the principal amount outstanding less the remaining unamortized debt issuance costs and unaccreted premium or discount, if any, associated with the loan as of the balance sheet date. |
| (2) | At both June 30, 2017 and December 31, 2016, the total available borrowings under the SBA debentures were $190.2 million, of which $41.2 million was available in HT II and $149.0 million was available in HT III. |
| (3) | The 2019 Notes were redeemed in full on February 24, 2017. |
| (4) | Availability subject to the Company meeting the borrowing base requirements. |
Debt issuance costs are fees and other direct incremental costs incurred by the Company in obtaining debt financing and are recognized as prepaid expenses and amortized over the life of the related debt instrument using the effective yield method or the straight line method, which closely approximates the effective yield method. In accordance with ASC Subtopic 835-30 (“Interest – Imputation of Interest”), debt issuance costs are presented as a reduction to the associated liability balance on the Consolidated Statement of Assets and Liabilities, except for debt issuance costs associated with line-of-credit arrangements. Debt issuance costs, net of accumulated amortization, were as follows as of June 30, 2017 and December 31, 2016:
| (in thousands) |
June 30, 2017 | December 31, 2016 | ||||||
| SBA Debentures |
$ | 2,376 | $ | 2,699 | ||||
| 2019 Notes |
— | 1,546 | ||||||
| 2024 Notes |
7,141 | 7,482 | ||||||
| 2021 Asset-Backed Notes |
813 | 1,233 | ||||||
| 2022 Convertible Notes |
3,969 | — | ||||||
| Wells Facility(1) |
337 | 501 | ||||||
| Union Bank Facility(1) |
543 | 768 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 15,179 | $ | 14,229 | ||||
|
|
|
|
|
|||||
| (1) | As the Wells Facility and Union Bank Facility are line-of-credit arrangements, the debt issuance costs associated with these instruments are presented separately as an asset on the Consolidated Statement of Assets and Liabilities in accordance with ASC Subtopic 835-30. |
F-139
Long-Term SBA Debentures
On September 27, 2006, HT II received a license to operate as a SBIC under the SBIC program and is able to borrow funds from the SBA against eligible investments and additional contributions to regulatory capital. Under the Small Business Investment Company Act and current SBA policy applicable to SBICs, a SBIC can have outstanding at any time SBA guaranteed debentures up to twice the amount of its regulatory capital. With the Company’s net investment of $44.0 million in HT II as of June 30, 2017, HT II has the capacity to issue a total of $41.2 million of SBA guaranteed debentures, subject to SBA approval, of which $41.2 million was outstanding as of June 30, 2017. As of June 30, 2017, HT II has paid the SBA commitment fees and facility fees of approximately $1.5 million and $3.6 million, respectively. As of June 30, 2017 the Company held investments in HT II in 33 companies with a fair value of approximately $98.7 million, accounting for approximately 7.1% of the Company’s total investment portfolio at June 30, 2017. HT II held approximately $104.8 million in assets and accounted for approximately 5.8% of the Company’s total assets prior to consolidation at June 30, 2017.
On May 26, 2010, HT III received a license to operate as a SBIC under the SBIC program and is able to borrow funds from the SBA against eligible investments and additional contributions to regulatory capital. With the Company’s net investment of $74.5 million in HT III as of June 30, 2017, HT III has the capacity to issue a total of $149.0 million of SBA guaranteed debentures, subject to SBA approval, of which $149.0 million was outstanding as of June 30, 2017. As of June 30, 2017, HT III has paid the SBA commitment fees and facility fees of approximately $1.5 million and $3.6 million, respectively. As of June 30, 2017, the Company held investments in HT III in 49 companies with a fair value of approximately $245.8 million, accounting for approximately 17.6% of the Company’s total investment portfolio at June 30, 2017. HT III held approximately $271.5 million in assets and accounted for approximately 14.9% of the Company’s total assets prior to consolidation at June 30, 2017.
SBICs are designed to stimulate the flow of private equity capital to eligible small businesses. Under present SBA regulations, eligible small businesses include businesses that have a tangible net worth not exceeding $19.5 million and have average annual fully taxed net income not exceeding $6.5 million for the two most recent fiscal years. In addition, SBICs must devote 25.0% of its investment activity to “smaller” enterprises as defined by the SBA. A smaller enterprise is one that has a tangible net worth not exceeding $6.0 million and has average annual fully taxed net income not exceeding $2.0 million for the two most recent fiscal years. SBA regulations also provide alternative size standard criteria to determine eligibility, which depend on the industry in which the business is engaged and are based on such factors as the number of employees and gross sales. According to SBA regulations, SBICs may make long-term loans to small businesses, invest in the equity securities of such businesses and provide them with consulting and advisory services. Through the Company’s wholly owned subsidiaries HT II and HT III, the Company plans to provide long-term loans to qualifying small businesses, and in connection therewith, make equity investments.
HT II and HT III are periodically examined and audited by the SBA’s staff to determine their compliance with SBA regulations. If HT II or HT III fails to comply with applicable SBA regulations, the SBA could, depending on the severity of the violation, limit or prohibit HT II’s or HT III’s use of debentures, declare outstanding debentures immediately due and payable, and/or limit HT II or HT III from making new investments. In addition, HT II or HT III may also be limited in their ability to make distributions to the Company if they do not have sufficient capital in accordance with SBA regulations. Such actions by the SBA would, in turn, negatively affect the Company because HT II and HT III are the Company’s wholly owned subsidiaries. HT II and HT III were in compliance with the terms of the SBIC’s leverage as of June 30, 2017 as a result of having sufficient capital as defined under the SBA regulations.
The rates of borrowings under various draws from the SBA beginning in March 2009 are set semiannually in March and September and range from 2.25% to 4.62% excluding annual fees. Interest payments on SBA debentures are payable semiannually. There are no principal payments required on these issues prior to maturity and no prepayment penalties. Debentures under the SBA generally mature ten years after being borrowed. Based
F-140
on the initial draw down date of March 2009, the initial maturity of SBA debentures will occur in March 2019. In addition, the SBA charges a fee that is set annually, depending on the Federal fiscal year the leverage commitment was delegated by the SBA, regardless of the date that the leverage was drawn by the SBIC. The annual fees related to HT II debentures that pooled on September 22, 2010 were 0.406% and 0.285%, depending upon the year in which the underlying commitment was closed. The annual fees on other debentures have been set at 0.906%. The annual fees related to HT III debentures that pooled on March 27, 2013 were 0.804%. The annual fees on other debentures have been set at 0.515%. The rates of borrowings on the Company’s SBA debentures range from 3.05% to 5.53% when including these annual fees.
The average amount of debentures outstanding for the three and six months ended June 30, 2017 for HT II was approximately $41.2 million with an average interest rate of approximately 4.51% and 4.48%, respectively. The average amount of debentures outstanding for the three and six months ended June 30, 2017 for HT III was approximately $149.0 million with an average interest rate of approximately 3.42% and 3.40%, respectively.
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the SBA debentures are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 1,737 | $ | 1,737 | $ | 3,456 | $ | 3,475 | ||||||||
| Amortization of debt issuance cost (loan fees) |
156 | 168 | 324 | 336 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 1,893 | $ | 1,905 | $ | 3,780 | $ | 3,811 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | — | $ | 3,442 | $ | 3,461 | ||||||||
As of June 30, 2017, the maximum statutory limit on the dollar amount of combined outstanding SBA guaranteed debentures is $350.0 million, subject to periodic adjustments by the SBA. In aggregate, at June 30, 2017, with the Company’s net investment of $118.5 million, HT II and HT III have the capacity to issue a total of $190.2 million of SBA-guaranteed debentures, subject to SBA approval. At June 30, 2017, the Company has issued $190.2 million in SBA-guaranteed debentures in the Company’s SBIC subsidiaries.
The Company reported the following SBA debentures outstanding principal balances as of June 30, 2017 and December 31, 2016:
| (in thousands) Issuance/Pooling Date |
Maturity Date | Interest Rate(1) | June 30, 2017 | December 31, 2016 | ||||||||||||
| March 25, 2009 |
March 1, 2019 | 5.53 | % | $ | 18,400 | $ | 18,400 | |||||||||
| September 23, 2009 |
September 1, 2019 | 4.64 | % | 3,400 | 3,400 | |||||||||||
| September 22, 2010 |
September 1, 2020 | 3.62 | % | 6,500 | 6,500 | |||||||||||
| September 22, 2010 |
September 1, 2020 | 3.50 | % | 22,900 | 22,900 | |||||||||||
| March 29, 2011 |
March 1, 2021 | 4.37 | % | 28,750 | 28,750 | |||||||||||
| September 21, 2011 |
September 1, 2021 | 3.16 | % | 25,000 | 25,000 | |||||||||||
| March 21, 2012 |
March 1, 2022 | 3.28 | % | 25,000 | 25,000 | |||||||||||
| March 21, 2012 |
March 1, 2022 | 3.05 | % | 11,250 | 11,250 | |||||||||||
| September 19, 2012 |
September 1, 2022 | 3.05 | % | 24,250 | 24,250 | |||||||||||
| March 27, 2013 |
March 1, 2023 | 3.16 | % | 24,750 | 24,750 | |||||||||||
|
|
|
|
|
|||||||||||||
| Total SBA Debentures |
$ | 190,200 | $ | 190,200 | ||||||||||||
|
|
|
|
|
|||||||||||||
| (1) | Interest rate includes annual charge |
2019 Notes
In April and July 2012, the Company issued $84.5 million in aggregate principal amount of 7.00% notes due 2019 (the “April 2019 Notes”). In September and October 2012, the Company issued $85.9 million in aggregate principal amount of 7.00% notes due 2019 (the “September 2019 Notes”). The April 2019 Notes and September 2019 Notes are together referred to as the “2019 Notes”.
F-141
In April 2015, the Company redeemed $20.0 million of the $84.5 million issued and outstanding aggregate principal amount of April 2019 Notes, as previously approved by the Board of Directors. In December 2015, the Company redeemed $40.0 million of the $85.9 million issued and outstanding aggregate principal amount of September 2019 Notes, as previously approved by the Board of Directors. The remaining 2019 Notes were fully redeemed on February 24, 2017.
As of December 31, 2016, the 2019 Notes payable outstanding principal balance consisted of:
| (in thousands) |
December 31, 2016 | |||
| April 2019 Notes |
$ | 64,490 | ||
| September 2019 Notes |
45,874 | |||
|
|
|
|||
| Total 2019 Notes principal outstanding |
$ | 110,364 | ||
|
|
|
|||
The April 2019 Notes bore interest at a rate of 7.00% per year and traded on the NYSE under the trading symbol “HTGZ.” The September 2019 Notes bore interest at a rate of 7.00% per year and traded on the NYSE under the trading symbol “HTGY.” For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the 2019 Notes are as follows:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 1,931 | $ | 1,159 | $ | 3,863 | ||||||||
| Amortization of debt issuance cost (loan fees) |
— | 160 | 1,546 | 320 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | — | $ | 2,091 | $ | 2,705 | $ | 4,183 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | 1,931 | $ | 1,911 | $ |
3,863 |
| |||||||
2024 Notes
On July 14, 2014, the Company and U.S. Bank, N.A. (the “2024 Trustee”), entered into the Third Supplemental Indenture (the “Third Supplemental Indenture”) to the Base Indenture between the Company and the 2024 Trustee, dated July 14, 2014, relating to the Company’s issuance, offer and sale of $100.0 million aggregate principal amount of 6.25% unsecured notes due 2024 (the “2024 Notes”). On August 6, 2014, the underwriters issued notification to exercise their over-allotment option for an additional $3.0 million in aggregate principal amount of the 2024 Notes.
On May 2, 2016, the Company closed an underwritten public offering of an additional $72.9 million in aggregate principal amount of the 2024 Notes. The $72.9 million in aggregate principal amount includes $65.4 million from the initial offering on April 21, 2016 and $7.5 million as a result of underwriters exercising a portion of their option to purchase up to an additional $9.8 million in aggregate principal to cover overallotments on April 29, 2016.
On June 27, 2016, the Company closed an underwritten public offering of an additional $60.0 million in aggregate principal amount of the 2024 Notes. On June 30, 2016, the underwriters exercised their option to purchase up to an additional $9.0 million in aggregate principal to cover overallotments, resulting in total aggregate principal of $69.0 million from the offering.
On October 11, 2016, the Company entered into a debt distribution agreement, pursuant to which it may offer for sale, from time to time, up to $150.0 million in aggregate principal amount of 2024 Notes through FBR Capital Markets & Co. acting as its sales agent (the “2024 Notes Agent”). Sales of the 2024 Notes may be made in negotiated transactions or transactions that are deemed to be “at the market offerings” as defined in Rule 415 under the Securities Act, including sales made directly on the NYSE, or similar securities exchange or sales made through a market maker other than on an exchange at prices related to prevailing market prices or at negotiated prices.
F-142
The 2024 Notes Agent receives a commission from the Company equal to up to 2.00% of the gross sales of any 2024 Notes sold through the 2024 Notes Agent under the debt distribution agreement. The 2024 Notes Agent is not required to sell any specific principal amount of 2024 Notes, but will use its commercially reasonable efforts consistent with its sales and trading practices to sell the 2024 Notes. The 2024 Notes are expected to trade “flat,” which means that purchasers in the secondary market will not pay, and sellers will not receive, any accrued and unpaid interest on the 2024 Notes that is not reflected in the trading price.
During the six months ended June 30, 2017, the Company sold 225,457 notes for approximately $5.6 million in aggregate principal amount. The Company did not sell any notes under the debt distribution agreement during the three months ended June 30, 2017. During the year ended December 31, 2016, the Company sold 317,125 notes for approximately $7.9 million in aggregate principal amount. As of June 30, 2017 approximately $136.4 million in aggregate principal amount remains available for issuance and sale under the debt distribution agreement.
All issuances of 2024 Notes rank equally in right of payment and form a single series of notes.
The 2024 Notes will mature on July 30, 2024 and may be redeemed in whole or in part at the Company’s option at any time or from time to time on or after July 30, 2017, upon not less than 30 days nor more than 60 days written notice by mail prior to the date fixed for redemption thereof, at a redemption price of 100% of the outstanding principal amount thereof plus accrued and unpaid interest payments otherwise payable for the then-current quarterly interest period accrued to but not including the date fixed for redemption. The 2024 Notes bear interest at a rate of 6.25% per year payable quarterly on January 30, April 30, July 30 and October 30 of each year, commencing on July 30, 2014, and trade on the NYSE under the trading symbol “HTGX”.
The 2024 Notes are the Company’s direct unsecured obligations and rank: (i) pari passu with the Company’s other outstanding and future senior unsecured indebtedness; (ii) senior to any of the Company’s future indebtedness that expressly provides it is subordinated to the 2024 Notes; (iii) effectively subordinated to all the Company’s existing and future secured indebtedness (including indebtedness that is initially unsecured to which the Company subsequently grants security), to the extent of the value of the assets securing such indebtedness; (iv) structurally subordinated to all existing and future indebtedness and other obligations of any of the Company’s subsidiaries.
The Base Indenture, as supplemented by the Third Supplemental Indenture, contains certain covenants including covenants requiring the Company to comply with (regardless of whether it is subject to) the asset coverage requirements set forth in Section 18(a)(1)(A) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act and to comply with the restrictions on dividends and other distributions as well as the purchase of capital stock set forth in Section 18(a)(1)(B) of the 1940 Act as modified by Section 61(a)(1) of the 1940 Act. These covenants are subject to important limitations and exceptions that are described in the Base Indenture, as supplemented by the Third Supplemental Indenture. The Base Indenture, as supplemented by the Third Supplemental Indenture, also contains certain reporting requirements, including a requirement that the Company provide financial information to the holders of the 2024 Notes and the 2024 Trustee if the Company should no longer be subject to the reporting requirements under the Exchange Act. The Base Indenture provides for customary events of default and further provides that the 2024 Trustee or the holders of 25% in aggregate principal amount of the outstanding 2024 Notes in a series may declare such 2024 Notes immediately due and payable upon the occurrence of any event of default after expiration of any applicable grace period. As of June 30, 2017, the Company was in compliance with the terms of the Base Indenture as supplemented by the Third Supplemental Indenture.
F-143
As of June 30, 2017 and December 31, 2016, the components of the carrying value of the 2024 Notes were as follows:
| (in thousands) |
June 30, 2017 | December 31, 2016 | ||||||
| Principal amount of debt |
$ | 258,510 | $ | 252,873 | ||||
| Unamortized debt issuance cost |
(7,141 | ) | (7,482 | ) | ||||
| Original issue premium, net of amortization |
109 | 99 | ||||||
|
|
|
|
|
|||||
| Carrying value of 2024 Notes |
$ | 251,478 | $ | 245,490 | ||||
|
|
|
|
|
|||||
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the 2024 Notes are as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 4,039 | $ | 2,375 | $ | 8,026 | $ | 3,984 | ||||||||
| Amortization of debt issuance cost (loan fees) |
252 | 135 | 501 | 218 | ||||||||||||
| Amortization of original issue premium |
(13 | ) | — | (29 | ) | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 4,278 | $ | 2,510 | $ | 8,498 | $ | 4,202 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 4,039 | $ | 1,609 | $ | 8,016 | $ | 3,219 | ||||||||
2021 Asset-Backed Notes
On November 13, 2014, the Company completed a $237.4 million term debt securitization in connection with which an affiliate of the Company made an offer of $129.3 million in aggregate principal amount of fixed rate asset-backed notes (the “2021 Asset-Backed Notes”), which were rated A(sf) by Kroll Bond Rating Agency, Inc. The 2021 Asset-Backed Notes were sold by Hercules Capital Funding Trust 2014-1 pursuant to a note purchase agreement, dated as of November 13, 2014, by and among the Company, Hercules Capital Funding 2014-1, LLC as trust depositor (the “2014 Trust Depositor”), Hercules Capital Funding Trust 2014-1 as issuer (the “2014 Securitization Issuer”), and Guggenheim Securities, LLC, as initial purchaser, and are backed by a pool of senior loans made to certain of the Company’s portfolio companies and secured by certain assets of those portfolio companies and are to be serviced by the Company. The securitization has an 18-month reinvestment period during which time principal collections may be reinvested into additional eligible loans. Interest on the 2021 Asset-Backed Notes is paid, to the extent of funds available, at a fixed rate of 3.524% per annum. The 2021 Asset-Backed Notes have a stated maturity of April 16, 2021.
As part of this transaction, the Company entered into a sale and contribution agreement with the 2014 Trust Depositor under which the Company has agreed to sell or have contributed to the 2014 Trust Depositor certain senior loans made to certain of the Company’s portfolio companies (the “2014 Loans”). The Company has made customary representations, warranties and covenants in the sale and contribution agreement with respect to the 2014 Loans as of the date of their transfer to the 2014 Trust Depositor.
In connection with the issuance and sale of the 2021 Asset-Backed Notes, the Company has made customary representations, warranties and covenants in the note purchase agreement. The 2021 Asset-Backed Notes are secured obligations of the 2014 Securitization Issuer and are non-recourse to the Company. The 2014 Securitization Issuer also entered into an indenture governing the 2021 Asset-Backed Notes, which includes customary representations, warranties and covenants. The 2021 Asset-Backed Notes were sold without being registered under the Securities Act of 1933, as amended, (the “Securities Act”) (A) in the United States to “qualified institutional buyers” as defined in Rule 144A under the Securities Act and to institutional “accredited investors” (as defined in Rules 501(a)(1), (2), (3) or (7) under the Securities Act) who in each case, are “qualified purchasers” as defined in Sec. 2 (a)(51)(A) of the 1940 Act and pursuant to an exemption under the Securities Act and (B) to non-U.S. purchasers acquiring interest in the 2021 Asset-Backed Notes outside the United States in accordance with Regulation S under the Securities Act. The 2014 Securitization Issuer is not registered under
F-144
the 1940 Act in reliance on an exemption provided by Section 3(c)(7) thereof and Rule 3a-7 thereunder. In addition, the 2014 Trust Depositor entered into an amended and restated trust agreement in respect of the 2014 Securitization Issuer, which includes customary representation, warranties and covenants.
The 2014 Loans are serviced by the Company pursuant to a sale and servicing agreement, which contains customary representations, warranties and covenants. The Company performs certain servicing and administrative functions with respect to the 2014 Loans. The Company is entitled to receive a monthly fee from the 2014 Securitization Issuer for servicing the 2014 Loans. This servicing fee is equal to the product of one-twelfth (or in the case of the first payment date, a fraction equal to the number of days from and including October 5, 2014 through and including December 5, 2014 over 360) of 2.00% and the aggregate outstanding principal balance of the 2014 Loans plus collections on deposit in the 2014 Securitization Issuer’s collections account, as of the first day of the related collection period (the period from the 5th day of the immediately preceding calendar month through the 4th day of the calendar month in which a payment date occurs, and for the first payment date, the period from and including October 5, 2014, to the close of business on December 5, 2014).The Company also serves as administrator to the 2014 Securitization Issuer under an administration agreement, which includes customary representations, warranties and covenants.
At June 30, 2017 and December 31, 2016, the 2021 Asset-Backed Notes had an outstanding principal balance of $87.7 million and $109.2 million, respectively.
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the 2021 Asset-Backed Notes are as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 807 | $ | 1,139 | $ | 1,695 | $ | 2,278 | ||||||||
| Amortization of debt issuance cost (loan fees) |
211 | 234 | 421 | 466 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 1,018 | $ | 1,373 | $ | 2,116 | $ | 2,744 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 848 | $ | 1,139 | $ | 1,788 | $ | 2,278 | ||||||||
Under the terms of the 2021 Asset-Backed Notes, the Company is required to maintain a reserve cash balance, funded through interest and principal collections from the underlying securitized debt portfolio, which may be used to pay monthly interest and principal payments on the 2021 Asset-Backed Notes. The Company has segregated these funds and classified them as restricted cash. There was approximately $17.2 million and $8.3 million of restricted cash as of June 30, 2017 and December 31, 2016, respectively, funded through interest collections.
Convertible Notes
2016 Convertible Notes
In April 2011, the Company issued $75.0 million in aggregate principal amount of 6.00% convertible notes due 2016 (the “2016 Convertible Notes”). The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016.
Prior to the close of business on October 14, 2015, holders were able to convert their 2016 Convertible Notes only under certain circumstances set forth in the indenture governing the 2016 Convertible Notes. On or after October 15, 2015 until the close of business on the scheduled trading day immediately preceding the maturity date, holders were able to convert their 2016 Convertible Notes at any time. Throughout the life of the 2016 Convertible Notes, holders of approximately $74.8 million of the 2016 Convertible Notes exercised their conversion rights. These 2016 Convertible Notes were settled with a combination of cash equal to the outstanding principal amount of the 2016 Convertible Notes and approximately 1.6 million shares of the Company’s common stock, or $24.3 million.
F-145
The 2016 Convertible Notes were accounted for in accordance with ASC Subtopic 470-20 (“Debt Instruments with Conversion and Other Options”). In accounting for the 2016 Convertible Notes, the Company estimated at the time of issuance that the values of the debt and the embedded conversion feature of the 2016 Convertible Notes were approximately 92.8% and 7.2%, respectively. The original issue discount of 7.2% attributable to the conversion feature of the 2016 Convertible Notes was recorded in “capital in excess of par value” in the Consolidated Statement of Assets and Liabilities. As a result, the Company recorded interest expense comprised of both stated interest expense as well as accretion of the original issue discount resulting in an estimated effective interest rate of approximately 8.1%.
For the three and six months ended June 30, 2016, the components of interest expense, fees and cash paid for interest expense for the 2016 Convertible Notes were as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 88 | $ | — | $ | 352 | ||||||||
| Amortization of debt issuance cost (loan fees) |
— | 11 | — | 43 | ||||||||||||
| Accretion of original issue discount |
— | 21 | — | 82 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | — | $ | 120 | $ | — | $ | 477 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | 440 | $ | — | $ | 440 | ||||||||
The estimated effective interest rate of the debt component of the 2016 Convertible Notes, equal to the stated interest of 6.0% plus the accretion of the original issue discount, was approximately 8.1% for the three and six months ended June 30, 2016.
2022 Convertible Notes
On January 25, 2017, the Company issued $230.0 million in aggregate principal amount of 4.375% Convertible Notes due 2022 (the “2022 Convertible Notes”), which amount includes the additional $30.0 million aggregate principal amount of 2022 Convertible Notes issued pursuant to the initial purchaser’s exercise in full of its overallotment option. The 2022 Convertible Notes were issued pursuant to an Indenture, dated January 25, 2017 (the “2022 Convertible Notes Indenture”), between the Company and U.S. Bank, National Association, as trustee (the “2022 Trustee”). The sale of the 2022 Convertible Notes generated net proceeds of approximately $225.7 million, including $4.3 million of debt issuance costs.
The 2022 Convertible Notes mature on February 1, 2022, unless previously converted or repurchased in accordance with their terms. The 2022 Convertible Notes bear interest at a rate of 4.375% per year payable semiannually in arrears on February 1 and August 1 of each year, commencing on August 1, 2017.
The 2022 Convertible Notes will be unsecured obligations of the Company and will rank senior in right of payment to the Company’s future indebtedness that is expressly subordinated in right of payment to the 2022 Convertible Notes; equal in right of payment to the Company’s existing and future indebtedness that is not so subordinated; effectively junior in right of payment to any of the Company’s secured indebtedness (including unsecured indebtedness that the Company later secures) to the extent of the value of the assets securing such indebtedness; and structurally junior to all existing and future indebtedness (including trade payables) incurred by the Company’s subsidiaries, financing vehicles or similar facilities.
Prior to the close of business on the business day immediately preceding August 1, 2021, holders may convert their 2022 Convertible Notes only under certain circumstances set forth in the 2022 Convertible Notes Indenture. On or after August 1, 2021 until the close of business on the scheduled trading day immediately preceding the Maturity Date, holders may convert their 2022 Convertible Notes at any time. Upon conversion, the Company will pay or deliver, as the case may be, at its election, cash, shares of its common stock or a combination of cash and shares of its common stock. The conversion rate is initially 60.9366 shares of common
F-146
stock per $1,000 principal amount of 2022 Convertible Notes (equivalent to an initial conversion price of approximately $16.41 per share of common stock). The conversion rate will be subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, if certain corporate events occur prior to the maturity date, the Company will increase the conversion rate for a holder who elects to convert its 2022 Convertible Notes in connection with such a corporate event in certain circumstances. As of June 30, 2017, the conversion rate was 60.9366 shares of common stock per $1,000 principal amount of Convertible Senior Notes (equivalent to an adjusted conversion price of approximately $16.41 per share of common stock).
The Company may not redeem the 2022 Convertible Notes at its option prior to maturity. No sinking fund is provided for the 2022 Convertible Notes. In addition, if certain corporate events occur in respect of the Company, holders of the 2022 Convertible Notes may require the Company to repurchase for cash all or part of their 2022 Convertible Notes at a repurchase price equal to 100% of the principal amount of the 2022 Convertible Notes to be repurchased, plus accrued and unpaid interest through, but excluding, the required repurchase date.
The 2022 Convertible Notes Indenture contains certain covenants, including covenants requiring the Company to comply with Section 18(a)(1)(A) as modified by Section 61(a)(1) of the 1940 Act and to provide financial information to the holders of the 2022 Convertible Notes and the 2022 Trustee if the Company ceases to be subject to the reporting requirements of the Exchange Act. These covenants are subject to important limitations and exceptions that are described in the 2022 Convertible Notes Indenture. The Company offered and sold the 2022 Convertible Notes to the initial purchaser in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act, for resale by the initial purchaser to qualified institutional buyers (as defined in the Securities Act) pursuant to the exemption from registration provided by Rule 144A under the Securities Act. The Company relied on these exemptions from registration based in part on representations made by the initial purchaser in connection with the sale of the 2022 Convertible Notes.
The 2022 Convertible Notes are accounted for in accordance with ASC Subtopic 470-20 (“Debt Instruments with Conversion and Other Options”). In accounting for the 2022 Convertible Notes, the Company estimated at the time of issuance that the values of the debt and the embedded conversion feature of the 2022 Convertible Notes were approximately 98.5% and 1.5%, respectively. The original issue discount of 1.5%, or $3.4 million, attributable to the conversion feature of the 2022 Convertible Notes was recorded in “capital in excess of par value” in the Consolidated Statement of Assets and Liabilities. As a result, the Company records interest expense comprised of both stated interest expense as well as accretion of the original issue discount resulting in an estimated effective interest rate of approximately 4.76%.
As of June 30, 2017, the components of the carrying value of the 2022 Convertible Notes were as follows:
| (in thousands) |
June 30, 2017 | |||
| Principal amount of debt |
$ | 230,000 | ||
| Unamortized debt issuance cost |
(3,969 | ) | ||
| Original issue discount, net of accretion |
(3,133 | ) | ||
|
|
|
|||
| Carrying value of 2022 Convertible Notes |
$ | 222,898 | ||
|
|
|
|||
For the three and six months ended June 30, 2017, the components of interest expense, fees and cash paid for interest expense for the 2022 Convertible notes were as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | 2,516 | $ | — | $ | 4,274 | $ | — | ||||||||
| Amortization of debt issuance cost (loan fees) |
212 | — | 345 | — | ||||||||||||
| Accretion of original issue discount |
168 | — | 280 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 2,896 | $ | — | $ | 4,899 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | — | $ | — | $ | — | $ | — | ||||||||
F-147
The estimated effective interest of the debt component of the 2022 Convertible Notes, equal to the stated interest rate of 4.375% plus the accretion of the original issue discount, was approximately 4.76% for the three and six months ended June 30, 2017. As of June 30, 2017, the Company is in compliance with the terms of the indentures governing the 2022 Convertible Notes.
Credit Facilities
As of June 30, 2017 and December 31, 2016, the Company has two available credit facilities, the Wells Facility and the Union Bank Facility.
Wells Facility
On June 29, 2015, the Company, through a special purpose wholly owned subsidiary, Hercules Funding II LLC (“Hercules Funding II”), entered into an Amended and Restated Loan and Security Agreement (the “Wells Facility”) with Wells Fargo Capital Finance, LLC, as a lender and as the arranger and the administrative agent, and the lenders party thereto from time to time.
The Wells Facility matures on August 2, 2019, unless terminated sooner in accordance with its terms.
Under the Wells Facility, Wells Fargo Capital Finance, LLC made commitments of $75.0 million, Alostar Bank of Commerce made commitments of $20.0 million, and Everbank Commercial Finance Inc. made commitments of $25.0 million. The Wells Facility contains an accordion feature, in which the Company can increase the credit line up to an aggregate of $300.0 million, funded by additional lenders and with the agreement of Wells Fargo and subject to other customary conditions. The Company expects to continue discussions with various other potential lenders to join the facility; however, there can be no assurances that additional lenders will join the Wells Facility. Borrowings under the Wells Facility generally bear interest at a rate per annum equal to LIBOR plus 3.25%, and the Wells Facility has an advance rate of 50% against eligible debt investments. The Wells Facility is secured by all of the assets of Hercules Funding II. The Wells Facility requires payment of a non-use fee on a scale of 0.0% to 0.50% depending on the average monthly outstanding balance under the facility relative to the maximum amount of commitments at such time. For the three and six months ended June 30, 2017, this non-use fee was $152,000 and $297,000, respectively. For the three and six months ended June 30, 2016, this non-use fee was $115,000 and $181,000, respectively.
The Wells Facility also includes various financial and other covenants applicable to the Company and the Company’s subsidiaries, in addition to those applicable to Hercules Funding II, including covenants relating to certain changes of control of the Company and Hercules Funding II. Among other things, these covenants also require the Company to maintain certain financial ratios, including a maximum debt to worth ratio, minimum interest coverage ratio, minimum portfolio funding liquidity, and a minimum tangible net worth in an amount, when added to outstanding subordinated indebtedness, that is in excess of $500.0 million plus 90% of the cumulative amount of equity raised after June 30, 2014. As of June 30, 2017, the minimum tangible net worth covenant increased to $718.6 million as a result of the March 2015 follow-on public offering of 7.6 million shares of common stock for total gross proceeds of approximately $100.4 million, the issuance of 7.3 million shares of common stock issued under the At-The-Market (“ATM”) equity distribution agreement (the “Equity Distribution Agreement”) with JMP Securities (“JMP”) for gross proceeds of $95.0 million during the year ended December 31, 2016, and the issuance of 3.3 million shares of common stock issued under the Equity Distribution Agreement for gross proceeds of $47.4 million during the six months ended June 30, 2017. The Wells Facility provides for customary events of default, including, without limitation, with respect to payment defaults, breach of representations and covenants, certain key person provisions, cross acceleration provisions to certain other debt, lien and judgment limitations, and bankruptcy.
On June 20, 2011, the Company paid $1.1 million in structuring fees in connection with the original Wells Facility. In connection with an amendment to the original Wells Facility in August 2014, the Company paid an
F-148
additional $750,000 in structuring fees and in connection with the amendment in December 2015, the Company paid an additional $188,000 in structuring fees. These fees are being amortized through the end of the term of the Wells Facility.
The Company had aggregate draws of $8.5 million on the available facility during the six months ended June 30, 2017 offset by repayments of $13.5 million. At December 31, 2016 there was $5.0 million, respectively, of borrowings outstanding on this facility. There were no borrowings outstanding on the facility as of June 30, 2017.
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the Wells Facility are as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 226 | $ | 2 | $ | 500 | ||||||||
| Amortization of debt issuance cost (loan fees) |
106 | 122 | 213 | 227 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 106 | $ | 348 | $ | 215 | $ | 727 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 214 | $ | 333 | $ | 470 | $ | 577 | ||||||||
Union Bank Facility
On May 5, 2016, the Company, through a special purpose wholly owned subsidiary, Hercules Funding III LLC (“Hercules Funding III”), as borrower, entered into the credit facility (the “Union Bank Facility”) with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Union Bank Facility from time to time. The Union Bank Facility replaced the company’s credit facility (the “Prior Union Bank Facility”) entered into on August 14, 2014 (as amended and restated from time to time) with MUFG Union Bank, as the arranger and administrative agent, and the lenders party to the Prior Union Bank Facility from time to time. Any references to amounts related to the Union Bank Facility prior to May 5, 2016 were incurred and relate to the Prior Union Bank Facility.
On July 18, 2016, the Company entered into the First Amendment to the Loan and Security Agreement, dated as of May 5, 2016 with MUFG Union Bank, N.A. The Amendment amends certain definitions relating to borrowings which accrue interest based on the London Interbank Offered Rate (“LIBOR Loans”) and (ii) the method(s) for calculating interest on and the paying of certain fees related to such LIBOR Loans.
Under the Union Bank Facility, MUFG Union Bank made commitments of $75.0 million. The Union Bank Facility contains an accordion feature, in which the Company can increase the credit line up to an aggregate of $200.0 million, funded by additional lenders and with the agreement of MUFG Union Bank and subject to other customary conditions. There can be no assurances that additional lenders will join the Union Bank Facility to increase available borrowings. Borrowings under the Union Bank Facility generally bear interest at either (i) if such borrowing is a base rate loan, a base rate per annum equal to the federal funds rate plus 1.00%, LIBOR plus 1.00% or MUFG Union Bank’s prime rate, in each case, plus a margin of 1.25% or (ii) if such borrowing is a LIBOR loan, a rate per annum equal to LIBOR plus 3.25%, and the Union Bank Facility generally has an advance rate of 50% against eligible debt investments. The Union Bank Facility is secured by all of the assets of Hercules Funding III.
The Company paid a one-time $562,500 structuring fee in connection with the Union Bank Facility. The Union Bank Facility requires payment of a non-use fee during the revolving credit availability period on a scale of 0.25% to 0.50% depending on the average monthly outstanding balance under the facility relative to the maximum amount of commitments at such time. For the three and six months ended June 30, 2017, the company incurred non-use of $95,000 and $189,000, respectively. For the three and six months ended June 30, 2016, the company incurred non-use fees under the Prior Union Bank Facility of $87,000 and $182,000, respectively.
F-149
The Union Bank Facility also includes various financial and other covenants applicable to the Company and its subsidiaries, in addition to those applicable to Hercules Funding III, including covenants relating to certain changes of control of the Company and Hercules Funding III. Among other things, these covenants also require the Company to maintain certain financial ratios, including a maximum debt to worth ratio, minimum interest coverage ratio, minimum portfolio funding liquidity, and a minimum tangible net worth in an amount that is in excess of $500.0 million plus 90% of the cumulative amount of equity raised after June 30, 2014. As of June 30, 2017, the minimum tangible net worth covenant increased to $765.9 million as a result of the March 2015 follow-on public offering of 7.6 million shares of common stock for total net proceeds of approximately $100.1 million, the issuance of 7.3 million shares of common stock issued under the Equity Distribution Agreement with JMP for net proceeds of $92.8 million during the year ended December 31, 2016, and the issuance of 3.3 million shares of common stock issued under the Equity Distribution Agreement with JMP for net proceeds of $46.9 million during the six months ended June 30, 2017. The Union Bank Facility provides for customary events of default, including with respect to payment defaults, breach of representations and covenants, servicer defaults, certain key person provisions, cross default provisions to certain other debt, lien and judgment limitations, and bankruptcy.
The Union Bank Facility matures on May 5, 2020, unless sooner terminated in accordance with its terms.
In connection with the Union Bank Facility, the Company and Hercules Funding III also entered into the Sale Agreement, by and among Hercules Funding III, as borrower, the Company, as originator and servicer, and MUFG Union Bank, as agent. Under the Sale Agreement, the Company agrees to (i) sell or transfer certain loans to HT III under the MUFG Union Bank Facility and (ii) act as servicer for the loans sold or transferred.
The Company did not make any draws or repayments on the available facility during the six months ended June 30, 2017. The Company had aggregate draws of $25.0 million on the available facility during the six months ended June 30, 2016 offset by repayments of $25.0 million. At June 30, 2017 and December 31, 2016, there were no borrowings outstanding on the Union Bank Facility.
For the three and six months ended June 30, 2017 and 2016, the components of interest expense and related fees and cash paid for interest expense for the previous and current Union Bank Facility are as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Interest expense |
$ | — | $ | 55 | $ | — | $ | 55 | ||||||||
| Amortization of debt issuance cost (loan fees) |
112 | 95 | 224 | 133 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total interest expense and fees |
$ | 112 | $ | 150 | $ | 224 | $ | 188 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash paid for interest expense and fees |
$ | 96 | $ | 333 | $ | 238 | $ | 577 | ||||||||
5. Income Taxes
The Company intends to operate so as to qualify to be subject to tax as a RIC under Subchapter M of the Code and, as such, will not be subject to U.S. federal income tax on the portion of taxable income and gains distributed to stockholders. Taxable income includes the Company’s taxable interest, dividend and fee income, reduced by certain deductions, as well as taxable net realized securities gains. Taxable income generally differs from net income for financial reporting purposes due to temporary and permanent differences in the recognition of income and expenses, and generally excludes net unrealized appreciation or depreciation, as such gains or losses are not included in taxable income until they are realized.
To qualify and be subject to tax as a RIC, the Company is required to meet certain income and asset diversification tests in addition to distributing dividends of an amount generally at least equal to 90% of its investment company taxable income, as defined by the Code and determined without regard to any deduction for distributions paid, to its stockholders. The amount to be paid out as a distribution is determined by the Board of
F-150
Directors each quarter and is based upon the annual earnings estimated by the management of the Company. To the extent that the Company’s earnings fall below the amount of dividend distributions declared, however, a portion of the total amount of the Company’s distributions for the fiscal year may be deemed a return of capital for tax purposes to the Company’s stockholders.
During the three months ended June 30, 2017, the Company declared a distribution of $0.31 per share. The determination of the tax attributes of the Company’s distributions is made annually as of the end of the Company’s taxable year generally based upon its taxable income for the full taxable year and distributions paid for the full taxable year. As a result, a determination made on a quarterly basis may not be representative of the actual tax attributes of the Company’s distributions for a full taxable year. If the Company had determined the tax attributes of our distributions taxable year-to-date as of June 30, 2017, 100% would be from our current and accumulated earnings and profits. However, there can be no certainty to stockholders that this determination is representative of what the actual tax attributes of the Company’s 2017 distributions to stockholders will be.
As a RIC, the Company will be subject to a 4% nondeductible U.S. federal excise tax on certain undistributed income unless the Company makes distributions treated as dividends for U.S. federal income tax purposes in a timely manner to its stockholders in respect of each calendar year of an amount at least equal to the sum of (1) 98% of the Company’s ordinary income (taking into account certain deferrals and elections) for each calendar year, (2) 98.2% of the Company’s capital gain net income (adjusted for certain ordinary losses) for the 1-year period ending October 31 of each such calendar year and (3) any ordinary income and capital gain net income realized, but not distributed, in preceding calendar years. The Company will not be subject to this excise tax on any amount on which the Company incurred U.S. federal corporate income tax (such as the tax imposed on a RIC’s retained net capital gains).
Depending on the level of taxable income earned in a taxable year, the Company may choose to carry over taxable income in excess of current taxable year distributions from such taxable income into the next taxable year and incur a 4% excise tax on such taxable income, as required. The maximum amount of excess taxable income that may be carried over for distribution in the next taxable year under the Code is the total amount of distributions paid in the following taxable year, subject to certain declaration and payment guidelines. To the extent the Company chooses to carry over taxable income into the next taxable year, distributions declared and paid by the Company in a taxable year may differ from the Company’s taxable income for that taxable year as such distributions may include the distribution of current taxable year taxable income, the distribution of prior taxable year taxable income carried over into and distributed in the current taxable year, or returns of capital.
The Company has taxable subsidiaries which are designed to hold certain portfolio investments in an effort to limit potential legal liability and/or comply with source-income type requirements contained in the RIC tax provisions of the Code. These taxable subsidiaries are consolidated for U.S. GAAP financial reporting purposes and the portfolio investments held by the taxable subsidiaries are included in the Company’s consolidated financial statements, and recorded at fair value. These taxable subsidiaries are not consolidated with the Company for income tax purposes and may generate income tax expense, or benefit, and tax assets and liabilities as a result of their ownership of certain portfolio investments. Any income generated by these taxable subsidiaries would be taxed at normal corporate tax rates based on its taxable income.
Taxable income for the six months ended June 30, 2017 was approximately $42.3 million or $0.52 per share. Taxable net realized losses for the same period was $1.2 million or approximately $0.01 per share. Taxable income for the six months ended June 30, 2016 was approximately $43.8 million or $0.61 per share. Taxable net realized losses for the same period were $2.4 million or approximately $0.03 per share.
For the six months ended June 30, 2017, the Company paid approximately $1.0 million of tax expense and had no accrued but unpaid tax expense as of the balance sheet date. For the six months ended June 30, 2016, the Company paid approximately $18,000 of tax expense and had approximately $498,000 of accrued but unpaid tax expense as of the balance sheet date.
F-151
The Company intends to distribute 100% of spillover earnings, which consists of ordinary income and long-term capital gains, from the Company’s taxable year ended December 31, 2016 to the Company’s stockholders during 2017.
6. Stockholder’s Equity
On August 16, 2013, the Company entered into the Equity Distribution Agreement with JMP. On March 7, 2016, the Company renewed the Equity Distribution Agreement and on December 21, 2016, we further amended the agreement to increase the total shares available under the program. The Equity Distribution Agreement, as amended, provides that the Company may offer and sell up to 12.0 million shares of its common stock from time to time through JMP, as its sales agent. Sales of the Company’s common stock, if any, may be made in negotiated transactions or transactions that are deemed to be “at the market,” as defined in Rule 415 under the Securities Act, including sales made directly on the NYSE or similar securities exchange or sales made to or through a market maker other than on an exchange, at prices related to the prevailing market prices or at negotiated prices.
During the six months ended June 30, 2017,the Company sold 3.3 million shares of common stock for total accumulated net proceeds of approximately $46.9 million, including $532,000 of offering expenses. The Company did not sell any shares under the program during the three months ended June 30, 2017. During the three and six months ended June 30, 2016, the Company sold 1.0 million and 2.1 million shares of common stock for total accumulated net proceeds of approximately $11.3 million and $23.7 million, respectively, including $420,000 and $822,000 of offering expenses, respectively. The Company generally uses net proceeds from these offerings to make investments, to repurchase or pay down liabilities and for general corporate purposes. As of June 30, 2017 approximately 751,000 shares remain available for issuance and sale under the ATM program.
On August 27, 2015, the Company’s Board of Directors authorized a stock repurchase plan permitting the Company to repurchase up to $50.0 million of its common stock until August 23, 2016, after which the plan expired. In January 2016, the Company repurchased 449,588 shares of its common stock at an average price per share of $10.64 per share and a total cost of approximately $4.8 million.
The Company has issued stock options for common stock subject to future issuance, of which 642,011 and 668,171 were outstanding at June 30, 2017 and December 31, 2016, respectively.
7. Equity Incentive Plan
The Company and its stockholders have authorized and adopted the 2004 Equity Incentive Plan (the “2004 Plan”) for purposes of attracting and retaining the services of its executive officers and key employees. Under the 2004 Plan, the Company is authorized to issue 12.0 million shares of common stock.
The Company and its stockholders have authorized and adopted the 2006 Non-Employee Director Plan (the “2006 Plan” and, together with the 2004 Plan, the “Plans”) for purposes of attracting and retaining the services of its Board of Directors. Under the 2006 Plan, the Company is authorized to issue 1.0 million shares of common stock. The Company filed an exemptive relief request with the Securities and Exchange Commission (“SEC”) to allow options to be issued under the 2006 Plan which was approved on October 10, 2007.
On June 21, 2007, the stockholders approved amendments to the 2004 Plan and the 2006 Plan allowing for the grant of restricted stock. The amended Plans limit the combined maximum amount of restricted stock that may be issued under both Plans to 10% of the outstanding shares of the Company’s stock on the effective date of the Plans plus 10% of the number of shares of stock issued or delivered by the Company during the terms of the Plans. The amendments further specify that no one person shall be granted awards of restricted stock relating to more than 25% of the shares available for issuance under the 2004 Plan. Further, the amount of voting securities that would result from the exercise of all of the Company’s outstanding warrants, options and rights, together
F-152
with any restricted stock issued pursuant to the Plans, at the time of issuance shall not exceed 25% of its outstanding voting securities, except that if the amount of voting securities that would result from such exercise of all of the Company’s outstanding warrants, options and rights issued to the Company’s directors, officers and employees, together with any restricted stock issued pursuant to the Plans, would exceed 15% of the Company’s outstanding voting securities, then the total amount of voting securities that would result from the exercise of all outstanding warrants, options and rights, together with any restricted stock issued pursuant to the Plans, at the time of issuance shall not exceed 20% of the Company’s outstanding voting securities.
During 2012, the Compensation Committee adopted a policy that provided for awards with different vesting schedules for short and long-term awards. All restricted stock grants under the 2004 Plan made prior to March 4, 2013 continue to vest on a monthly basis following their one year anniversary over the succeeding 36 months. Under the 2004 Plan, restricted stock awarded subsequent to March 3, 2013 vests subject to continued employment based on two vesting schedules: short-term awards vest one-half on the one year anniversary of the date of the grant and quarterly over the succeeding 12 months, and long-term awards vest one-fourth on the one year anniversary of the date of grant and quarterly over the succeeding 36 months. No restricted stock was granted pursuant to the 2004 Plan prior to 2009.
On December 29, 2016, the Company’s Board of Directors approved a further amendment and restatement of the 2004 Plan. The amended plan provides, in addition to the preexisting types of awards available for grant thereunder and among other things, (1) for the grant of restricted stock units; (2) for the deferral of the receipt of the shares of the Company’s common stock underlying vested restricted stock units; (3) that grantees may receive up to 10% of the value of the tentative restricted stock unit grants proposed for any grantee in the form of an option to acquire shares of the Company’s common stock; (4) that awards of restricted stock units may include performance vesting conditions; (5) that awards may require that all or a portion of the shares of the Company’s common stock delivered in respect of any vested restricted stock unit award be subject to a specified post-delivery holding period; and (6) that restricted stock unit awards may accrue dividend equivalents in respect of the Company’s common stock underlying any restricted stock unit award payable in the form of cash or additional shares of the Company’s common stock to the extent, and in respect of, any vested restricted stock units.
On June 21, 2017, the 2006 Plan expired in accordance with its terms and no additional awards may be granted under the 2006 Plan.
The following table summarizes the common stock option activities for the six months ended June 30, 2017 and 2016:
| Six Months Ended June 30, | ||||||||||||||||
| 2017 | 2016 | |||||||||||||||
| Common Stock Options |
Weighted Average Exercise Price |
Common
Stock Options |
Weighted Average Exercise Price |
|||||||||||||
| Outstanding at December 31, |
668,171 | $ | 13.73 | 622,171 | $ | 14.25 | ||||||||||
| Granted |
76,000 | $ | 14.69 | 128,000 | $ | 11.32 | ||||||||||
| Exercised |
(26,657 | ) | $ | 11.24 | (11,113 | ) | $ | 10.61 | ||||||||
| Forfeited |
(33,058 | ) | $ | 14.03 | (57,948 | ) | $ | 14.16 | ||||||||
| Expired |
(42,445 | ) | $ | 15.43 | (45,553 | ) | $ | 15.01 | ||||||||
|
|
|
|
|
|||||||||||||
| Outstanding at June 30, |
642,011 | $ | 13.82 | 635,557 | $ | 13.68 | ||||||||||
|
|
|
|
|
|||||||||||||
| Shares Expected to Vest at June 30, |
259,343 | $ | 13.82 | 325,833 | $ | 13.68 | ||||||||||
F-153
The following table summarizes common stock options outstanding and exercisable at June 30, 2017:
| (Dollars in thousands, except exercise price) |
Options Outstanding | Options Exercisable | ||||||||||||||||||||||||||||||
| Range of exercise prices |
Number of shares |
Weighted Average Remaining Contractual Life |
Aggregate Intrinsic Value |
Weighted Average Exercise Price |
Number of shares |
Weighted Average Remaining Contractual Life |
Aggregate Intrinsic Value |
Weighted Average Exercise Price |
||||||||||||||||||||||||
| $9.25 - $14.02 |
300,206 | 5.82 | $ | 372,064 | $ | 12.08 | 110,896 | 4.62 | $ | 196,178 | $ | 11.55 | ||||||||||||||||||||
| $14.56 - $16.34 |
341,805 | 3.99 | — | $ | 15.35 | 271,772 | 3.42 | — | $ | 15.44 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| $9.25 - $16.34 |
642,011 | 4.85 | $ | 372,064 | $ | 13.82 | 382,668 | 3.76 | $ | 196,178 | $ | 14.32 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
Options generally vest 33% one year after the date of grant and ratably over the succeeding 24 months.
All options may be exercised for a period ending seven years after the date of grant. At June 30, 2017 options for 382,668 shares were exercisable at a weighted average exercise price of approximately $14.32 per share with a weighted average remaining contractual term of 3.76 years.
The Company determined that the fair value of options granted under the Plans during the six months ended June 30, 2017 and 2016 was approximately $54,000 and $46,000, respectively. During the six months ended June 30, 2017 and 2016, approximately $39,000 and $100,000 of share-based cost due to stock option grants was expensed, respectively. As of June 30, 2017 there was approximately $110,000 of total unrecognized compensation costs related to stock options. These costs are expected to be recognized over a weighted average remaining vesting period of 2.04 years.
The Company follows ASC Topic 718 (“Compensation – Stock Compensation”) to account for stock options granted. Under ASC Topic 718, compensation expense associated with stock-based compensation is measured at the grant date based on the fair value of the award and is recognized over the vesting period. Determining the appropriate fair value model and calculating the fair value of stock-based awards at the grant date requires judgment, including estimating stock price volatility, forfeiture rate and expected option life. The fair value of options granted is based upon a Black Scholes option pricing model using the assumptions in the following table for the six months ended June 30, 2017 and 2016:
| Six Months Ended June 30, | ||||||||
| 2017 | 2016 | |||||||
| Expected Volatility |
23.07 | % | 23.73 | % | ||||
| Expected Dividends |
10 | % | 10 | % | ||||
| Expected term (in years) |
4.5 | 4.5 | ||||||
| Risk-free rate |
1.62% - 2.02% | 0.93% - 1.63% | ||||||
During the six months ended June 30, 2017 and 2016 the Company granted 10,111 shares and 547,214 shares, respectively, of restricted stock awards pursuant to the Plans. The Company determined that the fair value of restricted stock awards granted under the Plans during the six months ended June 30, 2017 and 2016 was approximately $150,000 and $6.6 million, respectively. As of June 30, 2017, there was approximately $5.2 million of total unrecognized compensation costs related to restricted stock awards. These costs are expected to be recognized over a weighted average remaining vesting period of 1.29 years.
F-154
The following table summarizes the activities for the Company’s unvested restricted stock awards for the six months ended June 30, 2017 and 2016:
| Six Months Ended June 30, | ||||||||||||||||
| 2017 | 2016 | |||||||||||||||
| Restricted Stock Awards |
Weighted Average Grant Date Fair Value |
Restricted Stock Awards |
Weighted Average Grant Date Fair Value |
|||||||||||||
| Unvested at December 31, |
799,558 | $ | 12.54 | 850,072 | $ | 13.59 | ||||||||||
| Granted |
10,111 | $ | 14.83 | 547,214 | $ | 12.01 | ||||||||||
| Vested |
(330,689 | ) | $ | 12.56 | (421,223 | ) | $ | 13.68 | ||||||||
| Forfeited |
(5,576 | ) | $ | 13.27 | (10,638 | ) | $ | 13.36 | ||||||||
|
|
|
|
|
|||||||||||||
| Unvested at June 30, |
473,404 | $ | 12.57 | 965,425 | $ | 12.65 | ||||||||||
|
|
|
|
|
|||||||||||||
During the six months ended June 30, 2017 the Company granted 600,461 shares of restricted stock units pursuant to the Plans based on the December 2016 amended terms. The Company determined that the fair value of restricted stock units granted under the Plans during the six months ended June 30, 2017 was approximately $8.5 million. As of June 30, 2017, there was approximately $7.3 million of total unrecognized compensation costs related to restricted stock units. These costs are expected to be recognized over a weighted average remaining vesting period of 2.57 years.
The following table summarizes the activities for the Company’s unvested restricted stock units for the six months ended June 30, 2017:
| Six Months Ended June 30, | ||||||||
| 2017 | ||||||||
| Restricted Stock Units |
Weighted Average Grant Date Fair Value |
|||||||
| Unvested at December 31, |
— | $ | — | |||||
| Granted |
600,461 | $ | 13.60 | |||||
| Distribution Equivalent Unit Granted |
26,717 | $ | 13.60 | |||||
| Vested |
— | $ | — | |||||
| Forfeited |
(2,874 | ) | $ | 13.92 | ||||
|
|
|
|||||||
| Unvested at June 30, |
624,304 | $ | 13.60 | |||||
|
|
|
|||||||
During the six months ended June 30, 2017, the Company expensed approximately $3.7 million of compensation expense related to restricted stock awards and restricted stock units. The Company had approximately $4.1 million in compensation expense related to restricted stock awards during the six months ended June 30, 2016.
The SEC, through an exemptive order granted on June 22, 2010, approved amendments to the Plans which allow participants to elect to have the Company withhold shares of the Company’s common stock to pay for the exercise price and applicable taxes with respect to an option exercise (“net issuance exercise”). The exemptive order also permits the holders of restricted stock to elect to have the Company withhold shares of the Company’s stock to pay the applicable taxes due on restricted stock at the time of vesting. Each individual can make a cash payment at the time of option exercise or to pay taxes on restricted stock.
F-155
8. Earnings Per Share
Shares used in the computation of the Company’s basic and diluted earnings per share are as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| (in thousands, except per share data) |
2017 | 2016 | 2017 | 2016 | ||||||||||||
| Numerator |
||||||||||||||||
| Net increase in net assets resulting from operations |
$ | 33,149 | $ | 9,475 | $ | 27,561 | $ | 23,770 | ||||||||
| Less: Distributions declared-common and restricted shares |
(25,663 | ) | (22,836 | ) | (51,330 | ) | (45,206 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Undistributed earnings |
7,486 | (13,361 | ) | (23,769 | ) | (21,436 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Undistributed earnings-common shares |
7,439 | (13,361 | ) | (23,769 | ) | (21,436 | ) | |||||||||
| Add: Distributions declared-common shares |
25,503 | 22,519 | 50,982 | 44,494 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Numerator for basic and diluted change in net assets per common share |
$ | 32,942 | $ | 9,158 | $ | 27,213 | $ | 23,058 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Denominator |
||||||||||||||||
| Basic weighted average common shares outstanding |
82,292 | 72,746 | 81,858 | 71,959 | ||||||||||||
| Common shares issuable |
103 | 16 | 95 | 6 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common shares outstanding assuming dilution |
82,395 | 72,762 | 81,953 | 71,965 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Change in net assets per common share |
||||||||||||||||
| Basic |
$ | 0.40 | $ | 0.13 | $ | 0.33 | $ | 0.32 | ||||||||
| Diluted |
$ | 0.40 | $ | 0.13 | $ | 0.33 | $ | 0.32 | ||||||||
In the table above, unvested share-based payment awards that have non-forfeitable rights to distributions or distribution equivalents are treated as participating securities for calculating earnings per share. Unvested common stock options and restricted stock units are also included in the denominator for the purpose of calculating diluted earnings per share.
For the three and six months ended June 30, 2017, the effect of the 2022 Convertible Notes under the treasury stock method is anti-dilutive and, accordingly, is excluded from the calculation of diluted earnings per share. The 2016 Convertible Notes were fully settled on or before their contractual maturity date of April 15, 2016, as such, there was no potential dilutive effect for the three and six months ended June 30, 2016.
The calculation of change in net assets resulting from operations per common share—assuming dilution, excludes all anti-dilutive shares. For the three months ended June 30, 2017, the number of anti-dilutive shares, as calculated based on the weighted average closing price of the Company’s common stock for the periods, consisted of 2.7 million shares related to 2022 Convertible Notes, 43,723 shares of unvested common stock options, and no shares of unvested restricted stock units. For the six months ended June 30, 2017, the number of anti-dilutive shares, as calculated based on the weighted average closing price of the Company’s common stock for the periods, consisted of 1.9 million shares related to 2022 Convertible Notes, 31,039 shares of unvested common stock options, and no shares of unvested restricted stock units. For three and six months ended June 30, 2016, the number of anti-dilutive shares related to unvested common stock options was 673,654 shares and 695,667 shares, respectively.
At June 30, 2017 and December 31, 2016, the Company was authorized to issue 200.0 million shares of common stock with a par value of $0.001. Each share of common stock entitles the holder to one vote.
F-156
9. Financial Highlights
Following is a schedule of financial highlights for the six months ended June 30, 2017 and 2016:
| Six Months Ended June 30, | ||||||||
| 2017 | 2016 | |||||||
| Per share data(1): |
||||||||
| Net asset value at beginning of period |
$ | 9.90 | $ | 9.94 | ||||
| Net investment income |
0.59 | 0.60 | ||||||
| Net realized gain on investments |
(0.03 | ) | (0.06 | ) | ||||
| Net unrealized depreciation on investments |
(0.22 | ) | (0.21 | ) | ||||
|
|
|
|
|
|||||
| Total from investment operations |
0.34 | 0.33 | ||||||
| Net increase (decrease) in net assets from capital share transactions(1) |
0.21 | (0.04 | ) | |||||
| Distributions of net investment income(6) |
(0.63 | ) | (0.63 | ) | ||||
| Stock-based compensation expense included in investment income(2) |
0.05 | 0.06 | ||||||
|
|
|
|
|
|||||
| Net asset value at end of period |
$ | 9.87 | $ | 9.66 | ||||
|
|
|
|
|
|||||
| Ratios and supplemental data: |
||||||||
| Per share market value at end of period |
$ | 13.24 | $ | 12.42 | ||||
| Total return(3) |
(2.04 | %) | 7.24 | % | ||||
| Shares outstanding at end of period |
82,819 | 74,320 | ||||||
| Weighted average number of common shares outstanding |
81,858 | 71,959 | ||||||
| Net assets at end of period |
$ | 817,451 | $ | 717,795 | ||||
| Ratio of total expense to average net assets(4) |
7.63 | % | 10.82 | % | ||||
| Ratio of net investment income before investment gains and losses to average net assets(4) |
11.50 | % | 12.05 | % | ||||
| Portfolio turnover rate(5) |
24.18 | % | 18.61 | % | ||||
| Weighted average debt outstanding |
$ | 707,323 | $ | 595,652 | ||||
| Weighted average debt per common share |
$ | 8.64 | $ | 8.28 | ||||
| (1) | All per share activity is calculated based on the weighted average shares outstanding for the relevant period, except net increase (decrease) in net assets from capital share transactions, which is based on the common shares outstanding as of the relevant balance sheet date. |
| (2) | Stock option expense is a non-cash expense that has no effect on net asset value. Pursuant to ASC Topic 718, net investment income includes the expense associated with the granting of stock options which is offset by a corresponding increase in paid-in capital. |
| (3) | The total return for the six months ended June 30, 2017 and 2016 equals the change in the ending market value over the beginning of the period price per share plus distributions paid per share during the period, divided by the beginning price assuming the distribution is reinvested on the date of the distribution. As such, the total return is not annualized. The total return does not reflect any sales load that must be paid by investors. |
| (4) | All ratios are calculated based on weighted average net assets for the relevant period and are annualized. |
| (5) | The portfolio turnover rate for the six months ended June 30, 2017 and 2016 equals the lesser of investment portfolio purchases or sales during the period, divided by the average investment portfolio value during the period. As such, portfolio turnover rate is not annualized. |
| (6) | Includes distributions on unvested shares. |
10. Commitments and Contingencies
The Company’s commitments and contingencies consist primarily of unused commitments to extend credit in the form of loans to the Company’s portfolio companies. A portion of these unfunded contractual commitments are dependent upon the portfolio company reaching certain milestones before the debt commitment becomes available. Furthermore, the Company’s credit agreements contain customary lending provisions which allow the Company relief from funding obligations for previously made commitments in instances where the underlying company experiences materially adverse events that affect the financial condition or business outlook for the Company. Since a portion of these commitments may expire without being drawn, unfunded contractual commitments do not necessarily represent future cash requirements. As such, the Company’s disclosure of unfunded contractual commitments includes only those which are available at the request of the portfolio company and unencumbered by milestones.
At June 30, 2017, the Company had approximately $57.6 million of unfunded commitments, including undrawn revolving facilities, which were available at the request of the portfolio company and unencumbered by milestones.
F-157
The Company also had approximately $70.0 million of non-binding term sheets outstanding at June 30, 2017. Non-binding outstanding term sheets are subject to completion of the Company’s due diligence and final investment committee approval process, as well as the negotiation of definitive documentation with the prospective portfolio companies. These non-binding term sheets generally convert to contractual commitments in approximately 90 days from signing. Not all non-binding term sheets are expected to close and do not necessarily represent future cash requirements.
The fair value of the Company’s unfunded commitments is considered to be immaterial as the yield determined at the time of underwriting is expected to be materially consistent with the yield upon funding, given that interest rates are generally pegged to market indices and given the existence of milestones, conditions and/or obligations imbedded in the borrowing agreements.
As of June 30, 2017, the Company’s unfunded contractual commitments available at the request of the portfolio company, including undrawn revolving facilities, and unencumbered by milestones are as follows:
| (in thousands) Portfolio Company |
Unfunded Commitments(1) |
|||
| NewVoiceMedia Limited |
$ | 15,000 | ||
| Evernote Corporation |
10,000 | |||
| Aquantia Corp. |
6,500 | |||
| Audentes Therapeutics, Inc. |
5,000 | |||
| Wrike, Inc. |
5,000 | |||
| Vela Trading Technologies |
4,800 | |||
| MDX Medical Inc. |
4,500 | |||
| 908 DEVICES INC. |
2,500 | |||
| Verastem, Inc. |
2,500 | |||
| RedSeal Inc. |
1,795 | |||
|
|
|
|||
| Total |
$ | 57,595 | ||
|
|
|
|||
| (1) | Amount represents unfunded commitments, including undrawn revolving facilities, which are available at the request of the portfolio company. Amount excludes unfunded commitments which are unavailable due to the borrower having not met certain milestones. |
Certain premises are leased under agreements which expire at various dates through March 2020. Total rent expense amounted to approximately $449,000 and $893,000 during the three and six months ended June 30, 2017. Total rent expense amounted to approximately $436,000 and $872,000 during the same period ended June 30, 2016.
The Company’s contractual obligations as of June 30, 2017 include:
| Payments due by period (in thousands) | ||||||||||||||||||||
| Contractual Obligations(1) |
Total | Less than 1 year | 1 - 3 years | 3 - 5 years | After 5 years | |||||||||||||||
| Borrowings(2)(3) |
$ | 766,388 | $ | 87,678 | $ | 21,800 | $ | 349,400 | $ | 307,510 | ||||||||||
| Operating Lease Obligations(4) |
2,616 | 1,744 | 872 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 769,004 | $ | 89,422 | $ | 22,672 | $ | 349,400 | $ | 307,510 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Excludes commitments to extend credit to the Company’s portfolio companies. |
| (2) | Includes $190.2 million in principal outstanding under the SBA debentures, $258.5 million of the 2024 Notes, $230.0 million of the 2022 Convertible Notes and $87.7 million of the 2021 Asset-Backed Notes as of June 30, 2017. |
| (3) | Amounts represent future principal repayments and not the carrying value of each liability. See Note 4 to the Company’s consolidated financial statements. |
| (4) | Facility leases. |
The Company may, from time to time, be involved in litigation arising out of its operations in the normal course of business or otherwise. Furthermore, third parties may try to seek to impose liability on the Company in connection with the activities of its portfolio companies. While the outcome of any current legal proceedings
F-158
cannot at this time be predicted with certainty, the Company does not expect any current matters will materially affect the Company’s financial condition or results of operations; however, there can be no assurance whether any pending legal proceedings will have a material adverse effect on the Company’s financial condition or results of operations in any future reporting period.
11. Recent Accounting Pronouncements
In January 2016, the FASB issued ASU 2016-01, “Financial Instruments—Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities,” which, among other things, requires that (i) all equity investments, other than equity-method investments, in unconsolidated entities generally be measured at fair value through earnings and (ii) an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments. Additionally, the ASU changes the disclosure requirements for financial instruments. ASU 2016-01 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2017. Early adoption is permitted for certain provisions. The Company does not believe that ASU 2016-01 will have a material impact on its consolidated financial statements and disclosures.
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842),” which, among other things, requires recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous GAAP. Additionally, the ASU requires the classification of all cash payments on leases within operating activities in the Consolidated Statement of Cash Flows. ASU 2016-02 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2018. Early adoption is permitted. The Company does not believe that ASU 2016-02 will have a material impact on its consolidated financial statements and disclosures.
In March 2016, the FASB issued ASU 2016-09, “Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting,” which, among other things, simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. ASU 2016-09 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2016. There is not a material impact from adopting this standard on the Company’s financial statements. The Company has adopted this standard for the six months ended June 30, 2017.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments,” which addresses eight specific cash flow issues including, among other things, the classification of debt prepayment or debt extinguishment costs. ASU 2016-15 is effective for annual reporting periods, and the interim periods within those periods, beginning after December 15, 2017. Early adoption is permitted. The Company does not believe that ASU 2016-15 will have a material impact on its consolidated financial statements and disclosures.
In November 2016, the FASB issued ASU 2016-18, “Statement of Cash Flows (Topic 230),” which requires that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The new guidance is effective for interim and annual periods beginning after December 15, 2017 and early adoption is permitted. The amendment should be adopted retrospectively. The Company does not believe that ASU 2016-18 will have a material impact on its consolidated financial statements and disclosures.
F-159
12. Subsequent Events
Distribution Declaration
On July 26, 2017 the Board of Directors declared a cash distribution of $0.31 per share to be paid on August 21, 2017 to stockholders of record as of August 14, 2017. This distribution represents the Company’s forty-eighth consecutive distribution since the Company’s IPO, bringing the total cumulative distribution to date to $13.40 per share.
Departure of Officer
On June 26, 2017, Andrew Olson announced his resignation, effective July 21, 2017, from his position as Vice President of Finance and Senior Controller. Gerard R. Waldt, Jr., our current Assistant Controller, assumed the position of Controller.
Portfolio Company Developments
As of July 31, 2017, the Company held warrants or equity positions in seven companies that have filed registration statements on Form S-1 with the SEC in contemplation of potential initial public offerings. All seven companies filed confidentially under the Jumpstart Our Business Startups Act. There can be no assurance that companies that have yet to complete their initial public offerings will do so in a timely matter or at all. In addition, subsequent to June 30, 2017, the following companies announced or completed liquidity events:
| 1. | In May 2017, the Company’s portfolio company Jaguar Animal Health, Inc. entered into a binding agreement merger agreement with Napo Pharmaceuticals. The merger became effective on July 31, 2017, at which point Jaguar Animal Health’s name changed to Jaguar Health, Inc. and Napo Pharmaceuticals began operating as a wholly-owned subsidiary of Jaguar Health, Inc. |
| 2. | In July 2017, the Company’s portfolio company JumpStart Games, Inc. was acquired by NetDragon Websoft Holding Limited. The acquisition was completed by NetDragon in Hong Kong. Terms of the transaction were not disclosed. |
F-160
HERCULES CAPITAL, INC.
SCHEDULE OF INVESTMENTS IN AND ADVANCES TO AFFILIATES
As of and for the Six Months Ended June 30, 2017
(in thousands)
| Portfolio Company |
Investment(1) | Amount of Interest Credited to Income(2) |
As of December 31, 2016 Fair Value |
Gross Additions(3) |
Gross Reductions(4) |
Net Change in Unrealized Appreciation/ (Depreciation) |
As of June 30, 2017 Fair Value |
|||||||||||||||||||||
| Control Investments |
||||||||||||||||||||||||||||
| Majority Owned Control Investments |
||||||||||||||||||||||||||||
| Achilles Technology Management Co II, Inc. |
Senior Debt | $ | 142 | $ | 1,304 | $ | 74 | $ | (450 | ) | $ | — | $ | 928 | ||||||||||||||
| Common Stock | — | 3,396 | — | — | (2,208 | ) | 1,188 | |||||||||||||||||||||
| HercGamma, Inc.(6) |
Common Stock | — | — | 1,169 | — | — | 1,169 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total Majority Owned Control Investments |
$ | 142 | $ | 4,700 | $ | 1,243 | $ | (450 | ) | $ | (2,208 | ) | $ | 3,285 | ||||||||||||||
| Other Control Investments |
||||||||||||||||||||||||||||
| SkyCross, Inc. |
Senior Debt | $ | — | $ | — | $ | — | $ | (1,842 | ) | $ | 1,842 | $ | — | ||||||||||||||
| Preferred Warrants | — | — | — | (394 | ) | 394 | — | |||||||||||||||||||||
| Tectura Corporation(5) |
Senior Debt | 899 | — | 20,231 | (240 | ) | — | 19,991 | ||||||||||||||||||||
| Preferred Warrants | — | — | 51 | (102 | ) | 51 | — | |||||||||||||||||||||
| Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.)(7) |
Common Stock | — | — | 61,502 | — | (53,214 | ) | 8,288 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total Other Control Investments |
$ | 899 | $ | — | $ | 81,784 | $ | (2,578 | ) | $ | (50,927 | ) | $ | 28,279 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total Control Investments |
$ | 1,041 | $ | 4,700 | $ | 83,027 | $ | (3,028 | ) | $ | (53,135 | ) | $ | 31,564 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Affiliate Investments |
||||||||||||||||||||||||||||
| Optiscan BioMedical, Corp. |
Preferred Stock | $ | — | $ | 4,529 | $ | 173 | $ | — | $ | 1,109 | $ | 5,811 | |||||||||||||||
| Preferred Warrants | — | 170 | — | — | 10 | 180 | ||||||||||||||||||||||
| Stion Corporation |
Senior Debt | 2 | 333 | — | (333 | ) | — | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total Affiliate Investments |
$ | 2 | $ | 5,032 | $ | 173 | $ | (333 | ) | $ | 1,119 | $ | 5,991 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total Control and Affiliate Investments |
$ | 1,043 | $ | 9,732 | $ | 83,200 | $ | (3,361 | ) | $ | (52,016 | ) | $ | 37,555 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Stock and warrants are generally non-income producing and restricted. The principal amount for debt is shown in the Consolidated Schedule of Investments as of June 30, 2017 |
| (2) | Represents the total amount of interest or dividends credited to income for the period an investment was an affiliate or control investment. |
| (3) | Gross additions include increases in the cost basis of investments resulting from new portfolio investments, paid-in-kind interest or dividends, the amortization of discounts and closing fees and the exchange of one or more existing securities for one or more new securities. |
| (4) | Gross reductions include decreases in the cost basis of investments resulting from principal repayments or sales and the exchange of one or more existing securities for one or more new securities. Gross reductions also include previously recognized depreciation on investments that become control or affiliate investments during the period. |
| (5) | As of March 31, 2017, the Company’s investment in Tectura Corporation became classified as a control investment as of result of obtaining more than 50% representation on the portfolio company’s board. |
| (6) | As of June 30, 2107, the Company’s wholly owned subsidiary HercGamma, Inc. became classified as a control investment as a result of an investment in a portfolio company whereby the subsidiary obtained a controlling financial interest. |
| (7) | As of June 30, 2107, the Company’s investment in Solar Spectrum Holdings LLC (p.k.a. Sungevity, Inc.) became classified as a control investment as a result of obtaining a controlling financial interest. |
F-161
$

% Notes due
PROSPECTUS SUPPLEMENT
Joint Book-Running Managers
| Citigroup | Jefferies |
The date of this prospectus supplement is October , 2017.